Outbreaks in the Neonatal Intensive Care Unit: Description and Management
Abstract
1. Introduction
- They frequently occur in high-risk departments;
- Responsible microorganisms are often resistant to common therapies;
- They are associated with high mortality rates;
- They are sometimes related to errors in care practices;
- They highlight often unknown or underestimated care issues.
2. The “Anatomy” of an Outbreak and Its Management
2.1. Risk Factors
- The presence of comorbidities;
- The need for various invasive procedures (mechanical ventilation, the use of a central catheter and enteral tubes, etc.);
- A high frequency of contact with personnel;
- Inappropriate/prolonged use of antibiotics;
- A prolonged hospital stay;
- Non-compliance with hygiene standards;
- Overcrowding in the ward;
- Structural or equipment deficiencies;
- An inadequate staff-to-patient ratio.
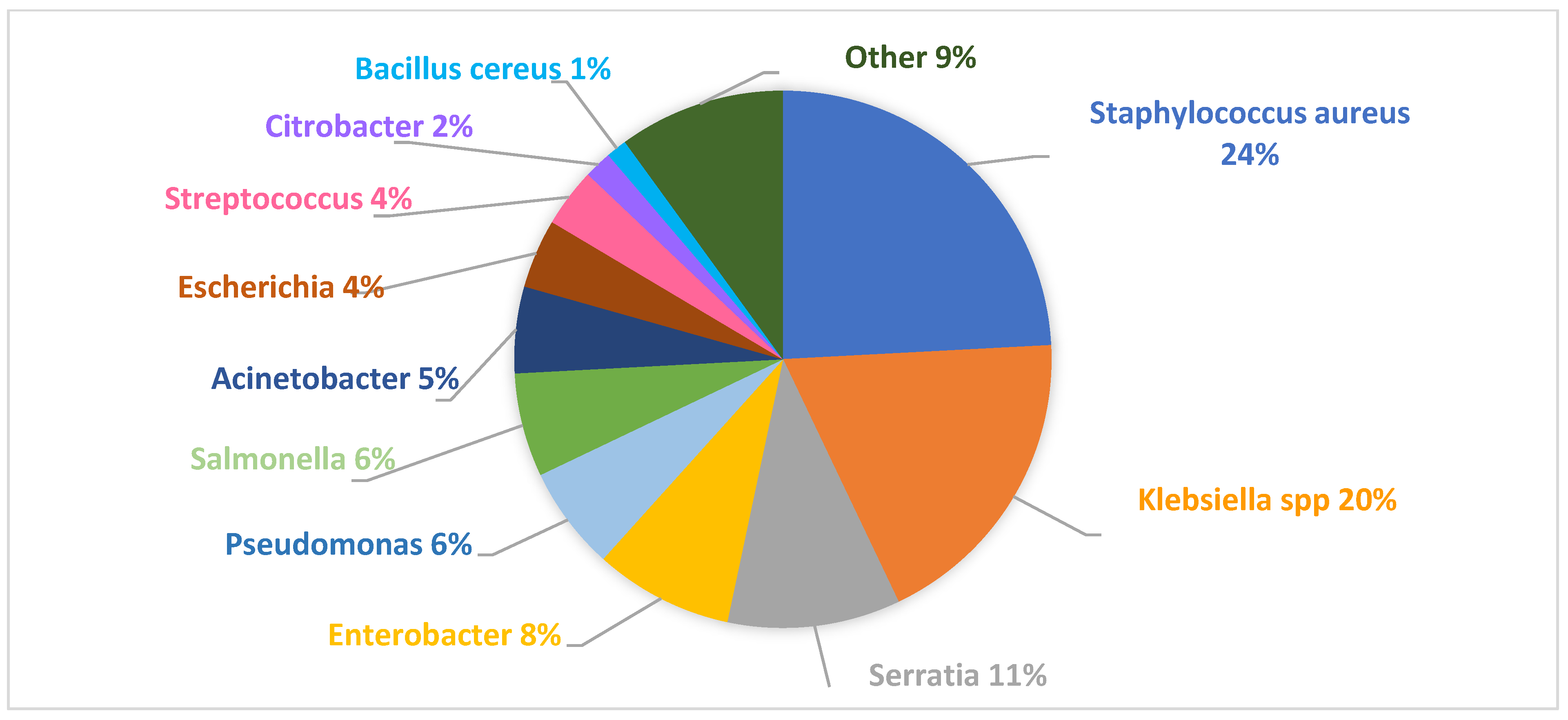
2.2. Pathogens Responsible for Outbreaks
- (a)
- Microorganisms with a propensity for transmission within healthcare facilities (e.g., Clostridium difficile, norovirus, respiratory syncytial virus, influenza virus, rotavirus, Enterobacter spp., and Serratia spp.);
- (b)
- Microorganisms that are resistant to first-line therapies;
- (c)
- Microorganisms that are difficult to treat due to their resistance to multiple classes of antimicrobials;
- (d)
- Both common and rare microorganisms with unusual resistance patterns within a healthcare facility;
- (e)
- New or re-emerging pathogens.
