Abstract
Bulinus snails surviving drought play a key role in the seasonal transmission of urogenital schistosomiasis, although our knowledge of their adaptation to dry season is still limited. We investigated the survival dynamic and infestation by the Schistosoma haematobium of Bulinus snails during the dry and rainy seasons in a single pond in an area of seasonal schistosomiasis transmission in Senegal. During the rainy season, 98 (94.23%) B. senegalensis and six (5.76%) B. umbilicatus were collected, respectively. In the dry season, B. umbilicatus outnumbered B. senegalensis, but all five (100%) B. senegalensis collected were viable and alive after the interruption of aestivation by immersion in water, while only 7 of 24 (29.16%) B. umbilicatus collected emerged from their dormant state. The rate of infestation with S. haeamatobium during the rainy season was 18.2% (19/104), while all the viable snails collected during the dry season were negative. B. senegalensis and B. umbilicatus have different seasonal dynamics with no evidence of maintaining S. haematobium infestation during the drought. Further studies including more survey sites and taking account both snails biology and ecological conditions are needed to better understand snail adaptation to seasonal changes and their ability to maintain S. haeamatobium infestation during drought.
1. Introduction
Schistosomiasis is a neglected tropical disease (NTD) caused by blood flukes of the genus Schistosoma. Schistosomiasis disease is highly associated with poor sanitation and lack of clean water in endemic rural areas [1]. According to the World Health Organization, it is estimated that more than 250 million people need preventive treatment worldwide [2]. In 2019, the disability-adjusted life years (DALY) lost due to schistosomiasis was estimated at 1.6 million [3]; thus, schistosomiasis is a major human health problem and an obstacle to the economic development of populations living in rural areas [4]. In Africa, schistosomiasis is transmitted to humans by freshwater snails of the genius of Biomphalaria and Bulinus. The epidemiology of the disease is closely linked to the presence of freshwater sources representing both snails breeding sites and schistosomiasis transmission places. In areas with perennial transmission, water sources are available throughout the year, maintaining high densities of snails [5], contrasting with seasonal transmission areas characterized by a period of drought, coinciding with the dry season [6,7]. In these ecosystems, the snail vectors survive the drought thanks to their aestivation capacities and are only active during the rainy season. The adaptation of snails to drought may vary according to species and bio-ecological conditions [8,9,10,11]. This feature helps maintain snail populations during the dry season and ensures the rapid repopulation of temporary pounds at the start of the rainy season [12]. This ability gives Bulinus snails a pivotal role in the epidemiology of the disease in areas of seasonal transmission. Many studies have used experimental procedures to highlight the differential adaptation of snails species to drought [11,13,14]. These experimental models do not accurately reflect the natural interactions between the different species of molluscs and their environment as most of the snail strains are breed in laboratories for a long period and may therefore lose their ability to adapt to natural conditions. In addition, the variability of natural conditions can be very different from that observed under experimental conditions. Even though it is possible to investigate snail adaptation and survival in natural conditions [15], to date, studies carried out under natural conditions are rare. Hence, the mechanisms underlying the survival of Bulinus snails during drought and their emergence in the rainy season remain largely unexplored under natural conditions in addition to the interaction between the parasite and dormant snail. Indeed, it is thought that the snails could maintain their infestation during the dry period and become infested immediately after the first rain [7]. Understanding the aestivation mechanisms of snails and their ability to retain the parasite during diapause could improve our knowledge of the emergence and transmission dynamics of the disease in areas with temporary ponds.
We carried out a study in Niakhar in central-western Senegal, where B. senegalensis and B. umbilicatus can withstand 7–8 months of drought [7], to understand these Bulinus population dynamics and infestation by S. heamatobium both in the rainy and dry seasons and their viability after stopping their aestivation. Their ability to survive the drought was also explored experimentally.
2. Materials and Methods
2.1. Study Area and Ponds
The investigation was carried out at the Niakhar commune located in the in the region of Fatick, central-western Senegal. Niakhar is located 140 Km to the south-east of Dakar, the capital city (Figure 1). The area is characterized by the presence of temporary ponds where a seasonal transmission of urogenital schistosomiasis occurs during the rainy season from June/July to October [7].
Figure 1.
Map of Senegal showing the geographical location of the Fatick and the Niakhar study area.
The Niakhar area includes 40 villages of which about 17 have a relatively detailed description of the different species of snail vectors of Schistosoma species. B. senegalensis and B. umbilicatus are the main species involved in the transmission of S. haematobium [7]. Among all the ponds surveyed in the area, only the pond located in the village of Ngangarlam hosts both B. senegalensis and B. umbilicatus [7]. This pond named “Mbel Khakhale” is one of the most infested by S. haematobium [16]. The pond has a depth of 1 m, and it is about 50 m long and 40 m wide and has two access points and is only supplied with water during the rainy season (Figure 2).

