Scale-Free Functional Brain Networks Exhibit Increased Connectivity, Are More Integrated and Less Segregated in Patients with Parkinson’s Disease following Dopaminergic Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Preprocessing
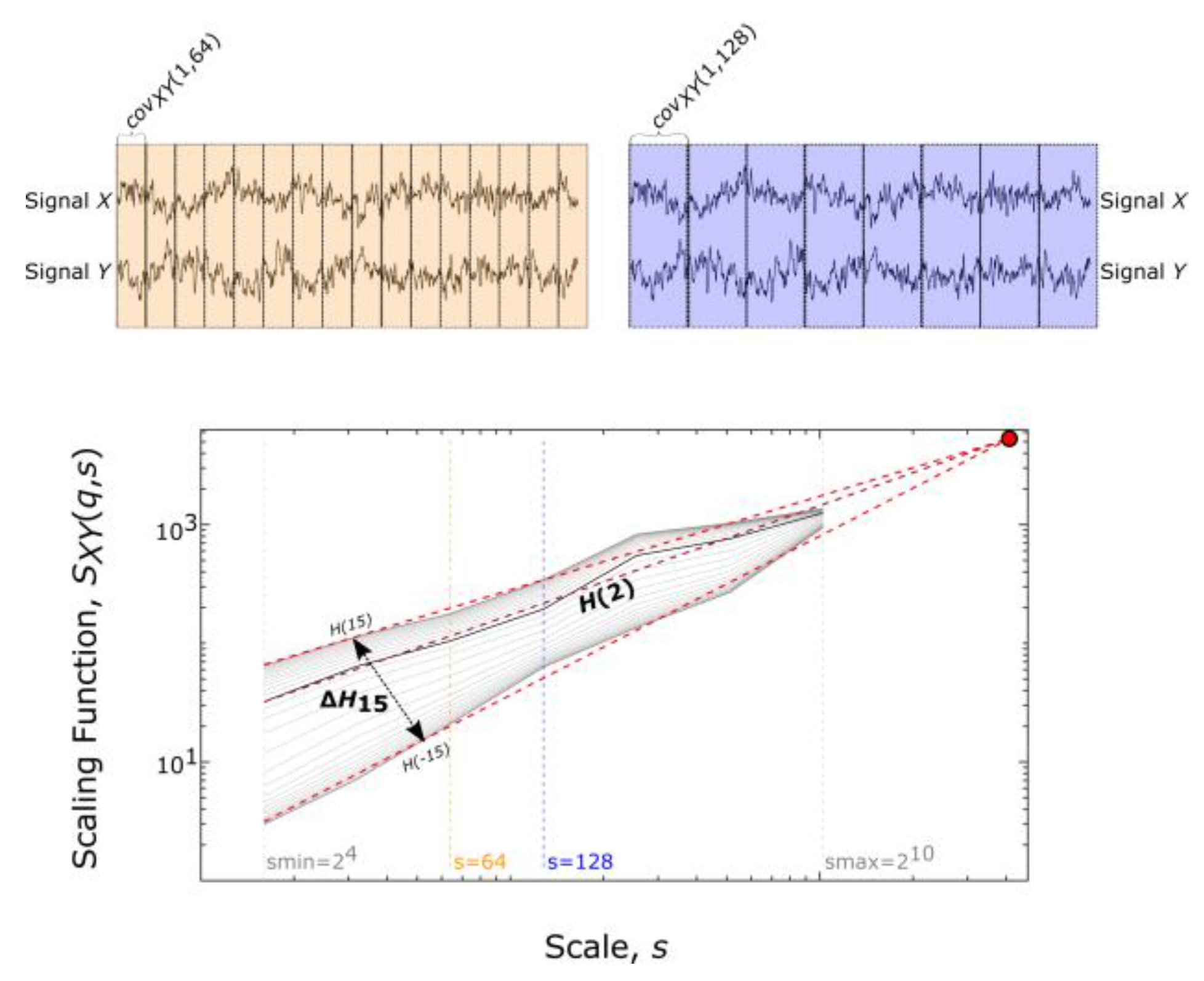
2.3. Bivariate Focus-Based Multifractal Analysis
2.4. Assessing Multifractality
2.5. Brain Networks
2.6. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beitz, J.M. Parkinson s disease a review. Front. Biosci. 2014, S6, S415. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W. Non-motor symptoms in Parkinson’s disease. Eur. J. Neurol. 2008, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wu, T. The study of brain functional connectivity in Parkinson’s disease. Transl. Neurodegener. 2016, 5, 18. [Google Scholar] [CrossRef]
- Cerasa, A.; Novellino, F.; Quattrone, A. Connectivity Changes in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2016, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Utianski, R.L.; Caviness, J.N.; van Straaten, E.C.; Beach, T.G.; Dugger, B.N.; Shill, H.A.; Driver-Dunckley, E.D.; Sabbagh, M.N.; Mehta, S.; Adler, C.H.; et al. Graph theory network function in Parkinson’s disease assessed with electroencephalography. Clin. Neurophysiol. 2016, 127, 2228–2236. [Google Scholar] [CrossRef]
- Tahmasian, M.; Bettray, L.M.; van Eimeren, T.; Drzezga, A.; Timmermann, L.; Eickhoff, C.R.; Eickhoff, S.B.; Eggers, C. A systematic review on the applications of resting-state fMRI in Parkinson’s disease: Does dopamine replacement therapy play a role? Cortex 2015, 73, 80–105. [Google Scholar] [CrossRef]
- Evangelisti, S.; Pittau, F.; Testa, C.; Rizzo, G.; Gramegna, L.L.; Ferri, L.; Coito, A.; Cortelli, P.; Calandra-Buonaura, G.; Bisquoli, F.; et al. L-Dopa Modulation of Brain Connectivity in Parkinson’s Disease Patients: A Pilot EEG-fMRI Study. Front. Neurosci. 2019, 13, 611. [Google Scholar] [CrossRef]
- Jalili, M. Graph theoretical analysis of Alzheimer’s disease: Discrimination of AD patients from healthy subjects. Inf. Sci. 2016, 384, 145–156. [Google Scholar] [CrossRef]
- Podobnik, B.; Stanley, H.E. Detrended Cross-Correlation Analysis: A New Method for Analyzing Two Nonstationary Time Series. Phys. Rev. Lett. 2008, 100, 084102. [Google Scholar] [CrossRef]
- Eke, A.; Herman, P.; Kocsis, L.; Kozak, L.R. Fractal characterization of complexity in temporal physiological signals. Physiol. Meas. 2002, 23, R1–R38. [Google Scholar] [CrossRef] [PubMed]
- Kristoufek, L. Spectrum-based estimators of the bivariate Hurst exponent. Phys. Rev. E 2014, 90, 062802. [Google Scholar] [CrossRef] [PubMed]
