1. Introduction
In recent decades, the explosive development of MgO-based cements has lied in an environmental consideration. Compared to the manufacture temperature of ordinary Portland cement, the lower temperature is required to produce MgO. Therefore, the energy saving associated with this decreased temperature makes MgO-based cements the core of eco-friendly cement production in the future. Magnesium oxysulfate cement (MOS) is a typical eco-friendly non-hydraulic material, first proposed by a Belgian scholar in 1957, similar to the magnesium oxychloride cement (MOC) [
1,
2]. MOS paste is prepared through the chemical reaction of active MgO powder and magnesium sulfate solution. MOS-based materials have attracted much attention due to their excellent performances, such as good fire resistance, good steel protection, low density and thermal conductivity, etc. [
3,
4]. MOS paste is similar in concept to MOC paste, except that MgSO
4 solution is used instead of MgCl
2 solution to form cementitious binder. Typically, the main chemical reactions in MOS cement system are shown as below:
As early as in 1957, Demediuk and Cole [
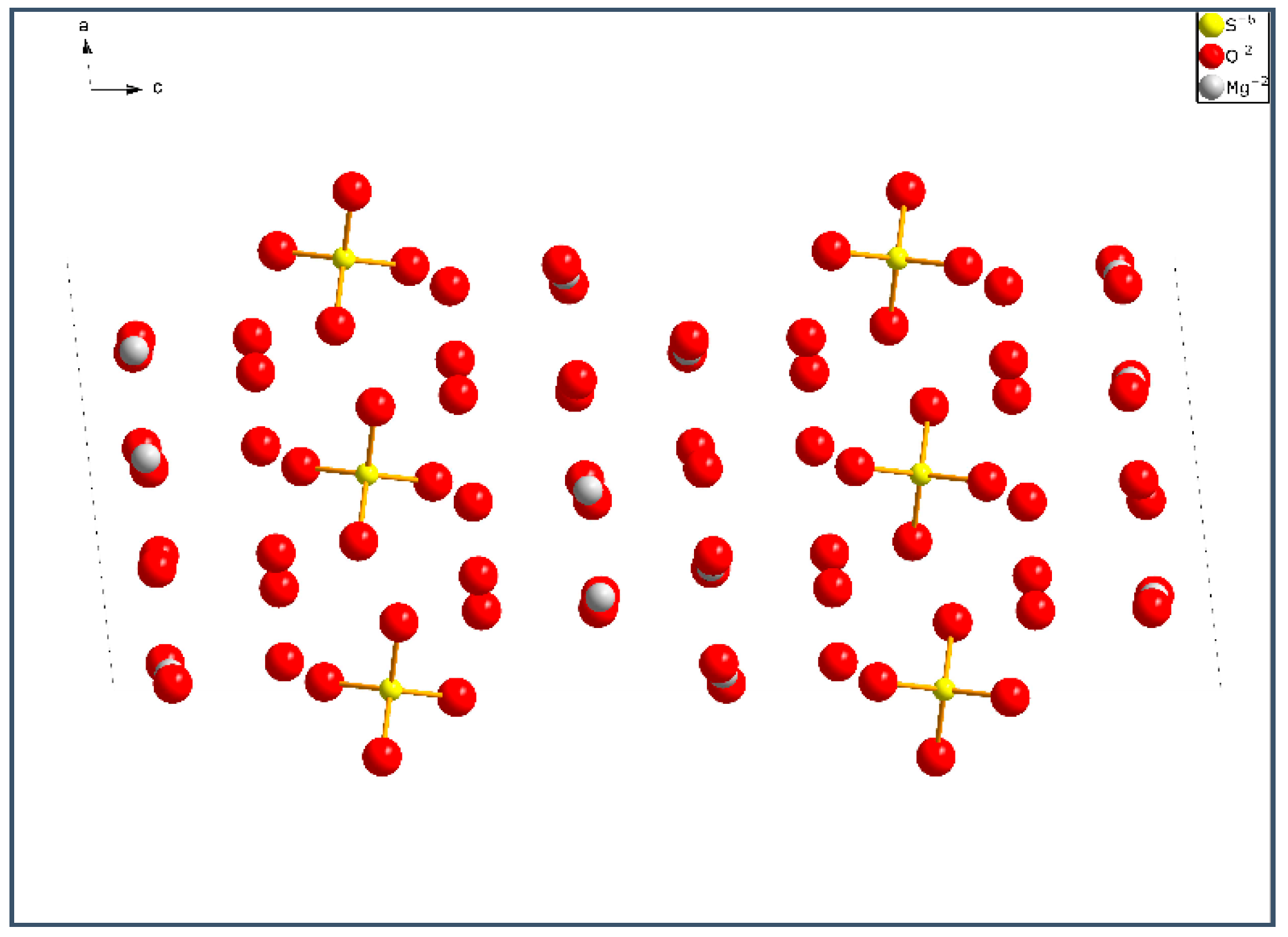
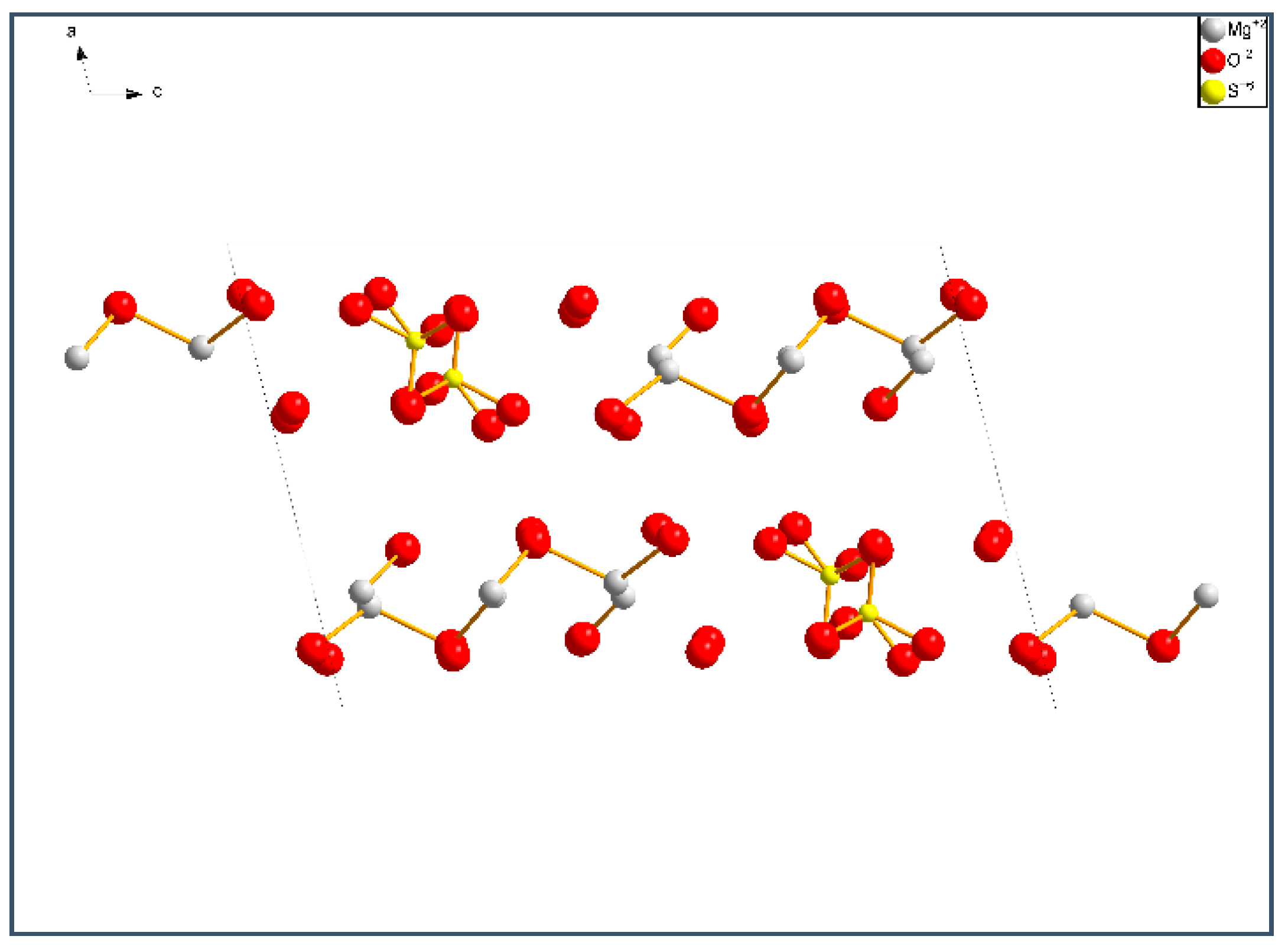