2.3. Modes of Pathogen Transmission, the Identification of the Infection Source, and the Recognition of an Outbreak
- (a)
- Contact transmission: This is the most common mode and can be divided into two categories:
- -
- Direct contact (the transmission of the pathogen from an infected person to another person without an intermediary object or a contaminated person).
- -
- Indirect contact (the transfer of an infectious agent through an intermediary object or a contaminated person, e.g., via the hands of healthcare personnel when proper hand hygiene is not performed or with devices or instruments used for patient care that are not adequately sanitized between patients).
- (b)
- Droplet transmission (through a short-distance airborne route).
- (c)
- Airborne transmission (from patients or the environment): This occurs with pathogens that remain infectious for a long time and over distances. Microorganisms transported this way can be dispersed over long distances by air currents and can be inhaled by susceptible individuals even if they have not had direct contact with the contagious individual.
- (d)
- Other sources of infection: These are associated with common environmental sources or vehicles (e.g., contaminated food, water, or medications).
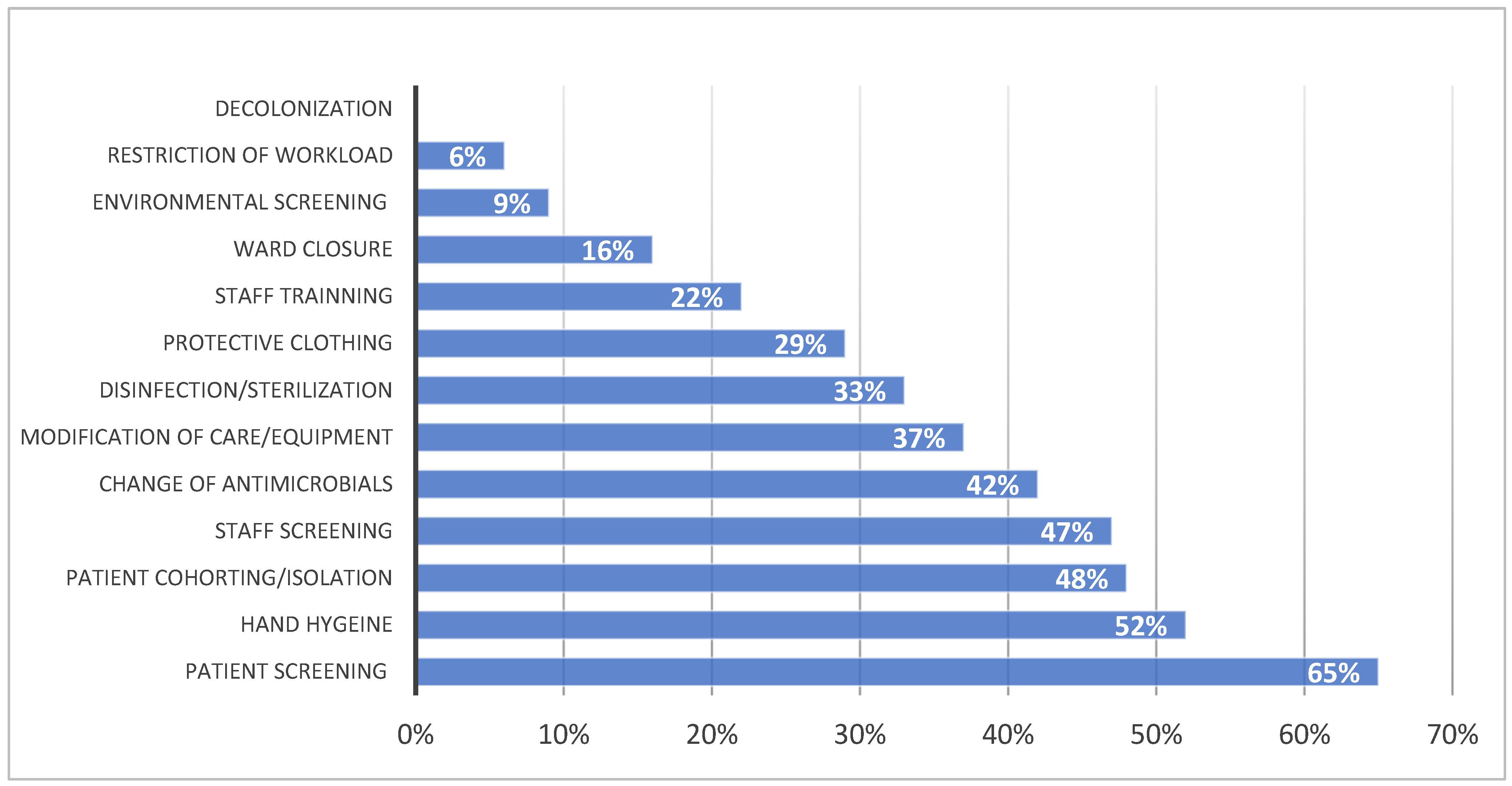
2.4. The Management of an Outbreak
- (a)
- Implement all standard precautions for the prevention of HAIs;
- (b)
- Conduct an epidemiological investigation to characterize the microorganism, define its biological characteristics and susceptibility to antimicrobial drugs, identify the origin and reservoir, trace the transmission routes, and identify possible risk factors;
- (c)
- Implement additional or specific measures, such as educating staff and parents, modifying care practices, adjusting parental access, ensuring compliance with space requirements to avoid overcrowding, and limiting admissions/closing the ward.
- Carrying out post-exposure prophylaxis with antiviral agents [47];
- Using vaccines for both pre- and post-exposure prevention;
- Screening and limiting visitors with signs of transmissible infections.
- The use of personal protective equipment (PPE). PPE refers to various devices used alone or in combination to protect mucous membranes, respiratory tracts, skin, and clothing from contact with infectious agents. The choice of PPE is based on the nature of patient interaction and/or the pathogen’s likely modes of transmission.
- The management of patient care equipment/devices. Equipment and medical instruments/devices should be cleaned and maintained according to the manufacturer’s instructions.
- The management of linens. Contaminated fabrics, including linens and patient clothing, can harbor pathogenic microorganisms. However, the risk of disease transmission is negligible if handled, transported, and washed safely.