- He, B.J. Scale-free brain activity: Past, present, and future. Trends Cogn. Sci. 2014, 18, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Kristoufek, L. Testing power-law cross-correlations: Rescaled covariance test. Eur. Phys. J. B 2013, 86, 418. [Google Scholar] [CrossRef]
- He, B.J.; Zempel, J.M.; Snyder, A.Z.; Raichle, M.E. The temporal structures and functional significance of scale-free brain activity. Neuron 2010, 66, 353–369. [Google Scholar] [CrossRef]
- Mukli, P.; Nagy, Z.; Racz, F.S.; Herman, P.; Eke, A. Impact of Healthy Aging on Multifractal Hemodynamic Fluctuations in the Human Prefrontal Cortex. Front. Physiol. 2018, 9, 1072. [Google Scholar] [CrossRef]
- Stylianou, O.; Racz, F.S.; Eke, A.; Mukli, P. Scale-Free Coupled Dynamics in Brain Networks Captured by Bivariate Focus-Based Multifractal Analysis. Front. Physiol. 2021, 11, 615961. [Google Scholar] [CrossRef]
- Stylianou, O.; Racz, F.S.; Kim, K.; Kaposzta, Z.; Czoch, A.; Yabluchanskiy, A.; Eke, A.; Mukli, P. Multifractal Functional Connectivity Analysis of Electroencephalogram Reveals Reorganization of Brain Networks in a Visual Pattern Recognition Paradigm. Front. Hum. Neurosci. 2021, 15, 608. [Google Scholar] [CrossRef]
- George, J.S.; Strunk, J.; Mak-McCully, R.; Houser, M.; Poizner, H.; Aron, A.R. Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. NeuroImage Clin. 2013, 3, 261–270. [Google Scholar] [CrossRef]
- Jackson, N.; Cole, S.R.; Voytek, B.; Swann, N.C. Characteristics of Waveform Shape in Parkinson’s Disease Detected with Scalp Electroencephalography. Eneuro 2019, 6. [Google Scholar] [CrossRef]
- Swann, N.C.; de Hemptinne, C.; Aron, A.R.; Ostrem, J.L.; Knight, R.T.; Starr, P.A. Elevated synchrony in Parkinson disease detected with electroencephalography. Ann. Neurol. 2015, 78, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Pernet, C.R.; Appelhoff, S.; Gorgolewski, K.J.; Flandin, G.; Phillips, C.; Delorme, A.; Oostenveld, R. EEG-BIDS, an extension to the brain imaging data structure for electroencephalography. Sci. Data 2019, 6, 103. [Google Scholar] [CrossRef] [PubMed]
- Appelhoff, S.; Sanderson, M.; Brooks, T.L.; van Vliet, M.; Quentin, R.; Holdgraf, C.; Chaumon, M.; Mikulan, E.; Tavabi, K.; Höchenberger, R.; et al. MNE-BIDS: Organizing electrophysiological data into the BIDS format and facilitating their analysis. J. Open Source Softw. 2019, 4, 1896. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.; Pernier, J.; Bertrand, O.; Echallier, J.F. Spherical splines for scalp potential and current density mapping. Electroencephalogr. Clin. Neurophysiol. 1989, 72, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Kayser, J.; Tenke, C.E. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clin. Neurophysiol. 2006, 117, 348–368. [Google Scholar] [CrossRef]