Figure 2.
Mbel Khakhale pond at Niakhar study site. (A) The pond filled with water during the rainy season. (B) The pond dried up during the dry season.
2.2. Bulinus Snail Collection and Testing for Schistosoma haematobium Infection
The snails were collected during the rainy and dry seasons. They were also tested to assess their infestation by Schistosoma haematobium (see in the Supplementary materials, Figure S1).
- Dry season
The snails were collected in May 2022 during the dry season. The snails were prospected around the centre and in the hollow areas after the pond had dried out. At each collection point, cores were performed using a core barrel 30 cm high and 10 cm wide. The ground was examined to collect the snails at 5, 10 and 15 cm to determine the sinking capacity of the snails.
At the same time, the entire surface of the pond was prospected to collect plastic waste, pieces of wood and even the dry bark of trees in order to collect the sand that covered them when the pond dried out.
All soil samples were lightly broken and sieved in a basin containing water to separate the snails from small stones and other debris. Then, the recovered snails were placed in tanks containing moistened sand from the pond to gradually prepare them for waking up while they were transported to the laboratory. Snails with empty shells were excluded from the study, while those with the body retracted to the bottom of the shell were placed in water for 24 h to check their viability. A snail was considered non-viable if it remained shrunken in its shell, unable to climb the wall of the container or died less than 24 after its immersion in water.
- Rainy season
The snail collection was carried out in October 2022 at the end of the rainy season. At this time of year, the pond is still wet. The snail survey was carried out on the bank and beyond to about 2 m. The snails were then collected during 15 min by two collectors with a dip net. Then, all snails collected were placed in a plastic container filled with some aquatic plants and pond water before being transferred to the laboratory.
All snails collected during the dry and the rainy season were morphologically identified according to Brown and Kristensen’s taxonomic keys [17].
- Cercarial shedding test
Cercariae shedding test for the detection of the Schistosoma haematobium parasite were performed on each snail collected during the rainy season and on all viable snails recovered during the dry season. Each snail was tested, once in the laboratory, by placing it in a glass tube with 10 mL of filtered water and exposing it to direct sunlight or electric light for 45 min to 1 h to induce cercariae shedding. Schistosome cercariae shed from infested snails were then observed under a dissecting microscope and identified according to the criteria developed by Fransden and Christensen [18].
- Molecular analysis
2.3. DNA Extraction
The cetyl trimethyl ammonium bromide (CTAB) method was used to extract genomic DNA from the head-to-foot portion [19]. This dissected part of each snail was ground with a pestle in 200 μL of 2% filtered CTAB and the mixture was incubated at 65 °C for 1 h. Then, 200 μL of chloroform was added for the nucleic acid clean-up. The mixture was then centrifuged at 12,000 rpm for 5 min, and the supernatant was recovered, with 200 µL of isopropanol added to precipitate the DNA. After 15 min of centrifugation at 12,000 rpm, the isopropanol was poured off and the tube was drained into paper towels. The procedure was repeated with 200 μL of 70° ethanol but the centrifugation took 5 min. The purified DNA was dried using a Speed-Vac concentrator for 5 min at 45 °C and resuspended in 100 μL of pure water. The DNA extracts were stored at −20 °C until use.
2.4. PCR Procedures
Two successive PCRs were performed to determine the prevalence of S. haeamatobium. Real-time PCR targeting the Dra1 gene was used first to highlight the presence of parasites of the haematobium group, followed by a standard PCR for the specific detection of S. haematobium.
2.4.1. Real-Time PCR (RT-PCR)
All snails collected during the rainy and dry seasons were tested with real-time PCR. The Dra1 tandem repeat sequence is the target region for real-time PCR; it allows the detection of the Schistosoma haematobium group parasite [20,21,22]. The PCR reaction was performed with a mixture of 20 μL including 5 μL of DNA, 3.5 μL of sterile pure water, 0.5 μL of forward primer (5′-GATCTCACCTATCAGACGAAAC-3′), 0.5 μL of reverse primer (5′-TCACAACGATACGACCAAC-3′), 0.5 μL of TaqManTM probe (Applied Biosystems, Foster City, CA, USA) (5′-TGTTGGTGAAGTGCCTGTTTCGCAA-3′), and 10 μL of master mix. The thermal cycler, CFX96 (Bio-Rad C 1000 Touch, Marnes-la-Coquette, France), was used to perform the amplification. The program consisted of an initial denaturation step of 2 min at 50 °C, followed by a 3 min denaturation at 95 °C, and then 40 cycles of 95 °C for 30 s and 60 °C for 1 min before holding the sample at 4 °C. The result was considered positive if the cycle threshold (Ct) value was less than 35. In each run, positive and negative controls were performed with the samples. DNA extracts from adult S. haematobium worms were used as positive controls and pure water as the negative control. Then, all Dra1-positive specimens were tested for S. haematobium using standard PCR.