- The placement of patients based on the mode of pathogen transmission, e.g., using single rooms, possibly with negative pressure, for patients with airborne infections.
- Respiratory hygiene/cough etiquette. This strategy, which became part of standard precautions after the SARS-CoV-2 pandemic, targets patients and accompanying family members with undiagnosed transmissible respiratory infections and applies to anyone showing signs of respiratory illness when entering a healthcare facility.
- For all inpatients from the date of the event’s onset or upon admission to the ward and then periodically until the event is eradicated;
- At the environmental level, including for surfaces and equipment (depending on the type of microorganism, such as Pseudomonas, Acinetobacter, Serratia, etc.);
- For staff (depending on the type of microorganism or if epidemiologically implicated in the microorganism’s transmission).
2.5. Special Issues: Prevention of Central Line-Associated Bloodstream Infections (CLABSIs)
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Members of the Study Group of Neonatal Infectious Diseases
References
- Cortese, F.; Scicchitano, P.; Gesualdo, M.; Filaninno, A.; De Giorgi, E.; Schettini, F.; Laforgia, N.; Ciccone, M.M. Early and late infections in newborns: Where do we stand? A review. Pediatr. Neonatol. 2016, 57, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the newborn and young infant from infectious diseases: Lessons from immune ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Beudeker, C.R.; Vijlbrief, D.C.; van Montfrans, J.M.; Rooijakkers, S.H.M.; van der Flier, M. Neonatal sepsis and transient immunodeficiency: Potential for novel immunoglobulin therapies? Front. Immunol. 2022, 13, 1016877. [Google Scholar] [CrossRef] [PubMed]
- Hooven, T.A.; Polin, R.A. Healthcare-associated infections in the hospitalized neonate: A review. Early Hum. Dev. 2014, 90 (Suppl. 1), S4–S6. [Google Scholar] [CrossRef]
- Marty, D.; Sorum, K.; Smith, K.; Nicoski, P.; Sayyed, B.A.; Amin, S. Nosocomial Infections in the Neonatal Intensive Care Unit. Neoreviews 2024, 25, e254–e264. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, C.F.; Rehn, Y.F.; Chen, J.C.; Chen, P.Y.; Chen, C.H.; Wang, T.M.; Huang, F.L. Reduced nosocomial infection rate in a neonatal intensive care unit during a 4-year surveillance period. J. Chin. Med. Assoc. 2017, 80, 427–431. [Google Scholar] [CrossRef]
- Jansen, S.J.; Lopriore, E.; van der Beek, M.T.; Veldkamp, K.E.; Steggerda, S.J.; Bekker, V. The road to zero nosocomial infections in neonates-a narrative review. Acta Paediatr. 2021, 110, 2326–2335. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Health-Care Associated Infections and Antimicrobial Use in European Acute Care Hospitals; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/point-prevalence-survey-healthcare-associated-infections-and-antimicrobial-use-5 (accessed on 30 June 2024).