- Kayser, J.; Tenke, C.E. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin. Neurophysiol. 2006, 117, 369–380. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Makeig, S.; Jung, T.-P.; Bell, A.J.; Ghahremani, D.; Sejnowski, T.J. Blind separation of auditory event-related brain responses into independent components. Proc. Natl. Acad. Sci. USA 1997, 94, 10979–10984. [Google Scholar] [CrossRef]
- Grech, D.; Pamuła, G. Multifractal Background Noise of Monofractal Signals. Acta Phys. Pol. A 2012, 121, B-34–B-39. [Google Scholar] [CrossRef]
- Mukli, P.; Nagy, Z.; Eke, A. Multifractal formalism by enforcing the universal behavior of scaling functions. Phys. A Stat. Mech. Its Appl. 2015, 417, 150–167. [Google Scholar] [CrossRef]
- Ashkenazy, Y.; Havlin, S.; Ivanov, P.C.; Peng, C.-K.; Schulte-Frohlinde, V.; Stanley, H.E. Magnitude and sign scaling in power-law correlated time series. Phys. A Stat. Mech. Its Appl. 2003, 323, 19–41. [Google Scholar] [CrossRef]
- Podobnik, B.; Jiang, Z.-Q.; Zhou, W.-X.; Stanley, H.E. Statistical tests for power-law cross-correlated processes. Phys. Rev. E 2011, 84, 066118. [Google Scholar] [CrossRef] [PubMed]
- Prichard, D.; Theiler, J. Generating surrogate data for time series with several simultaneously measured variables. Phys. Rev. Lett. 1994, 73, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shang, P.; Ge, W. Multifractal cross-correlation analysis based on statistical moments. Fractals 2012, 20, 271–279. [Google Scholar] [CrossRef]
- Wendt, H.; Scherrer, A.; Abry, P.; Achard, S. Testing fractal connectivity in multivariate long memory processes. In Proceedings of the 2009 IEEE International Conference on Acoustics, Speech and Signal Processing, Taipei, Taiwan, 19–24 April 2009; pp. 2913–2916. [Google Scholar]
- Arbabshirani, M.R.; Damaraju, E.; Phlypo, R.; Plis, S.; Allen, E.; Ma, S.; Mathalon, D.; Preda, A.; Vaidya, J.G.; Adali, T.; et al. Impact of autocorrelation on functional connectivity. Neuroimage 2014, 102, 294–308. [Google Scholar] [CrossRef]
- Kristoufek, L. Can the bivariate Hurst exponent be higher than an average of the separate Hurst exponents? Phys. A Stat. Mech. Its Appl. 2015, 431, 124–127. [Google Scholar] [CrossRef]
