The Effect of Larval Exposure to Heavy Metals on the Gut Microbiota Composition of Adult Anopheles arabiensis (Diptera: Culicidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples for Sequencing
2.2. Bioinformatics
3. Results
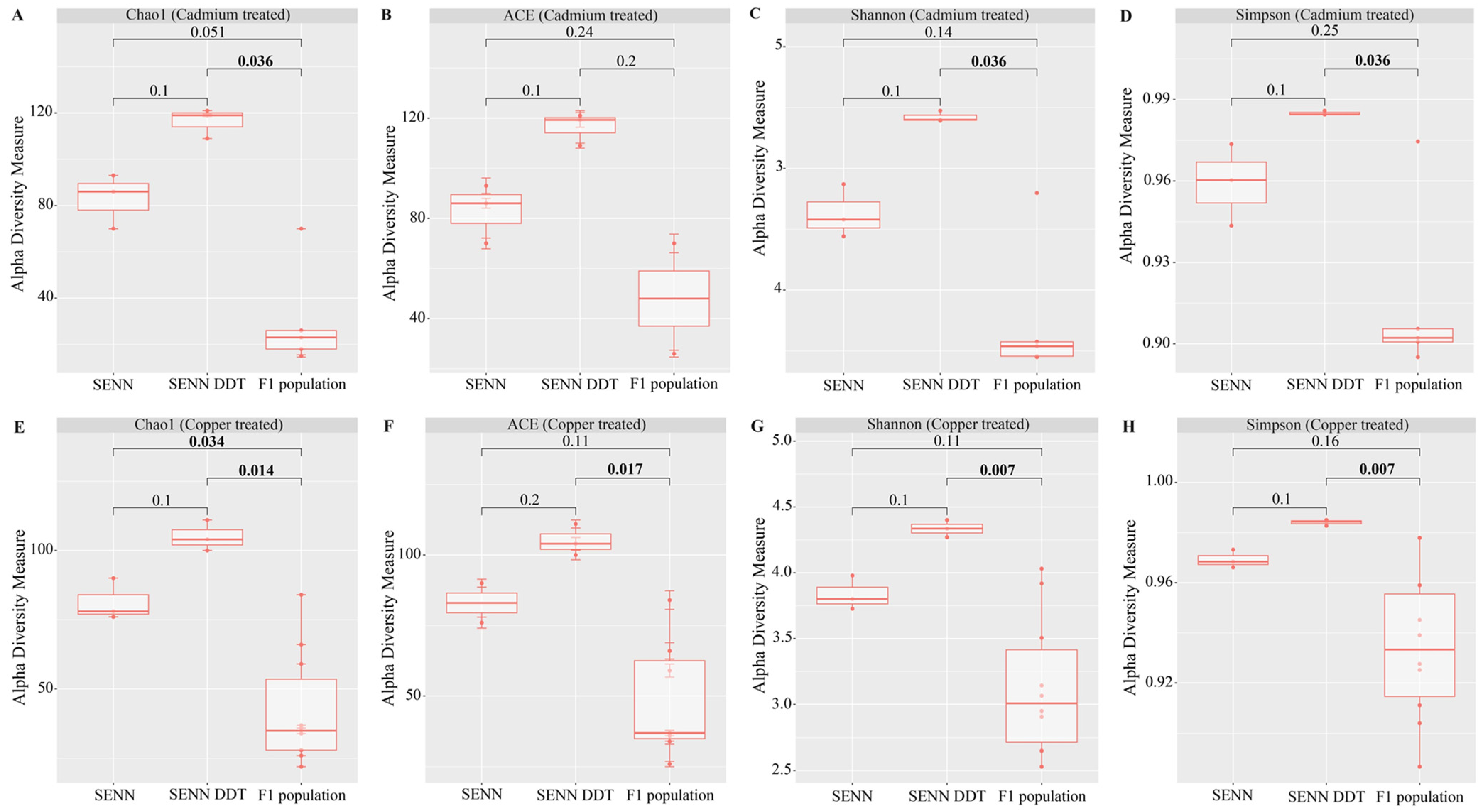
3.1. Alpha Diversity
3.2. Beta Diversity
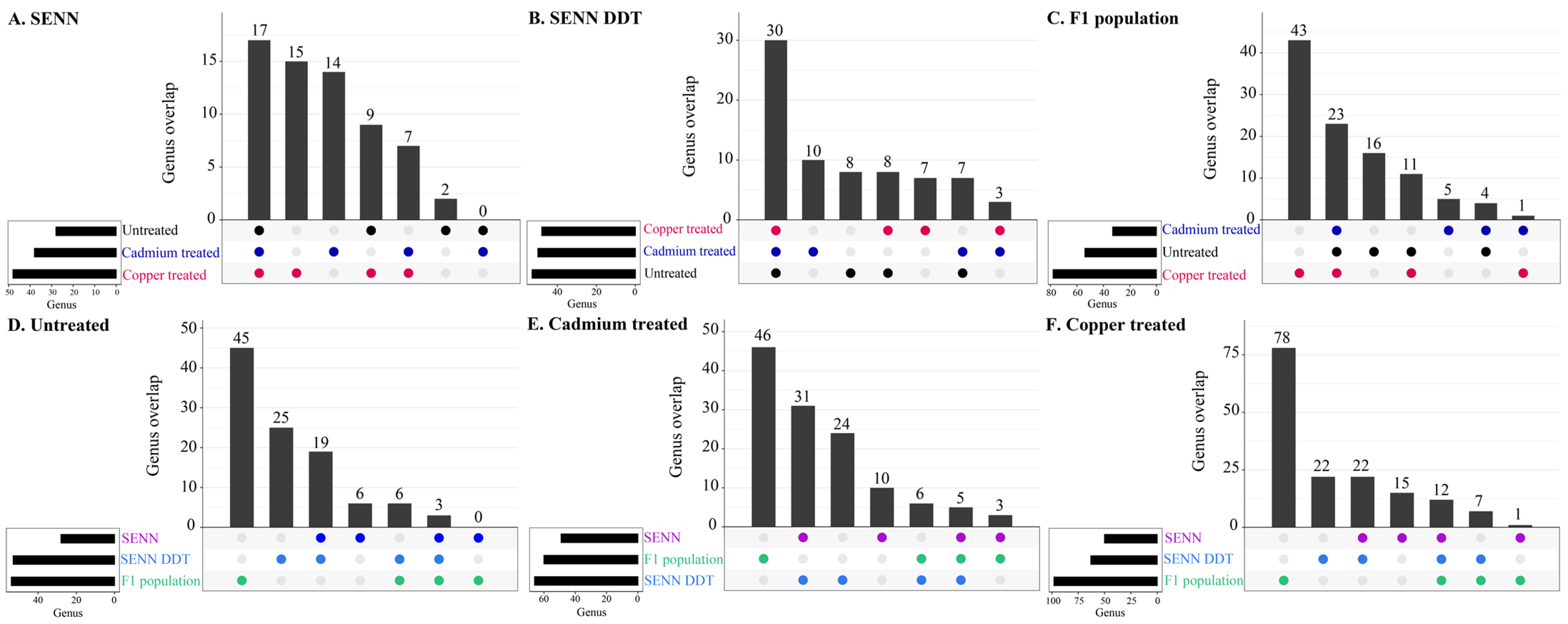
3.3. Overlapping Genera
3.4. Differential Abundance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raman, J.; Fakudze, P.; Sikaala, C.H.; Chimumbwa, J.; Moonasar, D. Eliminating malaria from the margins of transmission in Southern Africa through the Elimination 8 Initiative. Trans. R. Soc. S. Afr. 2021, 76, 137–145. [Google Scholar] [CrossRef]
- Kitau, J.; Oxborough, R.M.; Tungu, P.K.; Matowo, J.; Malima, R.C.; Magesa, S.M.; Bruce, J.; Mosha, F.W.; Rowland, M.W. Species shifts in the Anopheles gambiae complex: Do LLINs successfully control Anopheles arabiensis? PLoS ONE 2012, 7, e31481. [Google Scholar] [CrossRef] [PubMed]
- Killeen, G.F. Characterizing, controlling and eliminating residual malaria transmission. Malar. J. 2014, 13, 330. [Google Scholar] [CrossRef] [PubMed]
- Killeen, G.F.; Govella, N.J.; Lwetoijera, D.W.; Okumu, F.O. Most outdoor malaria transmission by behaviourally-resistant Anopheles arabiensis is mediated by mosquitoes that have previously been inside houses. Malar. J. 2016, 15, 225. [Google Scholar] [CrossRef] [PubMed]
- Munhenga, G.; Oliver, S.V.; Lobb, L.N.; Mazarire, T.T.; Sekgele, W.; Mashatola, T.; Mabaso, N.; Dlamini, D.M.; Zulu, M.; Moletsane, F.; et al. Malaria risk and receptivity: Continuing development of insecticide resistance in the major malaria vector Anopheles arabiensis in northern KwaZulu-Natal, South Africa. S. Afr. J. Sci. 2022, 118. [Google Scholar] [CrossRef]
- Kotnis, B.; Kuri, J. Evaluating the usefulness of paratransgenesis for malaria control. Math. Biosci. 2016, 277, 117–125. [Google Scholar] [CrossRef]
- Wilke, A.B.; Marrelli, M.T. Paratransgenesis: A promising new strategy for mosquito vector control. Parasites Vectors 2015, 8, 342. [Google Scholar] [CrossRef]
- Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 2014, 23, 2727–2739. [Google Scholar] [CrossRef]
- Coon, K.L.; Valzania, L.; McKinney, D.A.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Bacteria-mediated hypoxia functions as a signal for mosquito development. Proc. Natl. Acad. Sci. USA 2017, 114, e5362–e5369. [Google Scholar] [CrossRef]
- Fofana, A.; Gendrin, M.; Romoli, O.; Yarbanga, G.A.B.; Ouédraogo, G.A.; Yerbanga, R.S.; Ouédraogo, J.B. Analyzing gut microbiota composition in individual Anopheles mosquitoes after experimental treatment. iScience 2021, 24, 103416. [Google Scholar] [CrossRef]
- Barnard, K.; Jeanrenaud, A.; Brooke, B.D.; Oliver, S.V. The contribution of gut bacteria to insecticide resistance and the life histories of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Sci. Rep. 2019, 9, 9117. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, P.; Caccia, S.; Varotto-Boccazzi, I.; Arnoldi, I.; Barbieri, G.; Comandatore, F.; Epis, S. Mosquito Trilogy: Microbiota, Immunity and Pathogens, and Their Implications for the Control of Disease Transmission. Front. Microbiol. 2021, 12, 630438. [Google Scholar] [CrossRef] [PubMed]
- Belachew, E.A. Immune Response and Evasion Mechanisms of Plasmodium falciparum Parasites. J. Immunol. Res. 2018, 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, F.H.; Gendrin, M.; Wyer, C.A.S.; Christophides, G.K. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog 2017, 13, e1006391. [Google Scholar] [CrossRef] [PubMed]
- Baia-da-Silva, D.C.; Alvarez, L.C.S.; Lizcano, O.V.; Costa, F.T.M.; Lopes, S.C.P.; Orfanó, A.S.; Pascoal, D.O.; Nacif-Pimenta, R.; Rodriguez, I.C.; Guerra, M.; et al. The role of the peritrophic matrix and red blood cell concentration in Plasmodium vivax infection of Anopheles aquasalis. Parasites Vectors 2018, 11, 148. [Google Scholar] [CrossRef]