- Cai, S.; Thompson, D.K.; Anderson, P.J.; Yang, J.Y. Short- and long- term neurodevelopmental outcomes of very preterm infants with neonatal sepsis: A systematic review and meta-analysis. Children 2019, 6, 131. [Google Scholar] [CrossRef]
- Donovan, E.F.; Sparling, K.; Lake, M.R.; Narendran, V.; Schibler, K.; Haberman, B.; Rose, B.; Meinzen-Derr, J. The investment case for preventing NICU-associated infections. Am. J. Perinatol. 2013, 30, 179–184. [Google Scholar] [CrossRef]
- Cernada, M.; De Alba Romero, C.; Fernández-Colomer, B.; González-Pacheco, N.; González, M.; Couce, M.L.; en representación del Comité de Estándares y la Comisión de Infección Neonatal de la Sociedad; Española de Neonatología. Health care-associated infections in neonatology. An. Pediatría Engl. Ed. 2024, 100, 46–56. [Google Scholar] [CrossRef]
- Huang, J.; Cayabyab, R.; Cielo, M.; Ramanathan, R. Incidence, risk factors, short-term outcomes, and microbiome of ventilator-associated pneumonia in very-low-birth-weight infants: Experience at a single level III neonatal intensive care unit. Pediatr. Infect. Dis. J. 2024, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, E.C.; Xiao, S.; Colantuoni, E.; Clark, R.H.; Johnson, J.; Mukhopadhyay, S.; Kalu, I.C.; Zerr, D.M.; Reich, P.J.; Roberts, J.; et al. CDC Prevention Epicenters Program. Hospital-onset bacteremia among neonatal intensive care unit patients. JAMA Pediatr. 2024, 178, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and burden of sepsis acquired in hospitals and intensive care units: A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1536–1551. [Google Scholar] [CrossRef]
- Srivastava, S.; Shetty, N. Healthcare-associated infections in neonatal units: Lessons from contrasting worlds. J. Hosp. Infect. 2007, 65, 292–306. [Google Scholar] [CrossRef]
- CDC. Principles of Epidemiology: Lesson 6: Investigating an Outbreak, Section 2|Self-Study Course SS1978; CDC: Atlanta, GA, USA, 2016. Available online: https://archive.cdc.gov/www_cdc_gov/csels/dsepd/ss1978/lesson6/section2.html (accessed on 30 June 2024).
- Haas, J.P.; Trezza, L.A. Outbreak investigation in a neonatal intensive care unit. Semin. Perinatol. 2002, 26, 367–378. [Google Scholar] [CrossRef]
- World Health Organization. Managing Epidemics: Key Facts about Major Deadly Diseases, 2nd ed.; World Health Organization: Geneva, Switzerland, 2023; Available online: https://books.google.com.au/books?hl=zh-CN&lr=&id=daMOEQAAQBAJ&oi=fnd&pg=PR9&dq=18.%09World+Health+Organization.+Managing+Epidemics:+Key+Facts+about+Major+Deadly+Diseases,+2nd+ed.%3B+World+Health+Organization:+Geneva,+Zwitserland,+2023.+&ots=80tpzFO4rz&sig=8G6envmPdUqoSXpWKm4v6ru0YHc&redir_esc=y#v=onepage&q&f=false (accessed on 30 June 2024).
- Sood, G.; Perl, T.M. Outbreaks in health care settings. Infect. Dis. Clin. North Am. 2021, 35, 631–666. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. The Healthcare Infection Control Practices Advisory Committee. In 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings; CDC: Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html (accessed on 30 June 2024).