- Kristoufek, L. Power-law cross-correlations estimation under heavy tails. Commun. Nonlinear Sci. Numer. Simul. 2016, 40, 163–172. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Hirano, S. Clinical implications for dopaminergic and functional neuroimage research in cognitive symptoms of Parkinson’s disease. Mol. Med. 2021, 27, 40. [Google Scholar] [CrossRef]
- Beaulieu, J.-M.; Gainetdinov, R.R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Neve, K.A. Dopamine Receptors. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 169–173. [Google Scholar]
- Stoffers, D.; Bosboom, J.L.W.; Deijen, J.B.; Wolters, E.C.; Stam, C.J.; Berendse, H.W. Increased cortico-cortical functional connectivity in early-stage Parkinson’s disease: An MEG study. Neuroimage 2008, 41, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Olde Dubbelink, K.T.; Hillebrand, A.; Stoffers, D.; Deijen, J.B.; Twisk, J.W.; Stam, C.J.; Berendse, H.W. Disrupted brain network topology in Parkinson’s disease: A longitudinal magnetoencephalography study. Brain 2014, 137, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D.S.; Meyer-Lindenberg, A.; Achard, S.; Duke, T.; Bullmore, E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl. Acad. Sci. USA 2006, 103, 19518–19523. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. The human connectome: A complex network. Ann. N. Y. Acad. Sci. 2011, 1224, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Pappalettera, C.; Miraglia, F.; Alù, F.; Orticoni, A.; Judica, E.; Cotelli, M.; Pistoia, F.; Rossini, P.M. Graph Theory on Brain Cortical Sources in Parkinson’s Disease: The Analysis of ‘Small World’ Organization from EEG. Sensors 2021, 21, 7266. [Google Scholar] [CrossRef]
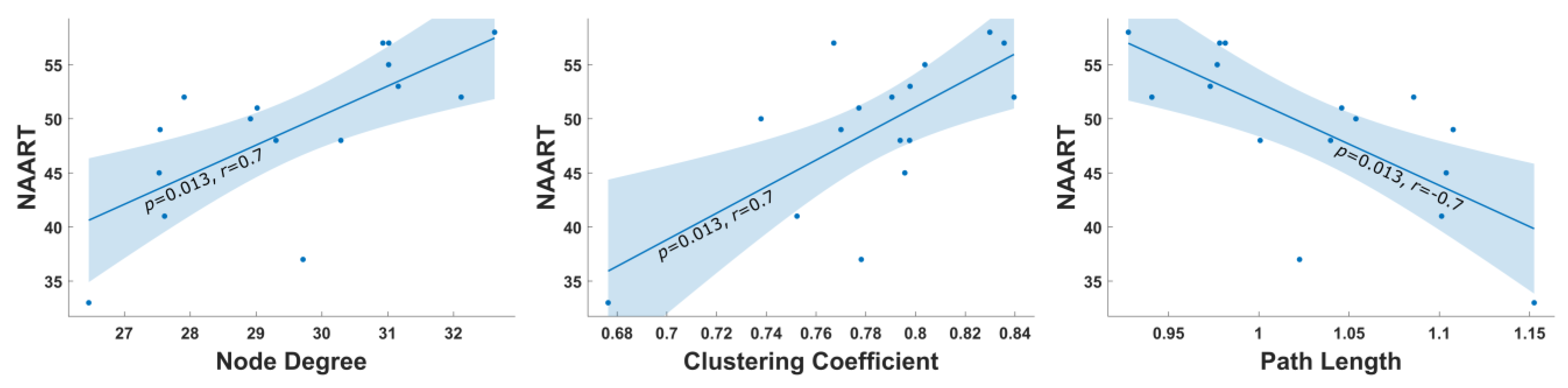
- Uttl, B. North American Adult Reading Test: Age Norms, Reliability, and Validity. J. Clin. Exp. Neuropsychol. 2002, 24, 1123–1137. [Google Scholar] [CrossRef]
- Stefanovski, L.; Triebkorn, P.; Spiegler, A.; Diaz-Cortes, M.A.; Solodkin, A.; Jirsa, V.; McIntosh, A.R.; Ritter, P.; Alzheimer’s Disease Neuroimaging Initiative. Linking Molecular Pathways and Large-Scale Computational Modeling to Assess Candidate Disease Mechanisms and Pharmacodynamics in Alzheimer’s Disease. Front. Comput. Neurosci. 2019, 13, 54. [Google Scholar] [CrossRef]
- Schirner, M.; McIntosh, A.R.; Jirsa, V.; Deco, G.; Ritter, P. Inferring multi-scale neural mechanisms with brain network modelling. Elife 2018, 7, e28927. [Google Scholar] [CrossRef]
- Arbabyazd, L.; Shen, K.; Wang, Z.; Hofmann-Apitius, M.; Ritter, P.; McIntosh, A.R.; Battaglia, D.; Jirsa, V. Virtual Connectomic Datasets in Alzheimer’s Disease and Aging Using Whole-Brain Network Dynamics Modelling. Eneuro 2021, 8. [Google Scholar] [CrossRef]