2.4.2. Rapid Diagnostic PCR (RD-PCR) to Detect S. haematobium
Dra1 real-time PCR-positive samples were tested for S. haematobium by RD-PCR [23]. Amplification was performed in a 25 μL reaction volume comprising 2 μL of DNA, 12.5 μL AmpliTaq Gold®360 PCR Mix (Applied Biosystems™, Waltham, MA, USA), 8.5 μL of sterile distilled H2O, 1 μL of universal direct Asmit1 primer (TTTTTTGGTCATCCTGAGGTGTAT) and 1 μL of reverse primer (Sh. R: 5′-TGATAATCAATGACCCTGCAATAA-3′). The amplification protocol was performed with initial denaturation at 95 °C for 15 min, with 39 cycles at 95 °C for 30 s, 58 °C for 1 min, 72 °C for 1 min, and a final step at 72 °C for 7 min using a thermal cycler (Applied Biosystems, Foster City, CA, USA). Migration was performed for 1 h and 15 min at 180 V in a 1.5% agarose gel with a SYBR Safe dye and readout was performed using the Gel Doc system (Bio-Rad Universal Hood II, Hercules, CA, USA).
- Evaluation of the Bulinus survival to drought under semi-experimental conditions
A semi-natural experimental device reproducing the drying of ponds was set up for this purpose (Figure 3). For the setting up of the device to reproduce the progressive drying of the ponds, two plastic tanks (“artificial ponds”) were each filled with sand from the study site to a height of 15 cm. Then, 5 L of water was added to each container before they were left to gradually dry in a period of one month. After that, the tanks were refilled with the same amount of water, and then 15 B. senegalensis and 8 B. umbilicatus specimens derived from laboratory breeding were added in each. The tanks were then left to dry out and were completely dry after 15 days. Two months after they had dried out completely, snails were recovered from the dry sand and put in water to induce the emergence of the specimens undergoing aestivation (Figure 3) and to monitor their mortality and reproduction.

Figure 3.
Tanks used to reproduce drought in semi-natural conditions. (A) Tank filled with water; (B) tank dried out after two months.
2.5. Statistical Analysis
Fisher’s exact test was used to compare the snail population during the rainy and the dry season and also between the snail populations in semi-natural conditions. All analyses involved two-sided p values, with statistical significance defined by p ≤ 0.05.
3. Results
3.1. Snail Species Collected and Infestation Rates
- Dry season
During the dry season, a total of 29 snails were collected, of which 24 (82.75%) and 5 (17.24%) were morphologically identified as B. umbilicatus and B. senegalensis, respectively. Both species were collected from the sand using the core barrel and mostly at 5 cm of depth. However, some B. umbilicatus were found buried under plastic waste, while some B. senegalensis were collected from tree trunk bark.
After careful observation, all snail specimens had intact shells. Among the 24 B. umbilicatus specimens, 10 (41.66%) showed no sign of life once put in water despite the presence of their soft part, 7 (29.16%) came out of their dormant state but did not survive beyond 24 h and 7 (29.16%) were maintained alive until giving two consecutive generations. All five specimens of B. senegalensis survived after 24 h exposure in water and gave offspring that survived for a month.
- Rainy season
During the rainy season, 104 snails were collected. The snails were mainly collected on the leaves of the white water-lily (Nymphaea alba), a plant that grows in the pond during the rainy season, to which the snails tend to cling, and also on solid objects found in the pond. Of the 104 snails collected, 98 individuals (94.23%) were identified as B. senegalensis and 6 individuals (5.76%) as B. umbilicatus.
The presence of B. senegalensis was significantly important compared with B. umbilicatus during the rainy season, while during the dry season, B. umbilicatus was the predominant species (Fisher’s exact test, p < 0.0001) (Figure 4).

Figure 4.
Distribution of B. senegalensis and B. umbilicatus in the wet and dry seasons. A total of 5 B. senegalensis and 24 B. umbilicatus were found during the dry season. During the rainy season, 96 B. senegalensis and 4 B. umbilicatus specimens were found. In the dry season, B. umbilicatus is the dominant species compared to B. senegalensis, while the reverse is observed during the rainy season (Fisher’s exact test, p < 0.0001).