- Anthony, M.; Bedford-Russell, A.; Cooper, T.; Fry, C.; Heath, P.T.; Kennea, N.; McCartney, M.; Patel, B.; Pollard, T.; Sharland, M.; et al. Managing and preventing outbreaks of Gram-negative infections in UK neonatal units. Arch. Dis. Child. Fetal Neonatal Ed. 2013, 98, F549–F553. [Google Scholar] [CrossRef] [PubMed]
- Ghirardi, B.; Pietrasanta, C.; Ciuffini, F.; Manca, M.F.; Uccella, S.; Lavizzari, A.; Pugni, L.; Mosca, F. Gestione degli eventi epidemici in Terapia Intensiva Neonatale—Management of outbreaks of nosocomial pathogens in neonatal intensive care unit. Pediatr. Medica Chir. 2013, 35, 263–268. [Google Scholar] [CrossRef]
- Beltempo, M.; Patel, S.; Platt, R.W.; Julien, A.S.; Blais, R.; Bertelle, V.; Lapointe, A.; Lacroix, G.; Gravel, S.; Cabot, M.; et al. Quebec investigators of the Canadian Neonatal Network; Quebec investigators of the Canadian Neonatal Network (CNN). Association of nurse staffing and unit occupancy with mortality and morbidity among very preterm infants: A multicentre study. Arch. Dis. Child.-Fetal Neonatal Ed. 2023, 108, 387–393. [Google Scholar] [CrossRef]
- NICE. NICE Neonatal Infection: Antibiotics for Prevention and Treatment (NG195); NICE guideline Published: London, UK, 2021; Available online: https://www.nice.org.uk/guidance/ng195/resources/neonatal-infection-antibiotics-for-prevention-and-treatment-pdf-66142083827653 (accessed on 30 June 2024).
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L. Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in health care settings, 2006. Am. J. Infect. Control 2007, 35 (Suppl. 2), S165–S193. [Google Scholar] [CrossRef]
- Johnson, J.; Quach, C. Outbreaks in the neonatal ICU: A review of the literature. Curr. Opin. Infect. Dis. 2017, 30, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Vonberg, R.P.; Weitzel-Kage, D.; Behnke, M.; Gastmeier, P. Worldwide Outbreak Database: The largest collection of nosocomial outbreaks. Infection 2011, 39, 29–34. [Google Scholar] [CrossRef]
- Outbreak Database. Worldwide Database for Nosocomial Outbreaks. Available online: http://www.outbreak-database.com (accessed on 30 June 2024).
- Stapleton, P.J.; Murphy, M.; McCallion, N.; Brennan, M.; Cunney, R.; Drew, R.J. Outbreaks of extended spectrum beta-lactamase-producing Enterobacteriaceae in neonatal intensive care units: A systematic review. Arch. Dis. Child. -Fetal Neonatal Ed. 2016, 101, F72–F78. [Google Scholar] [CrossRef]
- Thatrimontrichai, A.; Apisarnthanarak, A. Active surveillance culture program in asymptomatic patients as a strategy to control multidrug-resistant gram-negative organisms: What should be considered? J. Formos. Med. Assoc. 2020, 119, 1581–1585. [Google Scholar] [CrossRef]
- Prevel, R.; Boyer, A.; M’Zali, F.; Lasheras, A.; Zahar, J.R.; Rogues, A.M.; Gruson, D. Is systematic fecal carriage screening of extended-spectrum beta-lactamase-producing Enterobacteriaceae still useful in intensive care unit: A systematic review. Crit. Care 2019, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Zahar, J.R.; Blot, S.; Nordmann, P.; Martischang, R.; Timsit, J.F.; Harbarth, S.; Barbier, F. Screening for Intestinal Carriage of Extended-spectrum Beta-lactamase-producing Enterobacteriaceae in Critically Ill Patients: Expected Benefits and Evidence-based Controversies. Clin. Infect. Dis. 2019, 68, 2125–2130. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. European Society of Clinical Microbiology. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. 1), 1–55. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter Baumannii and Pseudomonas Aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241550178 (accessed on 30 June 2024).