- Bohara, G.; Lambert, D.; West, B.J.; Grigolini, P. Crucial events, randomness, and multifractality in heartbeats. Phys. Rev. E 2017, 96, 062216. [Google Scholar] [CrossRef] [PubMed]
- Culbreth, G.; West, B.; Grigolini, P. Entropic Approach to the Detection of Crucial Events. Entropy 2019, 21, 178. [Google Scholar] [CrossRef] [PubMed]


| Group | Tests | |||||
|---|---|---|---|---|---|---|
| SS | PR | SΔH15 | S-H(2) | DCCC | Biv-Univ | |
| HC | 78 ± 4% | 97 ± 4% | 100 ± 0% | 100 ± 0% | 11 ± 7% | 10 ± 5% |
| PD-OFF | 77 ± 4% | 99 ± 1% | 100 ± 0% | 100 ± 0% | 8 ± 4% | 11 ± 7% |
| PD-ON | 72 ± 15% | 99 ± 2% | 100 ± 0% | 94 ± 14% | 11 ± 9% | 20 ± 19% |
| Network | Group | ||
|---|---|---|---|
| HC | PD-OFF | PD-ON | |
| H(2) | 0.7 ± 1% | 0.4 ± 0.4% | 0.8 ± 0.7% |
| ΔH15 | 0.7 ± 0.8% | 0.4 ± 0.4% | 0.8 ± 0.7 % |
| HC vs. PD-OFF | 0.43 | 0.62 | 0.48 | 0.76 | 0.34 | 0.62 |
| HC vs. PD-ON | 0.86 | 0.03 * | 0.26 | 0.01 * | 0.83 | 0.03 * |
| PD-OFF vs. PD-ON | 0.19 | 0.04 * | 0.85 | 0.02 * | 0.19 | 0.04 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stylianou, O.; Kaposzta, Z.; Czoch, A.; Stefanovski, L.; Yabluchanskiy, A.; Racz, F.S.; Ritter, P.; Eke, A.; Mukli, P. Scale-Free Functional Brain Networks Exhibit Increased Connectivity, Are More Integrated and Less Segregated in Patients with Parkinson’s Disease following Dopaminergic Treatment. Fractal Fract. 2022, 6, 737. https://doi.org/10.3390/fractalfract6120737
Stylianou O, Kaposzta Z, Czoch A, Stefanovski L, Yabluchanskiy A, Racz FS, Ritter P, Eke A, Mukli P. Scale-Free Functional Brain Networks Exhibit Increased Connectivity, Are More Integrated and Less Segregated in Patients with Parkinson’s Disease following Dopaminergic Treatment. Fractal and Fractional. 2022; 6(12):737. https://doi.org/10.3390/fractalfract6120737
Chicago/Turabian StyleStylianou, Orestis, Zalan Kaposzta, Akos Czoch, Leon Stefanovski, Andriy Yabluchanskiy, Frigyes Samuel Racz, Petra Ritter, Andras Eke, and Peter Mukli. 2022. "Scale-Free Functional Brain Networks Exhibit Increased Connectivity, Are More Integrated and Less Segregated in Patients with Parkinson’s Disease following Dopaminergic Treatment" Fractal and Fractional 6, no. 12: 737. https://doi.org/10.3390/fractalfract6120737
APA StyleStylianou, O., Kaposzta, Z., Czoch, A., Stefanovski, L., Yabluchanskiy, A., Racz, F. S., Ritter, P., Eke, A., & Mukli, P. (2022). Scale-Free Functional Brain Networks Exhibit Increased Connectivity, Are More Integrated and Less Segregated in Patients with Parkinson’s Disease following Dopaminergic Treatment. Fractal and Fractional, 6(12), 737. https://doi.org/10.3390/fractalfract6120737