3.2. Snail Infestation Rates
All viable snails collected during the dry season, seven B. umbilicatus and five B. senegalensis, were found to be negative upon cercarial emissions and molecular tests. Of the 104 snails collected during the rainy season, 29.80% (31/104) emitted Schistosoma cercariae. Among the 31 snails found to be positive using the Schistosoma cercariae shedding test, 90.32% (28/31) and 9.76% (3/31) were identified as B. senegalensis and B. umbilicatus, respectively. However, a total positivity rate of 35.5% (37/104) was obtained using Dra1 RT-PCR. Among the 37 specimens positive to Dra1 RT-PCR, 32 and 5 were identified as B. senegalensis and B. umbilicatus, respectively. The use of RD-PCR showed that among these Dra1-positive specimens, 50% (16/32) and 60% (3/5) of B. senegalensis and B. umbilicatus, respectively, were positive to S. haematobium DNA. Considering both species, the positivity rate of S. haematobium was 18.2% (19/104) in the pond prospected in October in the rainy season.
3.3. Evaluation of the Bulinus Survival to Drought under Semi-Experimental Conditions
In the artificial ponds, after they had been drained and re-watered, only 1 specimen of the original population of 15 B. senegalensis was found. For B. umbilicatus, 18 specimens were found, whereas the initial population was 8 individuals. All the species recovered were placed in tanks filled with water in order to monitor their development. After 15 days of observation, no mortality was recorded. Eggs and juveniles were recorded in the tank containing the specimen of B. senegalensis, whereas in the tank containing the species of B. umbilicatus, there were no eggs. The presence of B. senegalensis was significantly dominant compared with B. umbilicatus in the wet tank, while the opposite trend was observed when the tank dried (Fisher’s exact test, p < 0.0001) (Figure 5).
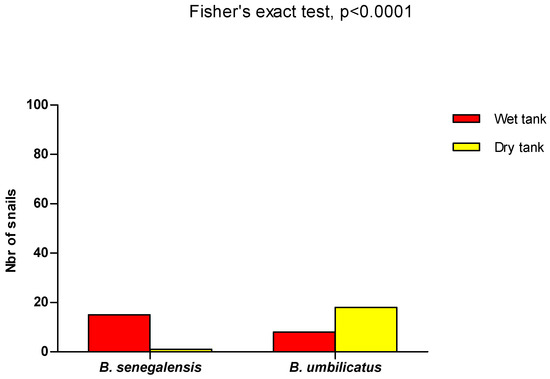
Figure 5.
B. senegalensis and B. umbilicatus in dry and wet reservoirs. Of the 15 initial B. senegalensis (wet reservoir), only 1 was found alive after the reservoir had dried out. For B. umbilicatus, there were 8 and 18 specimens alive before (wet reservoir) and after (dry reservoir), respectively. B. senegalensis was more dominant compared with B. umbilicatus in the wet tank, whereas B. umbilicatus was dominant in the dry tank (Fisher’s exact test, p < 0.0001).
4. Discussion
In the Niakhar area, located in central Senegal, schistosomiasis transmission occurs seasonally in temporary ponds. In this area, snail populations are highly dependent on rainfall and enter aestivation during the dry season [7]. In the course of this study, we reported on snail dynamics in a single pond at Niakhar during the rainy and the dry season. Our results, reporting the presence of B. senegalensis and B. umbilicatus, confirm previous work carried out during the rainy season in the same pond [7,16]. In addition, for the first time, we found, in the Niakhar area, the presence of both species during the dry season when the pond was totally dry. During the dry season, Bulinus specimens were not only collected exclusively in the dry mud but also in plastic residues covered with a layer muddy sand and plant waste. It would be interesting to assess the importance of these solid objects in the survival of snails when the ponds dry out and to see to what extent clean-up measures can be taken. This possibility of using clean-up action to eliminate drought-surviving snails would be an advantage over the use of chemicals during the rainy season when human activity is very high. In addition, it could be possible to raise public awareness of the risks of pond pollution on schistosomiasis transmission. However, more extensive studies, including more sites, would be needed to see whether plant and plastic debris would serve as significant refuges for snails, as most studies have reported that molluscs tend to bury themselves in the mud to survive drought, although some work has shown the possibility of molluscs burrowing between the roots of water lilies when ponds dry out [15] and also preferring the upper periphery of the gites [24].