1] analyzed the hydrated products in the MOS paste under the temperature range of 30–120 °C. They identified four magnesium oxysulfate phases (Phase 318, Phase 513, Phase 115 and Phase 123). Phases 512, 517, 131, 122, 251 and 514 were reported in other literatures [
5]. These phases observed in MOS pastes are shown in
Table 1.
The phase transformation and stability of hydrated phases in MOS paste, are expected to be primarily associated with two molar ratios, i.e., MgO/MgSO
4 (M) and H
2O/MgSO
4 (H). Generally, the following six main phases are generated within the temperature range from 30 °C to 120 °C [
9]: Phase 517 (5Mg(OH)
2·MgSO
4·7H
2O), Phase 318 (3Mg(OH)
2·MgSO
4·8H
2O), Phase 513 (5Mg(OH)
2·MgSO
4·3H
2O), Phase 115 (Mg(OH)
2·MgSO
4·5H
2O), Phase 123 (Mg(OH)
2·2MgSO
4·3H
2O) and magnesium hydroxide (MH). When M increases from 3 to 9, the main hydrated products are found to be Phase 318, Phase 517, and MH [
10,
11]. It is believed that Phase 517 has the high strength and good water resistance [
9]. However, it is noted that the low strength and chemical instability of eco-friendly MOS-based materials primarily hinder their large-scale applications to some extent.