- De Vooght, L.; Van Keer, S.; Van Den Abbeele, J. Towards improving tsetse fly paratransgenesis: Stable colonization of Glossina morsitans morsitans with genetically modified Sodalis. BMC Microbiol. 2018, 18, 165. [Google Scholar] [CrossRef]
- Grogan, C.; Bennett, M.; Lampe, D.J. An evaluation of fusion partner proteins for paratransgenesis in Asaia bogorensis. PLoS ONE 2022, 17, e0273568. [Google Scholar] [CrossRef]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef]
- Herren, J.K.; Mbaisi, L.; Mararo, E.; Makhulu, E.E.; Mobegi, V.A.; Butungi, H.; Mancini, M.V.; Oundo, J.W.; Teal, E.T.; Pinaud, S.; et al. A microsporidian impairs Plasmodium falciparum transmission in Anopheles arabiensis mosquitoes. Nat. Commun. 2020, 11, 2187. [Google Scholar] [CrossRef]
- Femi, A.; Tosin, S.O. The Potential for Wolbachia-Based Mosquito Biocontrol Strategies in Africa. In Mosquito Research; Henry, P.-G., Pablo, M.-S., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 8. [Google Scholar]
- Sandeu, M.M.; Maffo, C.G.T.; Dada, N.; Njiokou, F.; Hughes, G.L.; Wondji, C.S. Seasonal variation of microbiota composition in Anopheles gambiae and Anopheles coluzzii in two different eco-geographical localities in Cameroon. Med. Vet. Entomol. 2022, 36, 269–282. [Google Scholar] [CrossRef]
- Singh, A.; Patel, N.F.; Allam, M.; Chan, W.Y.; Mohale, T.; Ismail, A.; Oliver, S.V. Marked Effects of Larval Salt Exposure on the Life History and Gut Microbiota of the Malaria Vector Anopheles merus (Diptera: Culicidae). Insects 2022, 13, 1165. [Google Scholar] [CrossRef] [PubMed]
- Dandalo, L.C.; Brooke, B.D.; Munhenga, G.; Lobb, L.N.; Zikhali, J.; Ngxongo, S.P.; Zikhali, P.M.; Msimang, S.; Wood, O.R.; Mofokeng, M.; et al. Population Dynamics and Plasmodium falciparum (Haemosporida: Plasmodiidae) Infectivity Rates for the Malaria Vector Anopheles arabiensis (Diptera: Culicidae) at Mamfene, KwaZulu-Natal, South Africa. J. Med. Entomol. 2017, 54, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Msugupakulya, B.J.; Urio, N.H.; Jumanne, M.; Ngowo, H.S.; Selvaraj, P.; Okumu, F.O.; Wilson, A.L. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasites Vectors 2023, 16, 408. [Google Scholar] [CrossRef] [PubMed]
- Azrag, R.S.; Mohammed, B.H. Anopheles arabiensis in Sudan: A noticeable tolerance to urban polluted larval habitats associated with resistance to Temephos. Malar. J. 2018, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Nkondjio, C.; Fossog, B.T.; Ndo, C.; Djantio, B.M.; Togouet, S.Z.; Awono-Ambene, P.; Costantini, C.; Wondji, C.S.; Ranson, H. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): Influence of urban agriculture and pollution. Malar. J. 2011, 10, 154. [Google Scholar] [CrossRef]
- Dada, N.; Sheth, M.; Liebman, K.; Pinto, J.; Lenhart, A. Whole metagenome sequencing reveals links between mosquito microbiota and insecticide resistance in malaria vectors. Sci. Rep. 2018, 8, 2084. [Google Scholar] [CrossRef]
- Jones, C.M.; Toé, H.K.; Sanou, A.; Namountougou, M.; Hughes, A.; Diabaté, A.; Dabiré, R.; Simard, F.; Ranson, H. Additional selection for insecticide resistance in urban malaria vectors: DDT resistance in Anopheles arabiensis from Bobo-Dioulasso, Burkina Faso. PLoS ONE 2012, 7, e45995. [Google Scholar] [CrossRef]
- Awolola, T.S.; Oduola, A.O.; Obansa, J.B.; Chukwurar, N.J.; Unyimadu, J.P. Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. J. Vector Borne Dis. 2007, 44, 241–244. [Google Scholar]
- Mireji, P.O.; Keating, J.; Hassanali, A.; Impoinvil, D.E.; Mbogo, C.M.; Muturi, M.N.; Nyambaka, H.; Kenya, E.U.; Githure, J.I.; Beier, J.C. Expression of metallothionein and alpha-tubulin in heavy metal-tolerant Anopheles gambiae sensu stricto (Diptera: Culicidae). Ecotoxicol. Environ. Saf. 2010, 73, 46–50. [Google Scholar] [CrossRef]