During the rainy season, B. senegalensis was the dominant species compared to B. umbilicatus, and this trend is usually observed in Ngangarlam pond at Niakhar where these two Bulinus species are sympatric [7,16,25]. However, in the south-east of Senegal, it has been shown that the density of B. umbilicatus can be much higher than that of B. senegalensis in ephemeral ponds [15], suggesting that ecological conditions influence the density of different mollusc species on a local scale. In Niakhar, previous studies carried out during the rainy season have shown that B. snegalensis was the dominant species [7,16]. This trend is not observed during the dry season when B. umbilicatus outnumbered B. senegalensis. Thus, depending on the season, it is possible to have two dynamics linked to the ability of each species to survive the drought or the re-watering of the ponds. In the Niger Sahelian region, B. senegalensis and B. truncates, with very different aestivation capacities, have different population dynamics in persistent ponds filled with water for more than six months [26].
Based on the number of snails collected in the field during the dry season, it seems that B. umbilicatus is better adapted to drought than B. senegalensis. A previous study in south-eastern Senegal showed a higher density of B. umbilicatus compared with B. senegalensis just after the first rains of the season. This could mean that they predominate throughout the dry season [15]. Moreover, in the Gambia, in the Soudano-Sahelien area, a decrease in the survival of B. sengalensis in aestivation was observed over time during the dry period [27]. This trend was also observed in our semi-natural experiment, where B. umbilicatus was mainly found after the tanks had dried out, even though we used laboratory specimens which may behave differently from wild isolates. But, an extensive prospection should be conducted to gather data in other ponds and sites as B. senegalensis is described to be well adapted to drought [27]. The fact that there were more B. senegalensis during the rainy season could be explained by the greater capacity of this species to end its aestivation as soon as the rains return and its short generation time [28].
During this study, we observed a higher mortality rate in B. umbilicatus from natural conditions returned to water, whereas no mortality was observed in B. senegalensis. This could indicate that these two species do not have the same resilience to drought and to the pond re-watering and could reveal the complex dynamics of snails in temporary waterholes. In order to elucidate this complexity, more ecological and biological parameters need to be considered. Indeed, as ponds dry out, the increase in soil temperature can affect the ability of land snails to regulate their body temperature, and this is more pronounced in smaller snail species [29]. It is therefore likely that B. senegalensis, which is smaller than B. umbilicatus, is more sensitive to the increase in temperature during drought. At the species level, it is also well established that medium-sized molluscs are more resistant to drought [7,15,30]. However, during this study, we did not measure the size of the specimens to assess whether this plays a role in the survival of both B. umbilicatus and B. senegalensis during the dry season.
Also, the depth of their burial in the mud, the presence of vegetation, the water saturation of the mud at the time of burial, and soil quality combined with the anatomical specificities of each species may influence their aestivation capacity [27,31]. Therefore, combining experimental and field studies could help to better understand both the biological and ecological parameters influencing snails survival during the dry season. In our study, we observed the same trends between natural and semi-natural collections, indicating that it is possible to develop studies in controlled environments to understand the adaptation of molluscs to their changing environment. However, it is important to note that we used sand from natural ponds and recently colonised mollusc strains.
Previous studies carried out in the same pond showed a higher Dra1 positivity rate than that observed in this study [16]. However, the positivity rate of S. haematobium is currently higher than in collections made in 2015, where 2 out of 98 snails were positive in the molecular test [32]. This could be explained by the fact that in 2015, the snails were collected after praziquantel mass administration. During this study, none of the snail collected in the dry season were harbouring schistosome parasites despite the use of sensitive molecular techniques compared to the cercariae shedding method. In areas with seasonal transmission, it is assumed that the molluscs retain their infestation during the dry season and become infested as soon as the rains return [33]. In the Niakhar area, the possibility of B. umbilicatus maintaining its infestation for a period of 7 months of drought was highlighted [7]. In our study, although we used cercarial emission and molecular biology techniques, we did not find any molluscs infested during the drought. This could be due to the low number of molluscs collected during this season. This represents a limitation to this study, and a larger sampling including more ponds in diverse ecological areas would certainly be needed to determine the capacity of the snails to maintain the infestation during the dry seasons. In fact, compared with other studies, we did not test the snails at the beginning of the rainy season to assess their capacity to retain the parasite during the dry season. The beginning of the rainy season could be more favourable for finding more molluscs that have resisted the drought. On the other hand, it should also be borne in mind that infested molluscs may be more susceptible to desiccation than healthy molluscs, which could contribute to a reduction in their population during the dry season [34]. In addition the parasite survival is reduced during aestivation under experimental conditions [35]. However, in this study, we were only interested in S. haematobium. The possibility of snail infestation by non-S. haematobium was not evaluated. The presence of S. bovis and hybrids between S. haematobium and S. bovis have been highlighted in B. senegalensis in the pond we investigated [32]. A complete and specific detection non-S. haematobium infestation is therefore necessary to better understand the survival of snails and the parasites they harbour. In any case, a better understanding of the survival mechanisms of molluscs during the dry season should help us to understand the epidemiological system and the dynamics of infestation of human populations in areas of the seasonal transmission of schistosomiasis.