Thermodynamics is an indispensable tool for understanding and studying chemical reactions [
12]. It is generally accepted that the thermodynamic simulation can be applied to characterize the composition of hydrated products of cement pastes. Lothenbach et al. developed a model to simulate the composition of pore solution and the hydrated products in the hydration process of ordinary Portland cement based on the Parrot-Killoh hydration rate equation [
13] where the influences of sulfate attacks [
14], temperature [
15] and additives [
16] were considered. Similarly, Möschner et al. [
17] explored the effect of citric acid on the composition of solid and liquid phases in the hydrating cement pastes based on thermodynamic calculations. The citric acid was found to significantly change the composition of hydrated products but had little influence on the composition of the pore solution. Recently, Zhou et al. [
18] utilized a thermodynamic approach to study the formation conditions of two major hydrated products (Mg
3(OH)
5Cl·4H
2O and Mg
2(OH)
3Cl·4H
2O) of MOC pastes under room temperature. The results showed that the formation of hydrated products was controlled by the concentration of MgCl
2, activity of H
2O and pH values of the paste. The equilibrium of solid phase diagram and the solubility of different hydrated phases were consistent with those results obtained from experimental studies. Li et al. [
19] developed a temperature-dependent thermodynamic model for Mg(OH)
2 + MgSO
4 + H
2O system based on CALPHAD approach. Thermodynamic properties of several magnesium hydroxide sulfate hydrates were determined as functions of temperature. In summary, the present thermodynamic simulations have primarily been applied to simulate Portland cements and sulphoaluminate cements. The relevant thermodynamics database is only applicable for Portland cement and sulphoaluminate cements, and there is lack of report on the thermodynamic database dedicated to the MOS cement system. First principle calculation, as one powerful research tool inquiring the properties of materials from the atomic and molecular levels, has been successfully applied to investigate the thermodynamic properties of materials [
20,
21]. The most widely-used software based on the first principle calculation are WIEN [
22], Gaussian 98 [
23] and Material Studio [
24]. A comparison among them is listed in
Table 2. Therefore, it is very meaningful to establish a thermodynamic database that is applicable for the hydrating MOS-based materials through the first principle calculation.
Customarily, cement-based materials are considered as porous composite materials, perhaps consisting of a binding matrix and/or fragment of aggregates [
25,
26]. The pore structure affects the physical and chemical properties, and controls the durability of material [
27,
28,
29]. During past few years, several microstructure-based models, such as the durability models of concrete model (DuCOM) by Chaube et al. [
30], HYMOSTRUC by Van Breugel [
31], and μic by Bishnoi and Scrivener [
32], have been proposed to simulate the pore structure of cement paste at the micro level. Recently, a status-oriented computer model [
33] has been proposed to simulate the microstructure of OPC pastes. It is assumed that four phases (unhydrated cement grains, large capillary pores, inner and outer hydrated product layers) are contained in the hydrated cement paste. The microstructure of cement paste can be simulated as a function of cement particle distribution, water to cement ratio, and degree of hydration. Compared to early developed models, HYMOSTRUC and μic only focus on the early hydration stage of cement-based materials and DuCOM is inapplicable to the poly-size particle system. Therefore, the status-oriented computer model is adopted in this work. A comparison among these microstructure-based models is illustrated in
Table 3. The pores in cement-based materials are usually irregular and disordered, which show evident fractal features. The study on the fractal properties of cement-based materials is of great meaning as fractal theories enable to provide novel and effective ways to characterize of the microstructure of cement-based materials.
To address application scenarios of hydration and pore structure modeling on MOS cement paste, a preliminary research is carried out as follows: (1) the thermodynamic database relevant to MOS paste at 25 °C are collected and evaluated by the first principle calculation. (2) By using the established thermodynamic database and the geochemical speciation code PHREEQC, the influence of molar ratios on the equilibrium hydrated products assemblage is investigated. In addition, the basic relationship between the composition of hydrated products at equilibrium state and initial molar ratio is established. (3) Based on the obtained composition of equilibrium product, the visualization of microstructure is carried out based on the proposed models. A comparison among the porosities of MOS pastes with different molar ratios is given and a fractal analysis on the pore structure of MOS paste is conducted. In this work, a first principle calculation is originally adopted to evaluate the thermodynamic properties of phases in MOS cement system considering very little research on the evaluation of thermodynamic data among studies concerning MOS. The calculated thermodynamic data set the basis for the following thermodynamic analysis on the hydration products of MOS system, and thus facilitate the simulation and fractal analysis of pore structure of MOS paste.
4. Conclusions and Prospects
In this work, a novel multiscale modeling strategy is proposed to evaluate the pore structure and fractals in MOS cement system. A first principle calculation is initially performed to obtain the thermodynamic data for main phases and aqueous geochemical calculations are followed to evaluate the phase composition. Finally, the hydration status is visualized using microstructure-based method and porosity and pore fractals are analyzed. Based on the findings, several conclusions can be drawn:
(1) Based on the common molar ratios of magnesium oxysulfate cement, the basic thermodynamic data of MOS cement system under the temperature of 25 °C is obtained through literature and first principle calculation. The consistency in the phase composition between simulation results using PHREEQC and experimental results demonstrates that the thermodynamic simulation is applicable to the MOS cement system.