- Mireji, P.O.; Keating, J.; Hassanali, A.; Mbogo, C.M.; Muturi, M.N.; Githure, J.I.; Beier, J.C. Biological cost of tolerance to heavy metals in the mosquito Anopheles gambiae. Med. Vet. Entomol. 2010, 24, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Rono, M.K.; Muturi, C.N.; Ochieng, R.; Mwakubabanya, R.; Wachira, F.N.; Mwangangi, J.; Kinyanjui, S.; Njunge, J.; Mireji, P.O. Cadmium tolerance pathway in Anopheles gambiae senso stricto. Acta Trop. 2019, 198, 105033. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.V.; Brooke, B.D. The effect of metal pollution on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). PLoS ONE 2018, 13, e0192551. [Google Scholar] [CrossRef] [PubMed]
- Jeanrenaud, A.C.S.N.; Brooke, B.D.; Oliver, S.V. Second generation effects of larval metal pollutant exposure on reproduction, longevity and insecticide tolerance in the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Parasites Vectors 2020, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Jeanrenaud, A.C.S.N.; Brooke, B.D.; Oliver, S.V. Characterisation of the epigenetic architecture of the major malaria vector Anopheles arabiensis (Diptera: Culicidae) after treatment with epigenetic modulators and heavy metals. Acta Trop. 2022, 226, 106259. [Google Scholar] [CrossRef]
- Hunt, R.H.; Brooke, B.D.; Pillay, C.; Koekemoer, L.L.; Coetzee, M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Entomol. 2005, 19, 271–275. [Google Scholar] [CrossRef]
- Hemingway, J. Biochemical studies on malathion resistance in Anopheles arabiensis from Sudan. Trans. R. Soc. Trop. Med. Hyg. 1983, 77, 477–480. [Google Scholar] [CrossRef]
- Oliver, S.V.; Brooke, B.D. The effect of elevated temperatures on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Malar. J. 2017, 16, 73. [Google Scholar] [CrossRef]
- Oliver, S.V.; Brooke, B.D. The effect of larval nutritional deprivation on the life history and DDT resistance phenotype in laboratory strains of the malaria vector Anopheles arabiensis. Malar. J. 2013, 12, 44. [Google Scholar] [CrossRef]
- Coetzee, M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar. J. 2020, 19, 70. [Google Scholar] [CrossRef]
- Scott, J.A.; Brogdon, W.G.; Collins, F.H. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993, 49, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Li, X.-D.; Xin, L.; Rong, W.-T.; Liu, X.-Y.; Deng, W.-A.; Qin, Y.-C.; Li, X.-L. Effect of heavy metals pollution on the composition and diversity of the intestinal microbial community of a pygmy grasshopper (Eucriotettix oculatus). Ecotoxicol. Environ. Saf. 2021, 223, 112582. [Google Scholar] [CrossRef]
- Laviad-Shitrit, S.; Sharaby, Y.; Sela, R.; Thorat, L.; Nath, B.B.; Halpern, M. Copper and chromium exposure affect chironomid larval microbiota composition. Sci. Total Environ. 2021, 771, 145330. [Google Scholar] [CrossRef]
- Munhenga, G.; Brooke, B.D.; Spillings, B.; Essop, L.; Hunt, R.H.; Midzi, S.; Govender, D.; Braack, L.; Koekemoer, L.L. Field study site selection, species abundance and monthly distribution of anopheline mosquitoes in the northern Kruger National Park, South Africa. Malar. J. 2014, 13, 27. [Google Scholar] [CrossRef]
- Muturi, E.J.; Lagos-Kutz, D.; Dunlap, C.; Ramirez, J.L.; Rooney, A.P.; Hartman, G.L.; Fields, C.J.; Rendon, G.; Kim, C.-H. Mosquito microbiota cluster by host sampling location. Parasites Vectors 2018, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Schrieke, H.; Maignien, L.; Constancias, F.; Trigodet, F.; Chakloute, S.; Rakotoarivony, I.; Marie, A.; L’Ambert, G.; Makoundou, P.; Pages, N.; et al. The mosquito microbiome includes habitat-specific but rare symbionts. Comput. Struct. Biotechnol. J. 2022, 20, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Giachino, A.; Waldron, K.J. Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol. Microbiol. 2020, 114, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Lang, M. A Comprehensive Review on the Roles of Metals Mediating Insect-Microbial Pathogen Interactions. Metabolites 2023, 13, 839. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Wang, S.; Lin, Z.; Su, R.; Jin, F.; Zhang, Y. Responses of the gut microbiota to environmental heavy metal pollution in tree sparrow (Passer montanus) nestlings. Ecotoxicol. Environ. Saf. 2023, 264, 115480. [Google Scholar] [CrossRef]