5. Conclusions
This study shows that the dynamics of B. senegalensis and B. umbilicatus could be season- and species-dependent in ephemeral ponds. A better understanding of this differential adaptation between sympatric species could provide new insights into the role of Bulinus snails in seasonal transmission systems for bilharzia. The combination of experimental and field studies would provide relevant information on the complexity of the Bulinus snail’s development cycle in areas of seasonal transmission. The specific ecological conditions offered by dried-up ponds could represent a breakthrough for snail control.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed9060121/s1, Figure S1: Description of the procedure used to collect molluscs during the dry and rainy seasons, to assess their viability and their infestation by S. haematobium.
Author Contributions
Conceptualization, D.S. (Diara Sy), B.S. and S.D.; methodology, D.S. (Diara Sy), B.S. and S.D.; formal analysis, S.D. and A.N.W.; investigation, D.S. (Diara Sy) and B.S.; resources, C.S. and D.S (Doudou Sow); data curation, D.S. (Diara Sy); writing—original draft preparation, D.S. (Diara Sy) and S.D.; writing—review and editing, B.S., M.A.D., A.N.W. and D.S. (Doudou Sow); supervision, S.D. and D.S. (Doudou Sow); project administration, C.S.; funding acquisition, C.S. and D.S. (Doudou Sow) All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CEA-AGIR (Centre d’Exellence Africain “Environnement, Santé, Société)-Université Cheikh Anta DIOP and by the Unité Mixte de Recherche Vecteurs Infections Tropicales et Méditerranéennes (VITROME) Campus UCAD/IRD de Hann, BP 1386.
Institutional Review Board Statement
This study has received approval from the National Ethical Committee (CNERS) of Senegal (agreement no. 000017-MSAS/DPRS/CNERS).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to Malick Diop and Kokou Abotsi for their help during data collection and technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- Pennance, T.; Archer, J.; Lugli, E.B.; Rostron, P.; Llanwarne, F.; Ali, S.M.; Amour, A.K.; Suleiman, K.R.; Li, S.; Rollinson, D.; et al. Development of a Molecular Snail Xenomonitoring Assay to Detect Schistosoma haematobium and Schistosoma bovis Infections in Their Bulinus Snail Hosts. Molecules 2020, 25, 4011. [Google Scholar] [CrossRef]
- World Health Organization. Schistosomiase. Available online: https://www.who.int/fr/news-room/fact-sheets/detail/schistosomiasis (accessed on 28 March 2024).
- Montresor, A.; Mwinzi, P.; Mupfasoni, D.; Garba, A. Reduction in DALYs Lost Due to Soil-Transmitted Helminthiases and Schistosomiasis from 2000 to 2019 Is Parallel to the Increase in Coverage of the Global Control Programmes. PLoS Negl. Trop. Dis. 2022, 16, e0010575. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Sack, A.; Bakhoum, S.; Barrett, C.B.; Lopez-Carr, D.; Chamberlin, A.J.; Civitello, D.J.; Diatta, C.; Doruska, M.J.; De Leo, G.A.; et al. A Planetary Health Innovation for Disease, Food and Water Challenges in Africa. Nature 2023, 619, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Senghor, B.; Mathieu-Begné, E.; Rey, O.; Doucouré, S.; Sow, D.; Diop, B.; Sène, M.; Boissier, J.; Sokhna, C. Urogenital Schistosomiasis in Three Different Water Access in the Senegal River Basin: Prevalence and Monitoring Praziquantel Efficacy and Re-Infection Levels. BMC Infect. Dis. 2022, 22, 968. [Google Scholar] [CrossRef] [PubMed]
- Perez-Saez, J.; Mande, T.; Ceperley, N.; Bertuzzo, E.; Mari, L.; Gatto, M.; Rinaldo, A. Hydrology and Density Feedbacks Control the Ecology of Intermediate Hosts of Schistosomiasis across Habitats in Seasonal Climates. Proc. Natl. Acad. Sci. USA 2016, 113, 6427–6432. [Google Scholar] [CrossRef] [PubMed]
