Microscopic and Molecular Identification of Cyclospora cayetanensis and Cystoisospora belli in HIV-Infected People in Tabriz, Northwest of Iran
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design and Population
2.3. Laboratory Procedures
2.4. Parasitology Detection Procedure
2.5. Modified Acid Fast Technique
2.6. DNA Extraction and PCR
2.7. Genotyping and Phylogenetic Analysis
2.8. Statistical Analysis
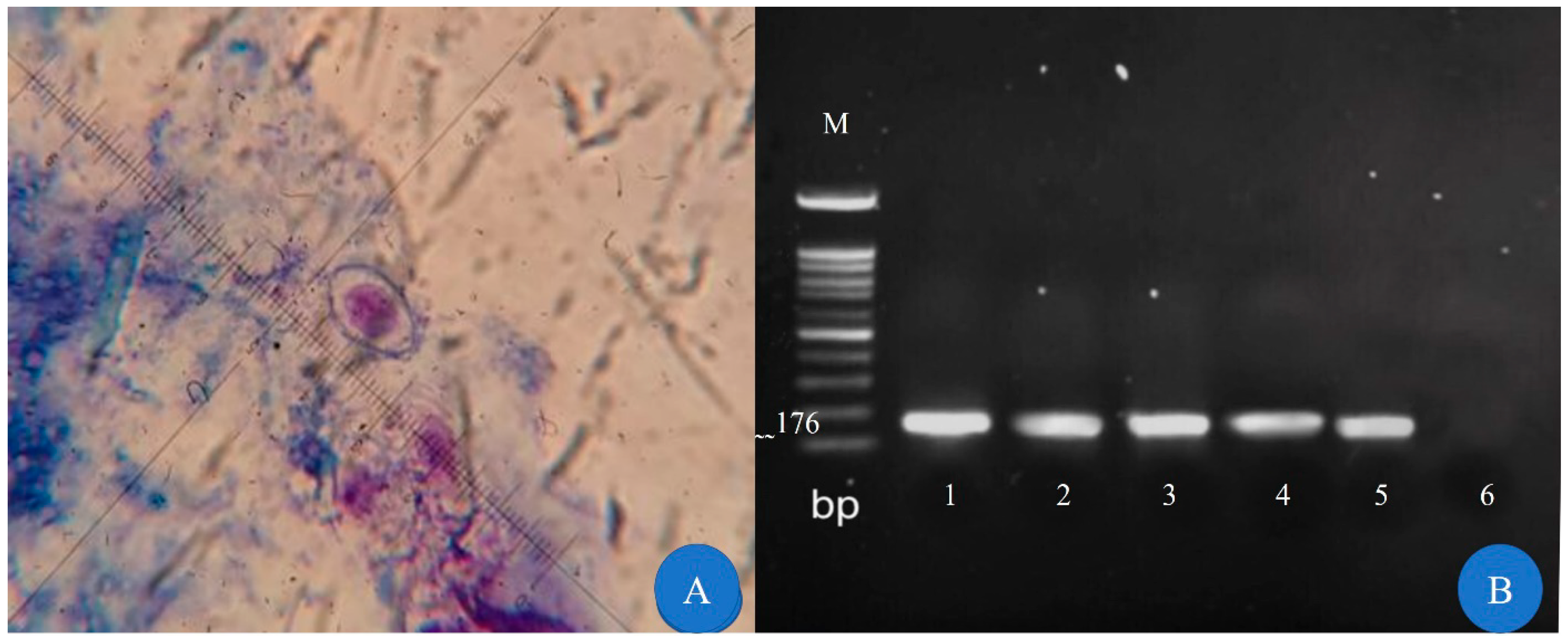
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wegayehu, T.; Tsalla, T.; Seifu, B.; Teklu, T. Prevalence of intestinal parasitic infections among highland and lowland dwellers in Gamo area, South Ethiopia. BMC Public Health 2013, 13, 151. [Google Scholar] [CrossRef] [PubMed]
- Daryani, A.; Hosseini-Teshnizi, S.; Hosseini, S.-A.; Ahmadpour, E.; Sarvi, S.; Amouei, A.; Mizani, A.; Gholami, S.; Sharif, M. Intestinal parasitic infections in Iranian preschool and school children: A systematic review and meta-analysis. Acta Trop. 2017, 169, 69–83. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Diseases; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Norhayati, M.; Fatmah, M.; Yusof, S.; Edariah, A. Intestinal parasitic infections in man: A review. Med. J. Malays. 2003, 58, 296–305. [Google Scholar]
- Wong, L.W.; Ong, K.S.; Khoo, J.R.; Goh, C.B.S.; Hor, J.W.; Lee, S.M. Human intestinal parasitic infection: A narrative review on global prevalence and epidemiological insights on preventive, therapeutic and diagnostic strategies for future perspectives. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1093–1105. [Google Scholar] [CrossRef]
- White, N. Malaria. In Manson’s Tropical Infectious Diseases; Elsevier: Philadelphia, PA, USA, 2014; pp. 532–600.e1. [Google Scholar]
- Barcelos, N.B.; Silva, L.d.F.; Dias, R.F.G.; Menezes Filho, H.R.d.; Rodrigues, R.M. Opportunistic and non-opportunistic intestinal parasites in HIV/AIDS patients in relation to their clinical and epidemiological status in a specialized medical service in Goiás, Brazil. Rev. Do Inst. De Med. Trop. De São Paulo 2018, 60, e13. [Google Scholar] [CrossRef]
- Frank, T.D.; Carter, A.; Jahagirdar, D.; Biehl, M.H.; Douwes-Schultz, D.; Larson, S.L.; Arora, M.; Dwyer-Lindgren, L.; Steuben, K.M.; Abbastabar, H. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019, 6, e831–e859. [Google Scholar] [CrossRef]
- Agholi, M.; Shahabadi, S.N.; Motazedian, M.H.; Hatam, G.R. Prevalence of enteric protozoan oocysts with special reference to Sarcocystis cruzi among fecal samples of diarrheic immunodeficient patients in Iran. Korean J. Parasitol. 2016, 54, 339. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Safarpour, H.; Xiao, L.; Zarean, M.; Hatam-Nahavandi, K.; Barac, A.; Picot, S.; Rahimi, M.T.; Rubino, S.; Mahami-Oskouei, M. Cryptosporidiosis in HIV-positive patients and related risk factors: A systematic review and meta-analysis. Parasite 2020, 27, 27. [Google Scholar] [CrossRef]