- Ambretti, S.; Bassetti, M.; Clerici, P.; Petrosillo, N.; Tumietto, F.; Viale, P.; Rossolini, G.M. Screening for carriage of carbapenem-resistant Enterobacteriaceae in settings of high endemicity: A position paper from an Italian working group on CRE infections. Antimicrob. Resist. Infect. Control 2019, 8, 136. [Google Scholar] [CrossRef]
- Verdugo-Paiva, F.; Otaiza, F.; Roson-Rodríguez, P.; Rojas-Gomez, A.M.; Galas, M.; El Omeiri, N.; Fuentes, Y.; Rada, G.; Ramón-Pardo, P. Effects of screening strategies to detect carbapenem-resistant gram-negative bacteria: A systematic review. Am. J. Infect. Control 2022, 50, 1381–1388. [Google Scholar] [CrossRef]
- Folgori, L.; Tersigni, C.; Hsia, Y.; Kortsalioudaki, C.; Heath, P.; Sharland, M.; Bielicki, J. The relationship between Gram-negative colonization and bloodstream infections in neonates: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2018, 24, 251–257. [Google Scholar] [CrossRef]
- Seidel, J.; Haller, S.; Eckmanns, T.; Harder, T. Routine screening for colonization by Gram-negative bacteria in neonates at intensive care units for the prediction of sepsis: Systematic review and meta-analysis. J. Hosp. Infect. 2018, 99, 367–380. [Google Scholar] [CrossRef]
- Almeida, T.L.; Mendo, T.; Costa, R.; Novais, C.; Marçal, M.; Martins, F.; Tuna, M. Carbapenemase-Producing Enterobacteriaceae (CPE) Newborn Colonization in a Portuguese Neonatal Intensive Care Unit (NICU): Epidemiology and Infection Prevention and Control Measures. Infect. Dis. Rep. 2021, 13, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Choudhury, D.D.; Gupta, K.; Rai, S.; Batra, P.; Manchanda, V.; Saha, R.; Kaur, I.R. Predictors for gut colonization of carbapenem-resistant Enterobacteriaceae in neonates in a neonatal intensive care unit. Am. J. Infect. Control 2018, 46, e31–e35. [Google Scholar] [CrossRef]
- Wang, J.; Lv, Y.; Yang, W.; Zhao, P.; Yin, C. Epidemiology and clinical characteristics of infection/colonization due to carbapenemase-producing Enterobacterales in neonatal patients. BMC Microbiol. 2022, 22, 177. [Google Scholar] [CrossRef]
- Orman, A.; Celik, Y.; Evik, G.; Ersöz, G.; Kuyucu, N.; Ozmen, B.O. Should perirectal swab culture be performed in cases admitted to the Neonatal Intensive Care Unit? Lessons learned from the Neonatal Intensive Care Unit. Children 2023, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- SIN Linee Guida per la Prevenzione Delle Infezioni Ospedaliere Nel Neonato. Available online: https://www.sin-neonatologia.it/linee-guida-per-la-prevenzione-delle-infezioni-ospedaliere-nel-neonato/ (accessed on 30 June 2024).
- Johnson, J.; Akinboyo, I.C.; Schaffzin, J.K. Infection Prevention in the Neonatal Intensive Care Unit. Clin. Perinatol. 2021, 48, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, D.; Ho, M.S.P.; Ting, J.; Shah, P.S. Antimicrobial Stewardship Programs in Neonates: A Meta-Analysis. Pediatrics 2024, 153, e2023065091. [Google Scholar] [CrossRef]
- McMullan, B.; Bryant, P.A.; Duffy, E.; Bielicki, J.; De Cock, P.; Science, M.; Zembles, T.; Timberlake, K.; Monsees, E.; Hamdy, R.F.; et al. Multinational consensus antimicrobial stewardship recommendations for children managed in hospital settings. Lancet Infect. Dis. 2023, 23, e199–e207. [Google Scholar] [CrossRef]
- Vain, N.E. Nosocomial Respiratory Viral Infection in the Neonatal Intensive Care Unit. Am. J. Perinatol. 2020, 37, S22–S25. [Google Scholar] [CrossRef]
- Milstone, A.M.; Elward, A.; Brady, M.T.; Cox, K.; Fauerbach, L.L.; Guzman-Cottrill, J.A.; Hogges, J.; Centers for Disease Control and Prevention (U.S.); National Center for Emerging and Zoonotic Infectious Diseases (U.S.); Division of Healthcare Quality Promotion; et al. Recommendations for Prevention and Control of Infections in Neonatal Intensive Care Unit Patients: Staphylococcus aureus (2020); Guideline: NICU: S. aureus; CDC: Atlanta, GA, USA, 2020. Available online: https://www.cdc.gov/infection-control/hcp/nicu-saureus/?CDC_AAref_Val=https://www.cdc.gov/infectioncontrol/guidelines/NICU-saureus (accessed on 30 June 2024).