- Senghor, B.; Diaw, O.T.; Doucoure, S.; Seye, M.; Talla, I.; Diallo, A.; Bâ, C.T.; Sokhna, C. Study of the Snail Intermediate Hosts of Urogenital Schistosomiasis in Niakhar, Region of Fatick, West Central Senegal. Parasites Vectors 2015, 8, 410. [Google Scholar] [CrossRef] [PubMed]
- Cridland, C.C. Results of Exposure of Batches from Highly Susceptible and Less-Susceptible Strains of Biomphalaria alexandrina alexandrina from Egypt to Strains of Schistosoma mansoni from Cairo and Alexandria. Bull. World Health Organ. 1968, 39, 955–961. [Google Scholar] [PubMed]
- Lancastre, F.; Vianey-Liaud, M.; Coutris, G.; Bolognini-Treney, J.; Mougeot, G.; Ouaghlissi, J.P. Resistance to desiccation of Biomphalaria glabrata adults infested by various miracidia of Schistosoma mansoni. Mem. Inst. Oswaldo. Cruz. 1989, 84, 205–212. [Google Scholar] [CrossRef]
- Starkloff, N.C.; Angelo, T.; Mahalila, M.P.; Charles, J.; Kinung’hi, S.; Civitello, D.J. Spatio-Temporal Variability in Transmission Risk of Human Schistosomes and Animal Trematodes in a Seasonally Desiccating East African Landscape. Proc. Biol. Sci. 2024, 291, 20231766. [Google Scholar] [CrossRef]
- Hang, D.-R.; Feng, Y.; Zhang, J.-F.; Wang, Y.-H.; Zhang, B.; Juma, S.; Sleiman, M.M.; Yang, K. Studies on the Ecology of Bulinus globosus Snails: Evidence against Burrowing into the Soil during the Dry Season. Front. Environ. Sci. 2022, 10, 925065. [Google Scholar] [CrossRef]
- Oyeyi, T.I.; Ndifon, G.T. A Note on the Post-Aestivation Biology of Bulinus rohlfsi (Clessin), an Intermediate Host of Schistosoma haematobium (Bilharz) in Northern Nigeria. Ann. Trop. Med. Parasitol. 1990, 84, 535–536. [Google Scholar] [CrossRef]
- Chu, K.Y.; Bihan, H.; Massoud, J. The Ability of Bulinus truncatus, Biomphalaria alexandrina and Lymnaea gedrosiana to Survive out of Water in the Laboratory. Ann. Trop. Med. Parasitol. 1967, 61, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.Y.; Arfaa, F.; Massoud, J. The Survival of Bulinus truncatus Buried in Mud under Experimental Outdoor Conditions. Ann. Trop. Med. Parasitol. 1967, 61, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Diaw, O.T.; Seye, M.; Sarr, Y. Resistance to drought of mollusks of the genus Bulinus, vectors of human and animal trematode infections in Senegal. II. Study under natural conditions in the North-Sudan area. Ecology and resistance to drought of Bulinus umbilicatus and B. senegalensis. Rev. Elev. Med. Vet. Pays Trop. 1989, 42, 177–187. [Google Scholar] [CrossRef]
- Gaye, P.M.; Doucoure, S.; Senghor, B.; Faye, B.; Goumballa, N.; Sembène, M.; L’Ollivier, C.; Parola, P.; Ranque, S.; Sow, D.; et al. Bulinus senegalensis and Bulinus umbilicatus Snail Infestations by the Schistosoma haematobium Group in Niakhar, Senegal. Pathogens 2021, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Kristensen, T. A Field Guide to African Freshwater Snails. 1. West African Species; Danish Bilharziasis Laboratory: Chatollenlund, Denmark, 1993. [Google Scholar]
- Frandsen, F.; Christensen, N.O. An Introductory Guide to the Identification of Cercariae from African Freshwater Snails with Special Reference to Cercariae of Trematode Species of Medical and Veterinary Importance. Acta Trop. 1984, 41, 181–202. [Google Scholar]
- Appleton, C.; Miranda, N. Locating Bilharzia Transmission Sites in South Africa: Guidelines for Public Health Personnel. S. Afr. J. Infect. Dis. 2015, 30, 95–102. [Google Scholar] [CrossRef]
- Cnops, L.; Soentjens, P.; Clerinx, J.; Van Esbroeck, M. A Schistosoma haematobium-Specific Real-Time PCR for Diagnosis of Urogenital Schistosomiasis in Serum Samples of International Travelers and Migrants. PLoS Negl. Trop. Dis. 2013, 7, e2413. [Google Scholar] [CrossRef]