- Wu, N.; Wang, X.; Xu, X.; Cai, R.; Xie, S. Effects of heavy metals on the bioaccumulation, excretion and gut microbiome of black soldier fly larvae (Hermetia illucens). Ecotoxicol. Environ. Saf. 2020, 192, 110323. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, L.; Tan, M.; Li, Y.; Xu, J.; Yan, S.; Jiang, D. Cd exposure-triggered susceptibility to Bacillus thuringiensis in Lymantria dispar involves in gut microbiota dysbiosis and hemolymph metabolic disorder. Ecotoxicol. Environ. Saf. 2022, 241, 113763. [Google Scholar] [CrossRef]
- Hu, M.; Li, X.; Li, Z.; Liu, B.; Yang, Z.; Tian, Y. Ochrobactrum teleogrylli sp. nov., a pesticide-degrading bacterium isolated from the insect Teleogryllus occipitalis living in deserted cropland. Int. J. Syst. Evol. Microbiol. 2020, 70, 2217–2225. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, D.; Chen, H.; Qiu, T.; Zhao, S.; Duan, C.; Cui, Y.; Zhu, X.; Chao, H.; Wang, Y.; et al. Synergistic interplay between Azospirillum brasilense and exogenous signaling molecule H2S promotes Cd stress resistance and growth in pak choi (Brassica chinensis L.). J. Hazard. Mater. 2023, 444, 130425. [Google Scholar] [CrossRef]
- Elizabeth George, S.; Wan, Y. Advances in characterizing microbial community change and resistance upon exposure to lead contamination: Implications for ecological risk assessment. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2223–2270. [Google Scholar] [CrossRef]
- Li, X.; Yu, Y.; Choi, L.; Song, Y.; Wu, M.; Wang, G.; Li, M. Phenylobacterium soli sp. nov., isolated from arsenic and cadmium contaminated farmland soil. Int. J. Syst. Evol. Microbiol. 2019, 69, 1398–1403. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Rosen, B.P. Organoarsenical tolerance in Sphingobacterium wenxiniae, a bacterium isolated from activated sludge. Environ. Microbiol. 2022, 24, 762–771. [Google Scholar] [CrossRef]
- Duan, C.; Liu, Y.; Zhang, H.; Chen, G.; Song, J. Cadmium Pollution Impact on the Bacterial Community of Haplic Cambisols in Northeast China and Inference of Resistant Genera. J. Soil Sci. Plant Nutr. 2020, 20, 1156–1170. [Google Scholar] [CrossRef]
- Wang, H.; Wu, P.; Liu, J.; Yang, S.; Ruan, B.; Rehman, S.; Liu, L.; Zhu, N. The regulatory mechanism of Chryseobacterium sp. resistance mediated by montmorillonite upon cadmium stress. Chemosphere 2020, 240, 124851. [Google Scholar] [CrossRef] [PubMed]
- Cavalca, L.; Zanchi, R.; Corsini, A.; Colombo, M.; Romagnoli, C.; Canzi, E.; Andreoni, V. Arsenic-resistant bacteria associated with roots of the wild Cirsium arvense (L.) plant from an arsenic polluted soil, and screening of potential plant growth-promoting characteristics. Syst. Appl. Microbiol. 2010, 33, 154–164. [Google Scholar] [CrossRef]
- Fries, J.; Pfeiffer, S.; Kuffner, M.; Sessitsch, A. Spirosomaendophyticum sp. nov., isolated from Zn- and Cd-accumulating Salix caprea. Int. J. Syst. Evol. Microbiol. 2013, 63, 4586–4590. [Google Scholar] [CrossRef] [PubMed]
- El-Ballat, E.A.-O.; Elsilk, S.E.; Ali, H.A.-O.; Ali, H.A.-O.; Hano, C.A.-O.; El-Esawi, M.A.-O. Metal-Resistant PGPR Strain Azospirillum brasilense EMCC1454 Enhances Growth and Chromium Stress Tolerance of Chickpea (Cicer arietinum L.) by Modulating Redox Potential, Osmolytes, Antioxidants, and Stress-Related Gene Expression. Plants 2023, 12, 2110. [Google Scholar] [CrossRef]
- Mohsin, H.; Asif, A.; Rehman, Y. Anoxic growth optimization for metal respiration and photobiological hydrogen production by arsenic-resistant Rhodopseudomonas and Rhodobacter species. J. Basic Microbiol. 2019, 59, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Grouzdev, D.S.; Babich, T.L.; Sokolova, D.S.; Tourova, T.P.; Poltaraus, A.B.; Nazina, T.N. Draft genome sequence data and analysis of Shinella sp. strain JR1-6 isolated from nitrate- and radionuclide-contaminated groundwater in Russia. Data Brief 2019, 25, 104319. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Xia, X.; Zhou, Z.; Zheng, S.; Wang, G. Reduction of tellurite in Shinella sp. WSJ-2 and adsorption removal of multiple dyes and metals by biogenic tellurium nanorods. Int. Biodeterior. Biodegrad. 2019, 144, 104751. [Google Scholar] [CrossRef]