- Akinboyo, I.C.; Zangwill, K.M.; Berg, W.M.; Cantey, J.B.; Huizinga, B.; Milstone, A.M. SHEA neonatal intensive care unit (NICU) white paper series: Practical approaches to Staphylococcus aureus disease prevention. Infect. Control Hosp. Epidemiol. 2020, 41, 1251–1257. [Google Scholar] [CrossRef]
- Tacconelli, E.; Mazzaferri, F.; de Smet, A.M.; Bragantini, D.; Eggimann, P.; Huttner, B.D.; Kuijper, E.J.; Lucet, J.C.; Mutters, N.T.; Sanguinetti, M.; et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant Gram-negative bacteria carriers. Clin. Microbiol. Infect. 2019, 25, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Kuti, B.P.; Ogunlesi, T.A.; Oduwole, O.; Oringanje, C.; Udoh, E.E.; Bello, S.; Horn, D.; Meremikwu, M.M. Hand Hygiene for the Prevention of Infections in Neonates. Cochrane Database Syst. Rev. 2023, 6, CD013326. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, C.; Tartari, E.; Deeves, M.; Pittet, D.; Allegranzi, B. World Health Organization World Hand Hygiene Day, 5 May 2024. SAVE LIVES: Clean Your Hands campaign: Promoting knowledge and capacity building on infection prevention and control, including hand hygiene, among health and care workers. Am. J. Infect. Control 2024, 52, 621. [Google Scholar] [CrossRef] [PubMed]
- Keneh, N.K.; Kenmoe, S.; Bowo-Ngandji, A.; Akoachere, J.T.K.; Kamga, H.G.; Ndip, R.N.; Ebogo-Belobo, J.T.; Kengne-Ndé, C.; Mbaga, D.S.; Tendongfor, N.; et al. Methicillin-Resistant Staphylococcus aureus Carriage among Neonate Mothers, Healthcare Workers, and Environmental Samples in Neonatal Intensive Care Units: A Systematic Review. Biomed. Res. Int. 2024, 5675786. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.; Sultan, O.; Mitchell, B.G.; Jenney, A.; Kiernan, M.; Brewster, D.J.; Russo, P.L. How long do nosocomial pathogens persist on inanimate surfaces? A scoping review. J. Hosp. Infect. 2024, 147, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Mitchell, B.G. Multimodal environmental cleaning strategies to prevent healthcare-associated infections. Antimicrob. Resist. Infect. Control 2023, 12, 83. [Google Scholar] [CrossRef]
- Dancer, S.J. Hospital cleaning: Past, present, and future. Antimicrob. Resist. Infect. Control 2023, 12, 80. [Google Scholar] [CrossRef]
- Hanna, M.; Shah, R.; Marquez, L.; Barzegar, R.; Gordon, A.; Pammi, M. Infant isolation and cohorting for preventing or reducing transmission of healthcare-associated infections in neonatal units. Cochrane Database Syst. Rev. 2023, 6, CD012458. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Roilides, E. Infection control measures against multidrug-resistant Gram-negative bacteria in children and neonates. Future Microbiol. 2023, 18, 751–765. [Google Scholar] [CrossRef]
- Rodríguez-Villodres, Á.; Ortiz de la Rosa, J.M.; Valencia-Martin, R.; Jiménez Parrilla, F.; Martín-Gutiérrez, G.; Márquez Patiño, N.; Perea Cruz, E.; Sánchez Jiménez, M.; Pavón Delgado, A.; Cisneros, J.M.; et al. Implementation of a PCR-based strategy to control an outbreak by Serratia marcescens in a Neonatal Intensive Care Unit. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 108. [Google Scholar] [CrossRef]
- Sciesielski, L.K.; Osang, L.K.M.; Dinse, N.; Weber, A.; Bührer, C.; Kola, A.; Dame, C. Validation of a New PCR-Based Screening Method for Prevention of Serratia marcescens Outbreaks in the Neonatal Intensive Care Unit. Neonatology 2023, 120, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, A.; Crombé, F.; Bosmans, P.; Cools, F.; Piérard, D.; Wybo, I. Serratia marcescens outbreak in a neonatal intensive care unit and the potential of whole-genome sequencing. J. Hosp. Infect. 