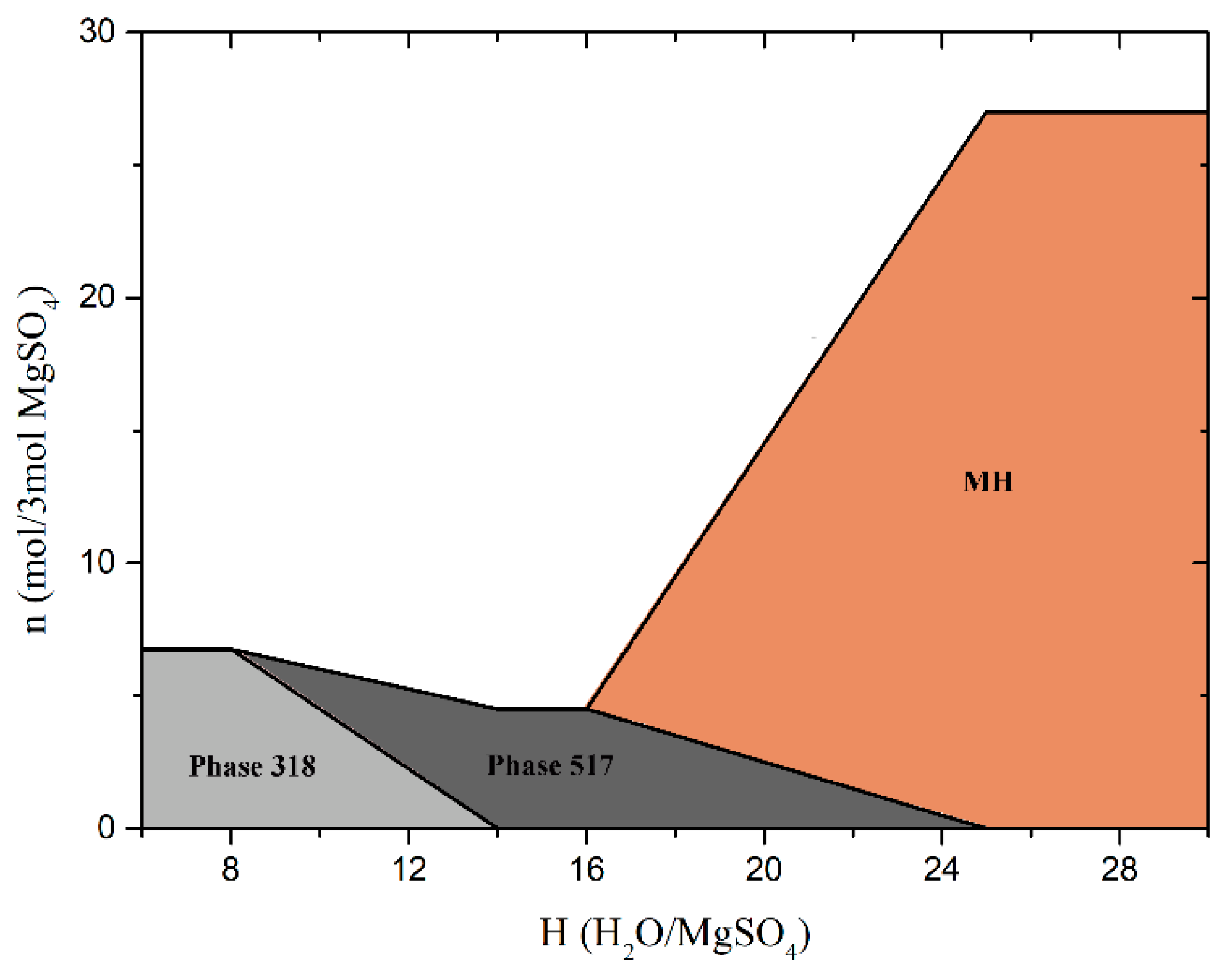
(2) Using the established thermodynamic database of MOS cement system, the hydration of MOS cement system and the stability of hydrated products under normal temperature and pressure are well studied. Results show that the composition of hydrated products in MOS cement system is sensitive to M and H. Phase 517 is precipitated with the increase of M and Phase 318 disappears gradually. When MgO is excessive, only MH will generate. Phase 318 solely exists when H is low, and Phase 517 appears gradually with increase of H. When H is large enough, MH comes to the only products, which corresponds to the low strength and water resistance of MOS pastes. It provides a certain benefit for mixture design: it is recommended that when H is equal to 18, M = 5 is beneficial to the strength and water resistance development of MOS cement pastes, meanwhile, when M is equal to 8, H = 14–16 is appropriate for mixture design.