- De Marco, P.; Pacheco, C.C.; Figueiredo, A.R.; Moradas-Ferreira, P. Novel pollutant-resistant methylotrophic bacteria for use in bioremediation. FEMS Microbiol. Lett. 2004, 234, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Bajaj, A.; Verma, H.; Kumar, R.; Singh, Y.; Lal, R. Complete genome sequence of Paracoccus sp. strain AK26: Insights into multipartite genome architecture and methylotropy. Genomics 2020, 112, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Maertens, L.; Cherry, P.; Tilquin, F.; Van Houdt, R.; Matroule, J.Y. Environmental Conditions Modulate the Transcriptomic Response of Both Caulobacter crescentus Morphotypes to Cu Stress. Microorganisms 2021, 9, 1116. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Chaturvedi, P.; Chandra, R.; Kumar, S. Identification of heavy metals tolerant Brevundimonas sp. from rhizospheric zone of Saccharum munja L. and their efficacy in in-situ phytoremediation. Chemosphere 2022, 295, 133823. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Qiao, P.; Liu, D.; Bai, Q.; Guan, W.; Yang, Y.; Zhao, T. Comparison of Copper-Tolerance Genes between Different Groups of Acidovorax citrulli. Microorganisms 2024, 12, 682. [Google Scholar] [CrossRef]
- Ryan, R.P.; Monchy, S.; Cardinale, M.; Taghavi, S.; Crossman, L.; Avison, M.B.; Berg, G.; van der Lelie, D.; Dow, J.M. The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 2009, 7, 514–525. [Google Scholar] [CrossRef]
- Boudehen, Y.-M.; Faucher, M.; Maréchal, X.; Miras, R.; Rech, J.; Rombouts, Y.; Sénèque, O.; Wallat, M.; Demange, P.; Bouet, J.-Y.; et al. Mycobacterial resistance to zinc poisoning requires assembly of P-ATPase-containing membrane metal efflux platforms. Nat. Commun. 2022, 13, 4731. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Mao, W.; Peng, Y.; Han, X.; Jin, H.; Xu, J.; Chang, L.; Hou, Y.; Shen, X.; et al. Functional versatility of Zur in metal homeostasis, motility, biofilm formation, and stress resistance in Yersinia pseudotuberculosis. Microbiol. Spectr. 2024, 12, e0375623. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, X.; Lian, Y.; Zhang, B.; He, X.; Xu, W.; Huang, K. Characterization of a cadmium resistance Lactococcus lactis subsp. lactis strain by antioxidant assays and proteome profiles methods. Environ. Toxicol. Pharmacol. 2016, 46, 286–291. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Choi, D.; Chang, Y.-C. Isolation and characterization of a biosurfactant-producing heavy metal resistant Rahnella sp. RM isolated from chromium-contaminated soil. Biotechnol. Bioprocess Eng. 2017, 22, 186–194. [Google Scholar] [CrossRef]
- Bisht, H.; Kumar, N. Characterization and Evaluation of the Nickel-Removal Capacity of Kluyvera cryocrescens M7 Isolated from Industrial Wastes. Pollution 2023, 9, 1059–1073. [Google Scholar] [CrossRef]
- Rezaee, A. Adsorption of Mercury from Synthetic Solutions by an Acetobacter xylinum Biofilm Abbas Rezaee, Jamshid Derayat, Hatam Godini and Gholamhossin Pourtaghi Department of Environmental Health, Faculty of Medical Sciences. J Res. J. Environ. Sci. 2008, 2, 401–407. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, V.K.; Qazi, G.N.; Kumar, A. Gluconobacter oxydans: Its biotechnological applications. J. Mol. Microbiol. Biotechnol. 2001, 3, 445–456. [Google Scholar] [PubMed]
- Ross, P.A.; Endersby-Harshman, N.M.; Hoffmann, A.A. A comprehensive assessment of inbreeding and laboratory adaptation in Aedes aegypti mosquitoes. Evol. Appl. 2019, 12, 572–586. [Google Scholar] [CrossRef]
- Cornet, S.; Gandon, S.; Rivero, A. Patterns of phenoloxidase activity in insecticide resistant and susceptible mosquitoes differ between laboratory-selected and wild-caught individuals. Parasites Vectors 2013, 6, 315. [Google Scholar] [CrossRef]
- Vézilier, J.; Nicot, A.; Lorgeril, J.; Gandon, S.; Rivero, A. The impact of insecticide resistance on Culex pipiens immunity. Evol. Appl. 2013, 6, 497–509. [Google Scholar] [CrossRef]