2021, 111, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.A.; Chinn, R.; Pong, A.; Schultz, K.; Kim, J.; Gade, L.; Jackson, B.R.; Beer, K.D.; Litvintseva, A.P. Use of whole-genome sequencing to detect an outbreak of Malasseziapachydermatis infection and colonization in a neonatal intensive care unit-California, 2015–2016. Infect. Control Hosp. Epidemiol. 2020, 41, 851–853. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Wong, S.C.; Cao, H.; Chen, J.H.K.; So, S.Y.C.; Wong, S.C.Y.; Sridhar, S.; Yuen, K.Y.; Ho, P.L. Whole-genome sequencing data-based modeling for the investigation of an outbreak of community-associated methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A.F.; Peter, D.; Mattner, F.; Weiss, M.; Hoppenz, M.; Wolf, S.; Bader, B.; Peter, S.; Liese, J. Surveillance of Enterobacter cloacae complex colonization and comparative analysis of different typing methods on a neonatal intensive care unit in Germany. Antimicrob. Resist. Infect. Control 2022, 11, 54. [Google Scholar] [CrossRef]
- Hsu, H.E.; Mathew, R.; Wang, R.; Broadwell, C.; Horan, K.; Jin, R.; Rhee, C.; Lee, G.M. Health care-associated infections among critically Ill children in the United States, 2013–2018. JAMA Pediatr. 2020, 174, 1176–1183. [Google Scholar] [CrossRef]
- Recommendations for Prevention and Control of Infections in Neonatal Intensive Care Unit Patients: Central-Line–Associated Bloodstream Infections. Centers for Disease Control and Prevention Website. Available online: https://www.cdc.gov/infectioncontrol/guidelines/nicu-clabsi/recommendations.html (accessed on 30 June 2024).
- Muller, M.; Bryant, K.A.; Espinosa, C.; Jones, J.A.; Quach, C.; Rindels, J.R.; Stewart, D.L.; Zangwill, K.M.; Sánchez, P.J. SHEA Neonatal Intensive Care Unit (NICU) White Paper Series: Practical approaches for the prevention of central-line–associated bloodstream infections. Infect. Control Hosp. Epidemiol. 2023, 44, 550–564. [Google Scholar] [CrossRef]
- Payne, V.; Hall, M.; Prieto, J.; Johnson, M. Care bundles to reduce central line-associated bloodstream infections in the neonatal unit: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F422–F429. [Google Scholar] [CrossRef]
- Hamza, W.S.; Hamed, E.A.M.; Alfadhli, M.A.; Ramadan, M.A. A multidisciplinary intervention to reduce central line-associated bloodstream infection in pediatrics and neonatal intensive care units. Pediatr. Neonatol. 2022, 63, 71–77. [Google Scholar] [CrossRef]
- Linam, M.; Wannemacher, L.; Hawthorne, A.; Calamaro, C.; Spafford, P.; Walson, K. Initiation of interdisciplinary prevention rounds: Decreasing CLABSIs in critically ill children. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e80. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzialla, C.; Berardi, A.; Mondì, V.; on behalf of the Study Group of Neonatal Infectious Diseases. Outbreaks in the Neonatal Intensive Care Unit: Description and Management. Trop. Med. Infect. Dis. 2024, 9, 212. https://doi.org/10.3390/tropicalmed9090212
Tzialla C, Berardi A, Mondì V, on behalf of the Study Group of Neonatal Infectious Diseases. Outbreaks in the Neonatal Intensive Care Unit: Description and Management. Tropical Medicine and Infectious Disease. 2024; 9(9):212. https://doi.org/10.3390/tropicalmed9090212
Chicago/Turabian StyleTzialla, Chryssoula, Alberto Berardi, Vito Mondì, and on behalf of the Study Group of Neonatal Infectious Diseases. 2024. "Outbreaks in the Neonatal Intensive Care Unit: Description and Management" Tropical Medicine and Infectious Disease 9, no. 9: 212. https://doi.org/10.3390/tropicalmed9090212
APA StyleTzialla, C., Berardi, A., Mondì, V., & on behalf of the Study Group of Neonatal Infectious Diseases. (2024). Outbreaks in the Neonatal Intensive Care Unit: Description and Management. Tropical Medicine and Infectious Disease, 9(9), 212. https://doi.org/10.3390/tropicalmed9090212