- Ibironke, O.; Koukounari, A.; Asaolu, S.; Moustaki, I.; Shiff, C. Validation of a New Test for Schistosoma haematobium Based on Detection of Dra1 DNA Fragments in Urine: Evaluation through Latent Class Analysis. PLoS Negl. Trop. Dis. 2012, 6, e1464. [Google Scholar] [CrossRef]
- Hamburger, J.; He-Na; Abbasi, I.; Ramzy, R.M.; Jourdane, J.; Ruppel, A. Polymerase Chain Reaction Assay Based on a Highly Repeated Sequence of Schistosoma haematobium: A Potential Tool for Monitoring Schistosome-Infested Water. Am. J. Trop. Med. Hyg. 2001, 65, 907–911. [Google Scholar] [CrossRef]
- Webster, B.L.; Rollinson, D.; Stothard, J.R.; Huyse, T. Rapid Diagnostic Multiplex PCR (RD-PCR) to Discriminate Schistosoma haematobium and S. Bovis. J. Helminthol. 2010, 84, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Webbe, G.; Msangi, A.S. Observations on Three Species of Bulinus on the East Coast of Africa. Ann. Trop. Med. Parasitol. 1958, 52, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Senghor, B.; Diaw, O.T.; Doucoure, S.; Seye, M.; Diallo, A.; Talla, I.; Bâ, C.T.; Sokhna, C. Impact of Annual Praziquantel Treatment on Urogenital Schistosomiasis in a Seasonal Transmission Focus in Central Senegal. PLoS Negl. Trop. Dis. 2016, 10, e0004557. [Google Scholar] [CrossRef] [PubMed]
- Vera, C.; Bremond, P.; Labbo, R.; Mouchet, F.; SELLIN, E.; Boulanger, D.; Pointier, J.P.; Delay, B.; Sellin, B. Seasonal Fluctuation in Populations Densities of Bulinus senegalensis and B. truncatus (Planorbidae) in Temporary Pools in a Focus of Schistosoma heamatobium in Niger: Implications for Control. J. Molluscan Stud. 1995, 61, 79–88. [Google Scholar] [CrossRef]
- Smithers, S.R. On the Ecology of Schistosome Vectors in the Gambia, with Evidence of Their Rôle in Transmission. Trans. R Soc. Trop. Med. Hyg. 1956, 50, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.S. Freshwater Snails of Africa And Their Medical Importance; CRC Press: London, UK, 2014; ISBN 978-0-429-09494-1. [Google Scholar]
- Knigge, T.; Di Lellis, M.A.; Monsinjon, T.; Köhler, H.-R. Relevance of Body Size and Shell Colouration for Thermal Absorption and Heat Loss in White Garden Snails, Theba Pisana (Helicidae), from Northern France. J. Therm. Biol. 2017, 69, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Diaw, O.T.; Vassiliades, G.; Seye, M.; Sarr, Y. Prolifération de mollusques et incidence sur les trématodoses dans la région du delta et du lac de Guiers après la construction du barrage de Diama sur le fleuve Sénégal. Rev. D’élevage Médecine Vétérinaire Des Pays Trop. 1990, 43, 499–502. [Google Scholar] [CrossRef]
- Oliver, L.; Barbosa, F.S. Observations on Vectors of Schistosomiasis mansoni Kept out of Water in the Laboratory. J. Parasitol. 1956, 42, 277–286. [Google Scholar] [CrossRef]
- Senghor, B.; Webster, B.; Pennance, T.; Sène, M.; Doucouré, S.; Sow, D.; Sokhna, C. Molecular Characterization of Schistosome Cercariae and Their Bulinus Snail Hosts from Niakhar, a Seasonal Transmission Focus in Central Senegal. Curr. Res. Parasitol. Vector. Borne Dis. 2023, 3, 100114. [Google Scholar] [CrossRef]
- Webbe, G. The Transmission of Schistosoma haematobium in an Area of Lake Province, Tanganyika. Bull. World Health Organ. 1962, 27, 59–85. [Google Scholar]
- Ohlweiler, F.P.; Kawano, T. Biomphalaria tenagophila (Orbigny, 1835) (Mollusca): Adaptation to Desiccation and Susceptibility to Infection with Schistosoma mansoni Sambon, 1907. Rev. Inst. Med. Trop. Sao Paulo 2002, 44, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Rubaba, O.; Chimbari, M.; Mukaratirwa, S. The Role of Snail Aestivation in Transmission of Schistosomiasis in Changing Climatic Conditions. Afr. J. Aquat. Sci. 2016, 41, 143–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).