- Dennison, N.J.; Saraiva, R.G.; Cirimotich, C.M.; Mlambo, G.; Mongodin, E.F.; Dimopoulos, G. Functional genomic analyses of Enterobacter, Anopheles and Plasmodium reciprocal interactions that impact vector competence. Malar. J. 2016, 15, 425. [Google Scholar] [CrossRef]
- Bassene, H.; Niang, E.H.A.; Fenollar, F.; Dipankar, B.; Doucouré, S.; Ali, E.; Michelle, C.; Raoult, D.; Sokhna, C.; Mediannikov, O. 16S Metagenomic Comparison of Plasmodium falciparum-Infected and Noninfected Anopheles gambiae and Anopheles funestus Microbiota from Senegal. Am. J. Trop. Med. Hyg. 2018, 99, 1489–1498. [Google Scholar] [CrossRef]
- Villegas, L.M.; Pimenta, P.F.P. Metagenomics, paratransgenesis and the Anopheles microbiome: A portrait of the geographical distribution of the anopheline microbiota based on a meta-analysis of reported taxa. Memórias Do Inst. Oswaldo Cruz 2014, 109, 672–684. [Google Scholar] [CrossRef]
- Kariithi, H.M.; Meki, I.K.; Schneider, D.I.; De Vooght, L.; Khamis, F.M.; Geiger, A.; Demirbaş-Uzel, G.; Vlak, J.M.; Ince, I.A.; Kelm, S.; et al. Enhancing vector refractoriness to trypanosome infection: Achievements, challenges and perspectives. BMC Microbiol. 2018, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bagdasarian, M.; Walker, E.D. Elizabethkingia anophelis: Molecular manipulation and interactions with mosquito hosts. Appl. Environ. Microbiol. 2015, 81, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Rodrigues, J.; Bilgo, E.; Tormo, J.R.; Challenger, J.D.; De Cozar-Gallardo, C.; Pérez-Victoria, I.; Reyes, F.; Castañeda-Casado, P.; Gnambani, E.J.; et al. Delftia tsuruhatensis TC1 symbiont suppresses malaria transmission by anopheline mosquitoes. Science 2023, 381, 533–540. [Google Scholar] [CrossRef]
- Rocha, E.M.; Marinotti, O.; Serrão, D.M.; Correa, L.V.; Katak, R.d.M.; de Oliveira, J.C.; Muniz, V.A.; de Oliveira, M.R.; do Nascimento Neto, J.F.; Pessoa, M.C.F.; et al. Culturable bacteria associated with Anopheles darlingi and their paratransgenesis potential. Malar. J. 2021, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Tchioffo, M.T.; Boissière, A.; Churcher, T.S.; Abate, L.; Gimonneau, G.; Nsango, S.E.; Awono-Ambéné, P.H.; Christen, R.; Berry, A.; Morlais, I. Modulation of Malaria Infection in Anopheles gambiae Mosquitoes Exposed to Natural Midgut Bacteria. PLoS ONE 2013, 8, e81663. [Google Scholar] [CrossRef]
- Yu, S.; Wang, P.; Qin, J.; Zheng, H.; Wang, J.; Liu, T.; Yang, X.; Wang, Y. Bacillus sphaericus exposure reduced vector competence of Anopheles dirus to Plasmodium yoelii by upregulating the Imd signaling pathway. Parasites Vectors 2020, 13, 446. [Google Scholar] [CrossRef]
- Favia, G.; Ricci, I.; Marzorati, M.; Negri, I.; Alma, A.; Sacchi, L.; Bandi, C.; Daffonchio, D. Bacteria of the Genus Asaia: A Potential Paratransgenic Weapon Against Malaria. In Transgenesis and the Management of Vector-Borne Disease; Aksoy, S., Ed.; Springer: New York, NY, USA, 2008; pp. 49–59. [Google Scholar]
- Qi, Z.; Han, X.; Zhang, Y.; Wang, J.; Cao, Y.M. Listeria monocytogenes inoculation protects mice against blood-stage Plasmodium yoelii infection. Tohoku J. Exp. Med. 2013, 229, 87–96. [Google Scholar] [CrossRef]
- Kosakamoto, H.; Yamauchi, T.; Akuzawa-Tokita, Y.; Nishimura, K.; Soga, T.; Murakami, T.; Mori, H.; Yamamoto, K.; Miyazaki, R.; Koto, A.; et al. Local Necrotic Cells Trigger Systemic Immune Activation via Gut Microbiome Dysbiosis in Drosophila. Cell Rep. 2020, 32, 107938. [Google Scholar] [CrossRef]
- Garver, L.S.; Bahia, A.C.; Das, S.; Souza-Neto, J.A.; Shiao, J.; Dong, Y.; Dimopoulos, G. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS Pathog. 2012, 8, e1002737. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, W.; Li, S.; Ma, J.; Wang, J.; Zhao, X. Microbial degradation mechanism and pathway of the novel insecticide paichongding by a newly isolated Sphingobacterium sp. P1-3 from soil. J. Agric. Food Chem. 2015, 63, 3823–3829. [Google Scholar] [CrossRef]