- Safarpour, H.; Cevik, M.; Zarean, M.; Barac, A.; Hatam-Nahavandi, K.; Rahimi, M.T.; Baghi, H.B.; Koshki, T.J.; Pagheh, A.S.; Shahrivar, F. Global status of Toxoplasma gondii infection and associated risk factors in people living with HIV. Aids 2020, 34, 469–474. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Ghanizadegan, M.A.; Razavi, A.; Kangari, M.; Seyfi, R.; Shahdust, M.; Yazdanian, A.; Safarpour, H.; Bannazadeh Baghi, H.; Zarean, M. Strongyloides stercoralis infection in human immunodeficiency virus-infected patients and related risk factors: A systematic review and meta-analysis. Transbound. Emerg. Dis. 2019, 66, 2233–2243. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Akalu, T.Y.; Aynalem, Y.A.; Shiferaw, W.S.; Merkeb Alamneh, Y.; Getnet, A.; Abebaw, A.; Atnaf, A.; Abate, A.; Tilahun, M.; Kassie, B. National burden of intestinal parasitic infections and its determinants among people living with HIV/AIDS on anti-retroviral therapy in Ethiopia: A systematic review and meta-analysis. SAGE Open Med. 2022, 10, 20503121221082447. [Google Scholar] [CrossRef]
- Assefa, S.; Erko, B.; Medhin, G.; Assefa, Z.; Shimelis, T. Intestinal parasitic infections in relation to HIV/AIDS status, diarrhea and CD4 T-cell count. BMC Infect. Dis. 2009, 9, 155. [Google Scholar] [CrossRef]
- Olopade, B.O.; Idowu, C.O. Intestinal parasites among HIV-infected patients at obafemi awolowo university teaching hospitals complex, Ile-Ife. Ann. Trop. Pathol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Paboriboune, P.; Phoumindr, N.; Borel, E.; Sourinphoumy, K.; Phaxayaseng, S.; Luangkhot, E.; Sengphilom, B.; Vansilalom, Y.; Odermatt, P.; Delaporte, E. Intestinal parasitic infections in HIV-infected patients, Lao People’s Democratic Republic. PLoS ONE 2014, 9, e91452. [Google Scholar] [CrossRef]
- Requena, I.; Añez, H.; Lacourt, E.; Blanco, Y.; Castillo, H.; Rivera, M.; Devera, R. Elevada prevalencia de coccidios intestinales en pacientes infectados con el Virus de la Inmunodeficiencia Humana en Ciudad Bolívar, Venezuela. Rev. Bioméd. 2007, 18, 73–75. [Google Scholar]
- Almeria, S.; Cinar, H.N.; Dubey, J.P. Cyclospora cayetanensis and cyclosporiasis: An update. Microorganisms 2019, 7, 317. [Google Scholar] [CrossRef]
- Hadjilouka, A.; Tsaltas, D. Cyclospora cayetanensis—Major outbreaks from ready to eat fresh fruits and vegetables. Foods 2020, 9, 1703. [Google Scholar] [CrossRef]
- Chacín-Bonilla, L. Epidemiology of Cyclospora cayetanensis: A review focusing in endemic areas. Acta Trop. 2010, 115, 181–193. [Google Scholar] [CrossRef]
- Masoumi-Asl, H.; Khanaliha, K.; Bokharaei-Salim, F.; Esteghamati, A.; Kalantari, S.; Hosseinyrad, M. Enteric opportunistic infection and the impact of antiretroviral therapy among hiv/aids patients from Tehran, Iran. Iran. J. Public Health 2019, 48, 730. [Google Scholar] [CrossRef]
- Ramezanzadeh, S.; Beloukas, A.; Pagheh, A.S.; Rahimi, M.T.; Hosseini, S.A.; Oliveira, S.M.R.; de Lourdes Pereira, M.; Ahmadpour, E. Global Burden of Cyclospora cayetanensis Infection and Associated Risk Factors in People Living with HIV and/or AIDS. Viruses 2022, 14, 1279. [Google Scholar] [CrossRef] [PubMed]
- Ortega, Y.R.; Sanchez, R. Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clin. Microbiol. Rev. 2010, 23, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Connor, B.A. Cyclosporiasis. In Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 719–721. [Google Scholar]