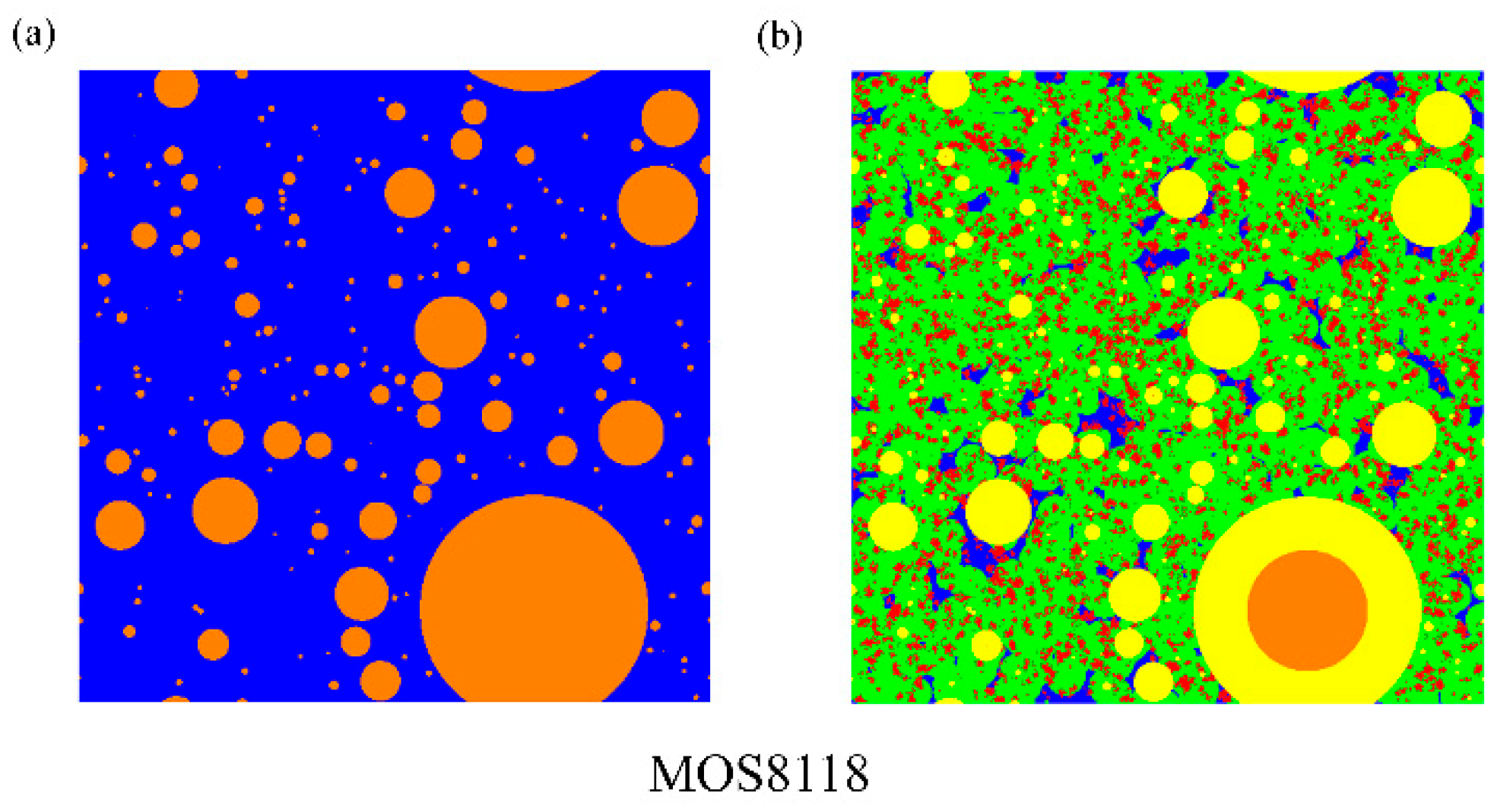
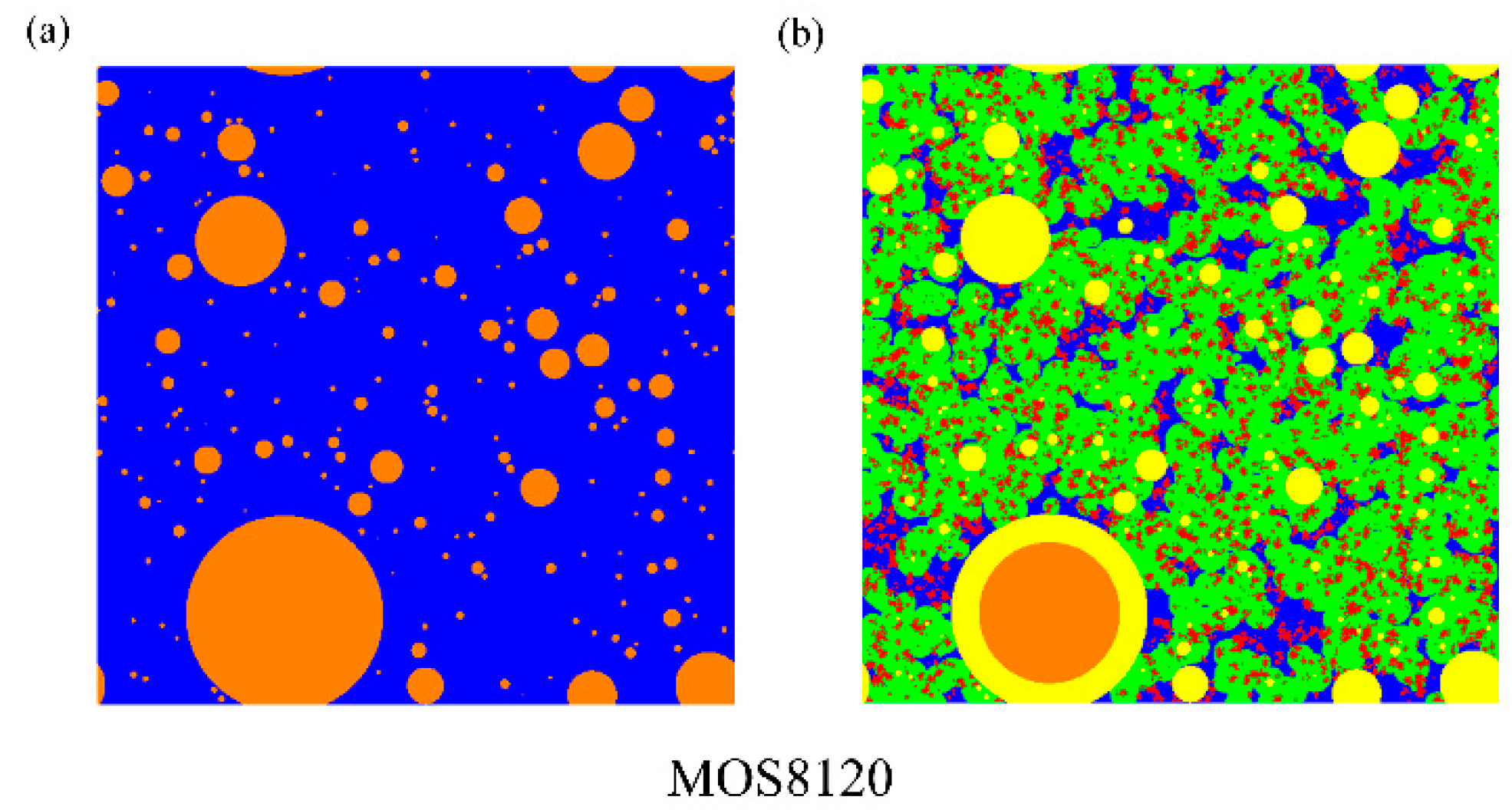
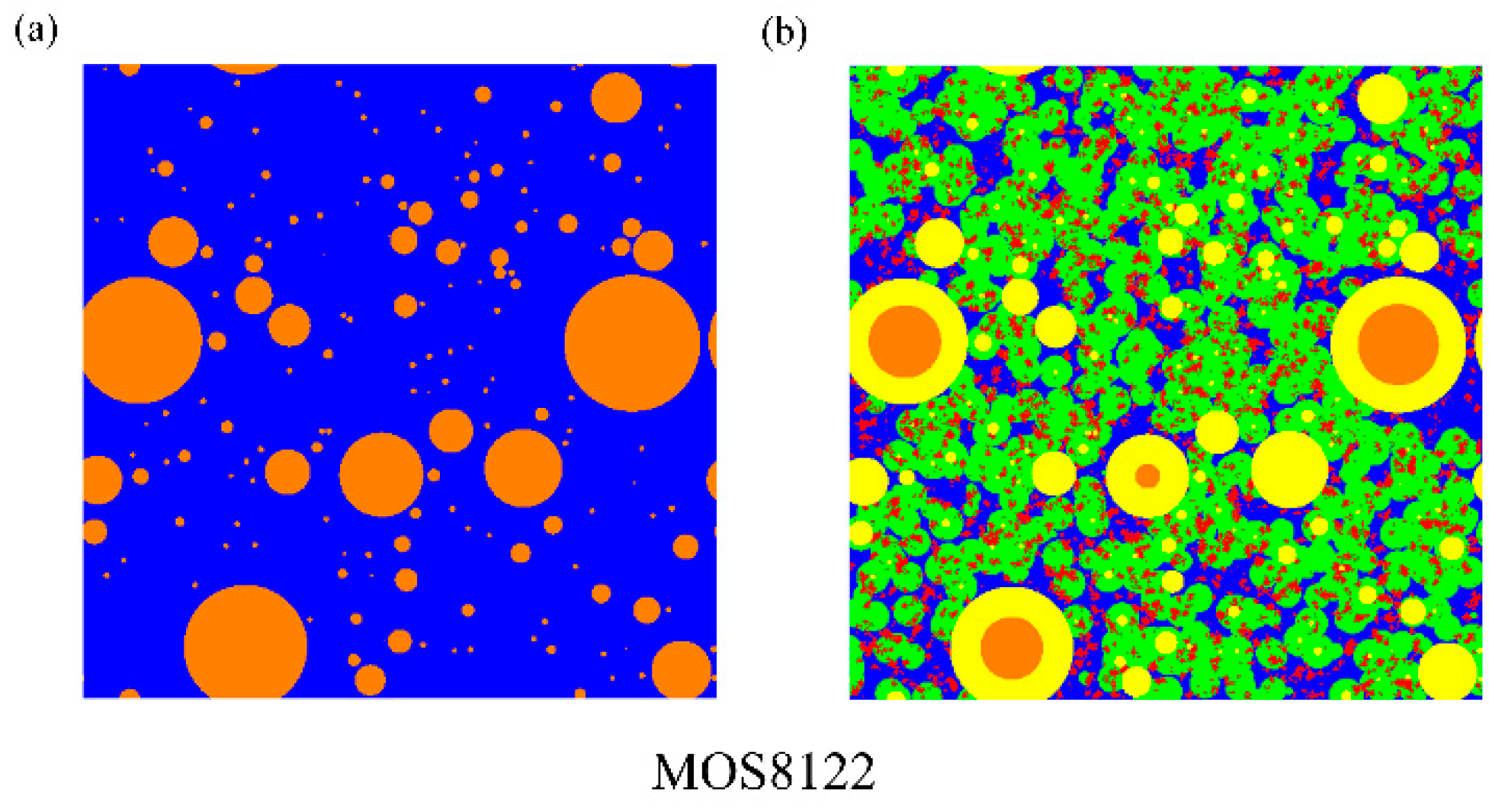
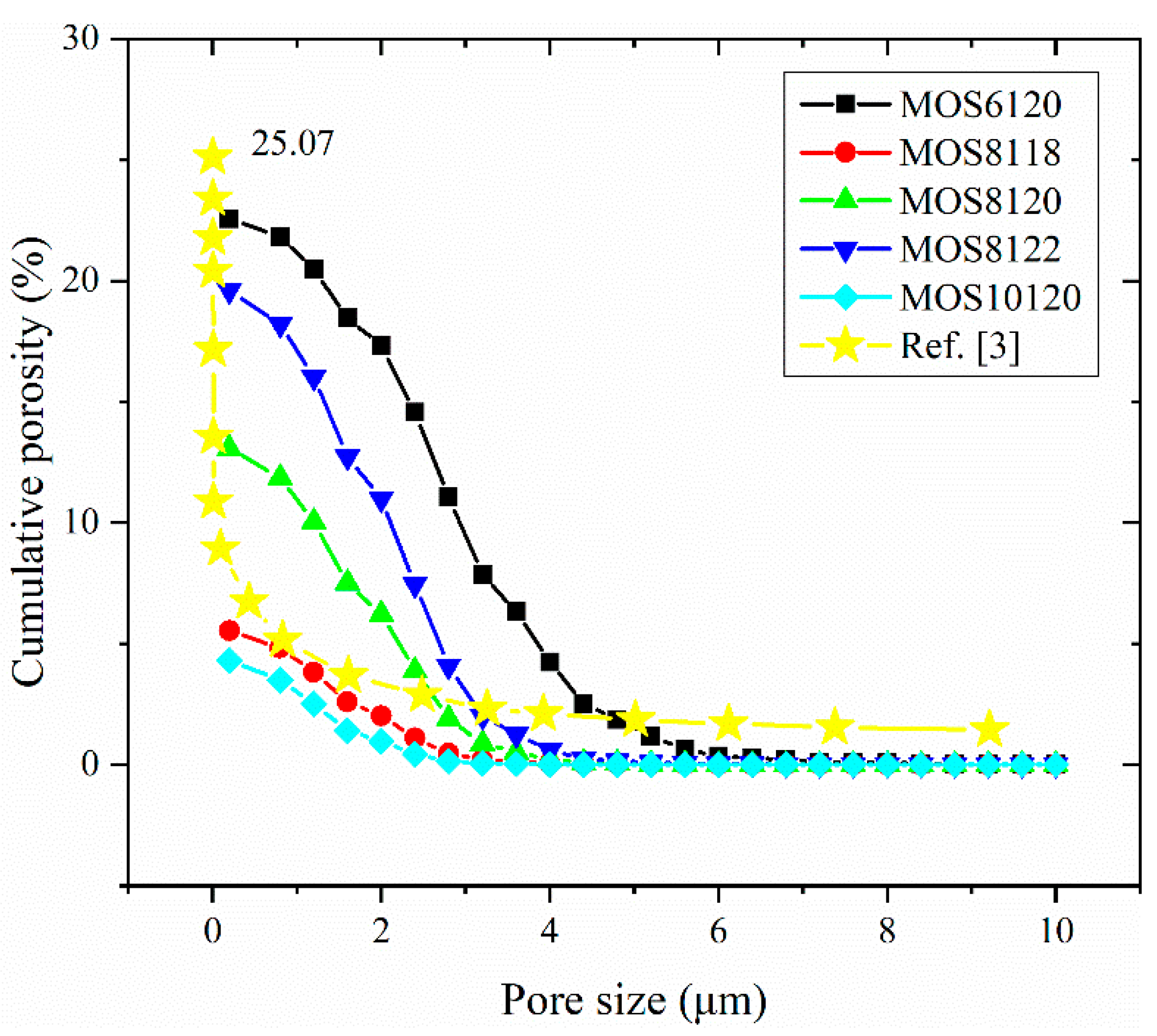
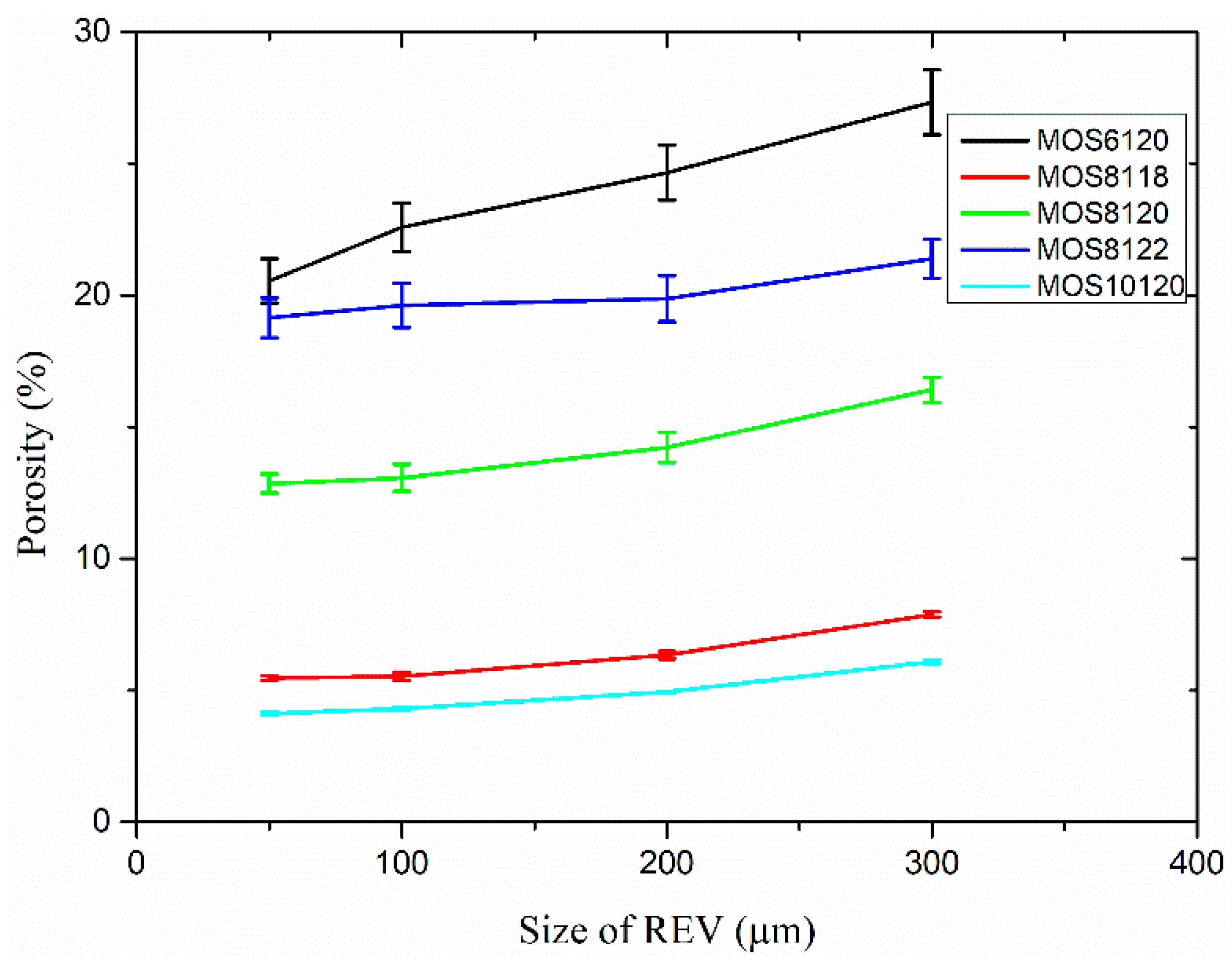
(3) The microstructure in initial and ultimate hydration stages of MOS pastes are visualized by means of MATLAB. Based on the hydration status, the evaluation of porosity and analysis of fractal feature of pore structure are conducted. The comparison of cumulative porosities in MOS pastes with different molar ratios shows that a large M is beneficial to reducing porosity and similar trend for the case of small H. Besides, fractal analysis reveals that Df of MOS paste is positively proportional to the porosity and large M, as well as small H is beneficial for the modification of microstructure of MOS paste by reducing the Df.
This work provides a novel modeling strategy for MOS cement system integrating different scales and methods. In the future, more attributes will be taken into consideration when simulating the hydration behavior of MOS cement, such as the influence of temperature, additives, etc. Besides, other important properties of MOS cement will be investigated including leaching, thermal conductivity, durability and other long-term performance of such material. In addition, the dynamic process of hydration of MOS cement system based on thermodynamics and kinetics will also be of great significance to understand the toughening mechanism for this eco-friendly construction materials.