- Gómez-Govea, M.A.; Ramírez-Ahuja, M.L.; Contreras-Perera, Y.; Jiménez-Camacho, A.J.; Ruiz-Ayma, G.; Villanueva-Segura, O.K.; Trujillo-Rodríguez, G.J.; Delgado-Enciso, I.; Martínez-Fierro, M.L.; Manrique-Saide, P.; et al. Suppression of Midgut Microbiota Impact Pyrethroid Susceptibility in Aedes aegypti. Front. Microbiol. 2022, 13, 761459. [Google Scholar] [CrossRef] [PubMed]
- Elochi, E.; Chinedu, O.S. Biodegradation of Chlorpyrifos Insecticide by Bacillus cereus ST06 and Chryseobacterium sp 6024 Isolated from Agricultural Soil, Nigeria. J. Adv. Microbiol. 2024, 24, 47–58. [Google Scholar] [CrossRef]
- Jabeur, R.; Guyon, V.; Toth, S.; Pereira, A.E.; Huynh, M.P.; Selmani, Z.; Boland, E.; Bosio, M.; Beuf, L.; Clark, P.; et al. A novel binary pesticidal protein from Chryseobacterium arthrosphaerae controls western corn rootworm by a different mode of action to existing commercial pesticidal proteins. PLoS ONE 2023, 18, e0267220. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhao, R.; Zhang, X.; Niu, T.; Cao, B.; Zhu, F.; Li, N.; Zhang, Y.; Wu, Y.; Wang, Y. Rhodopseudomonas capsulata enhances cleaning of chlorfenapyr from environment. J. Clean. Prod. 2020, 259, 120271. [Google Scholar] [CrossRef]
- Monard, C.; Vandenkoornhuyse, P.; Le Bot, B.; Binet, F. Relationship between bacterial diversity and function under biotic control: The soil pesticide degraders as a case study. ISME J. 2011, 5, 1048–1056. [Google Scholar] [CrossRef]
- Chu, C.; Yuan, C.; Liu, X.; Yao, L.; Zhu, J.; He, J.; Kwon, S.-W.; Huang, X. Phenylobacterium kunshanense sp. nov., isolated from the sludge of a pesticide manufacturing factory. Int. J. Syst. Evol. Microbiol. 2015, 65, 325–330. [Google Scholar] [CrossRef]
- Lu, P.; Jin, L.; Liang, B.; Zhang, J.; Li, S.; Feng, Z.; Huang, X. Study of Biochemical Pathway and Enzyme Involved in Metsulfuron-Methyl Degradation by Ancylobacter sp. XJ-412-1 Isolated from Soil. Curr. Microbiol. 2011, 62, 1718–1725. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Insecticide-tolerant and plant growth promoting Bradyrhizobium sp. (vigna) improves the growth and yield of greengram [Vigna radiata (L.) Wilczek] in insecticide-stressed soils. Symbiosis 2011, 54, 17–27. [Google Scholar] [CrossRef]
- Almeida, L.G.d.; Moraes, L.A.B.d.; Trigo, J.R.; Omoto, C.; Cônsoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, N.; Wang, L.; Guo, J.; Chen, K.; Dai, Y. Characterization of nitrilases from Variovorax boronicumulans that functions in insecticide flonicamid degradation and β-cyano-L-alanine detoxification. J. Appl. Microbiol. 2022, 133, 311–322. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhou, Q.-W.; Zhou, G.-C.; Cao, Y.-M.; Dai, Y.-J.; Ji, W.-W.; Shang, G.-D.; Yuan, S. Biotransformation of the Neonicotinoid Insecticide Thiacloprid by the Bacterium Variovorax boronicumulans Strain J1 and Mediation of the Major Metabolic Pathway by Nitrile Hydratase. J. Agric. Food Chem. 2012, 60, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Golby, S.; Ceri, H.; Gieg, L.M.; Chatterjee, I.; Marques, L.L.R.; Turner, R.J. Evaluation of microbial biofilm communities from an Alberta oil sands tailings pond. FEMS Microbiol. Ecol. 2012, 79, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.-F.; Liu, Z.-H.; Zhang, R.-M.; Zhou, D.; Sun, Y.; Ma, L.; Shen, B. Effect of the midgut symbiotic Aeromonas hydrophila on the deltamethrin resistance of Culex pipiens pallens. J. Pathog. Biol. 2021, 16, 661–666. [Google Scholar]
- Zhao, Y.; Wu, S.; Song, M.; Cao, Q.; Xia, Z.-Y.; Li, S.-P.; Huang, J.; Zhang, L.; Jiang, J.; Chen, K. Camelimonas fluminis sp. nov., a cyhalothrin-degrading bacterium isolated from river water. Int. J. Syst. Evol. Microbiol. 2015, 65, 3109–3114. [Google Scholar]
- Gao, Y.; Liu, M.; Zhao, X.; Zhang, X.; Zhou, F. Paracoccus and Achromobacter bacteria contribute to rapid biodegradation of imidacloprid in soils. Ecotoxicol. Environ. Saf. 2021, 225, 112785. [Google Scholar] [CrossRef] [PubMed]
- Anjum, R.; Grohmann, E.; Malik, A. Molecular characterization of conjugative plasmids in pesticide tolerant and multi-resistant bacterial isolates from contaminated alluvial soil. Chemosphere 2011, 84, 175–181. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Parapatla, H.; Nandavaram, A.; Palmer, T.; Siddavattam, D. Organophosphate Hydrolase Is a Lipoprotein and Interacts with Pi-specific Transport System to Facilitate Growth of Brevundimonas diminuta Using OP Insecticide as Source of Phosphate *. J. Biol. Chem. 2016, 291, 7774–7785. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Cheng, P.; Wang, Y.; Liu, H.; Wang, H.; Wang, H.; Gong, M. Differences in the intestinal microbiota between insecticide-resistant and -sensitive Aedes albopictus based on full-length 16S rRNA sequencing. Microbiol. Open 2021, 10, e1177. [Google Scholar] [CrossRef]
- Sutherland, T.D.; Horne, I.; Harcourt, R.L.; Russell, R.J.; Oakeshott, J.G. Isolation and characterization of a Mycobacterium strain that metabolizes the insecticide endosulfan. J. Appl. Microbiol. 2002, 93, 380–389. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, Y.; Zhang, W.; Yu, C.; Ji, W.; Xu, W.; Ni, J.; Yuan, S. Biotransformation of thianicotinyl neonicotinoid insecticides: Diverse molecular substituents response to metabolism by bacterium Stenotrophomonas maltophilia CGMCC 1.1788. Bioresour. Technol. 2010, 101, 3838–3843. [Google Scholar] [CrossRef]
- Bresolin, G.; Morgan, J.A.W.; Ilgen, D.; Scherer, S.; Fuchs, T.M. Low temperature-induced insecticidal activity of Yersinia enterocolitica. Mol. Microbiol. 2006, 59, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Ubalde, M.C.; Braña, V.; Sueiro, F.; Morel, M.A.; Martínez-Rosales, C.; Marquez, C.; Castro-Sowinski, S. The versatility of Delftia sp. isolates as tools for bioremediation and biofertilization technologies. Curr. Microbiol. 2012, 64, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Lawan, M.; Yakasai, H.M.; Babandi, A.; Ibrahim, S.; Shehu, D.; Ya’u, M.; Babagana, K.J.B.S.; Research, T. Characterization of Cypermethrin-degradation by a Novel Molybdenum-reducing Morganella sp. Isolated from Active Agricultural Land in Northwestern Nigeria. Bioremediation Sci. Technol. Res. 2021, 9, 25–30. [Google Scholar] [CrossRef]
- Leska, A.; Nowak, A.; Miśkiewicz, K.; Rosicka-Kaczmarek, J. Binding and Detoxification of Insecticides by Potentially Probiotic Lactic Acid Bacteria Isolated from Honeybee (Apis mellifera L.) Environment—An In Vitro Study. Cells 2022, 11, 3743. [Google Scholar] [CrossRef]
- Pelloquin, B.; Kristan, M.; Edi, C.; Meiwald, A.; Clark, E.; Jeffries, C.L.; Walker, T.; Dada, N.; Messenger, L.A.-O. Overabundance of Asaia and Serratia Bacteria Is Associated with Deltamethrin Insecticide Susceptibility in Anopheles coluzzii from Agboville, Côte d’Ivoire. Microbiol. Spectr. 2021, 9, e00157-21. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Misser, S.; Allam, M.; Chan, W.-Y.; Ismail, A.; Munhenga, G.; Oliver, S.V. The Effect of Larval Exposure to Heavy Metals on the Gut Microbiota Composition of Adult Anopheles arabiensis (Diptera: Culicidae). Trop. Med. Infect. Dis. 2024, 9, 249. https://doi.org/10.3390/tropicalmed9100249
Singh A, Misser S, Allam M, Chan W-Y, Ismail A, Munhenga G, Oliver SV. The Effect of Larval Exposure to Heavy Metals on the Gut Microbiota Composition of Adult Anopheles arabiensis (Diptera: Culicidae). Tropical Medicine and Infectious Disease. 2024; 9(10):249. https://doi.org/10.3390/tropicalmed9100249
Chicago/Turabian StyleSingh, Ashmika, Shristi Misser, Mushal Allam, Wai-Yin Chan, Arshad Ismail, Givemore Munhenga, and Shüné V. Oliver. 2024. "The Effect of Larval Exposure to Heavy Metals on the Gut Microbiota Composition of Adult Anopheles arabiensis (Diptera: Culicidae)" Tropical Medicine and Infectious Disease 9, no. 10: 249. https://doi.org/10.3390/tropicalmed9100249
APA StyleSingh, A., Misser, S., Allam, M., Chan, W.-Y., Ismail, A., Munhenga, G., & Oliver, S. V. (2024). The Effect of Larval Exposure to Heavy Metals on the Gut Microbiota Composition of Adult Anopheles arabiensis (Diptera: Culicidae). Tropical Medicine and Infectious Disease, 9(10), 249. https://doi.org/10.3390/tropicalmed9100249