- Wang, Z.-D.; Liu, Q.; Liu, H.-H.; Li, S.; Zhang, L.; Zhao, Y.-K.; Zhu, X.-Q. Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: A global systematic review and meta-analysis. Parasites Vectors 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.; Almeria, S. Cystoisospora belli infections in humans: The past 100 years. Parasitology 2019, 146, 1490–1527. [Google Scholar] [CrossRef] [PubMed]
- Tatfeng, Y.; Usuanlele, M.; Orukpe, A.; Digban, A.; Okodua, M.; Oviasogie, F.; Turay, A. Mechanical transmission of pathogenic organisms: The role of cockroaches. J. Vector Borne Dis. 2005, 42, 129. [Google Scholar] [PubMed]
- Laksemi, D.; Suwanti, L.T.; Mufasirin, M.; Suastika, K.; Sudarmaja, M. Opportunistic parasitic infections in patients with human immunodeficiency virus/acquired immunodeficiency syndrome: A review. Vet. World 2020, 13, 716. [Google Scholar] [CrossRef]
- Gebrecherkos, T.; Kebede, H.; Gelagay, A.A. Intestinal parasites among HIV/AIDS patients attending University of Gondar Hospital, northwest Ethiopia. Ethiop. J. Health Dev. 2019, 33, 65–72. [Google Scholar]
- Agholi, M.; Hatam, G.R.; Motazedian, M.H. HIV/AIDS-associated opportunistic protozoal diarrhea. AIDS Res. Hum. Retrovir. 2013, 29, 35–41. [Google Scholar] [CrossRef]
- Taghipour, H.; Mosaferi, M. Characterization of medical waste from hospitals in Tabriz, Iran. Sci. Total Environ. 2009, 407, 1527–1535. [Google Scholar] [CrossRef]
- Garcia, L.S.; Procop, G.W. Diagnostic medical parasitology. In Manual of Commercial Methods in Clinical Microbiology: International Edition; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 284–308. [Google Scholar]
- Oguoma, V.; Ekwunife, C. The need for a better method: Comparison of direct smear and formol-ether concentration techniques in diagnosing intestinal parasites. Internet J. Trop. Med. 2007, 3, 1–6. [Google Scholar]
- Lalonde, L.F.; Gajadhar, A.A. Highly sensitive and specific PCR assay for reliable detection of Cyclospora cayetanensis oocysts. Appl. Environ. Microbiol. 2008, 74, 4354–4358. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Eisenberg, J.N.; Scott, J.C.; Porco, T. Integrating disease control strategies: Balancing water sanitation and hygiene interventions to reduce diarrheal disease burden. Am. J. Public Health 2007, 97, 846–852. [Google Scholar] [CrossRef]
- Brown, J.; Cairncross, S.; Ensink, J.H. Water, sanitation, hygiene and enteric infections in children. Arch. Dis. Child. 2013, 98, 629–634. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendations on the Management of Diarrhoea and Pneumonia in HIV-Infected Infants and Children: Integrated Management of Childhood Illness (IMCI); World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Utami, W.S.; Murhandarwati, E.H.; Artama, W.T.; Kusnanto, H. Cryptosporidium infection increases the risk for chronic diarrhea among people living with HIV in Southeast Asia: A systematic review and meta-analysis. Asia Pac. J. Public Health 2020, 32, 8–18. [Google Scholar] [CrossRef]
- Gendrel, D.; Treluyer, J.; Richard-Lenoble, D. Parasitic diarrhea in normal and malnourished children. Fundam. Clin. Pharmacol. 2003, 17, 189–197. [Google Scholar] [CrossRef]
- DuPont, H.L. Persistent diarrhea: A clinical review. JAMA 2016, 315, 2712–2723. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Sepahvand, A.; Khatami, M.; Moayyedkazemi, A. Prevalence and associated risk factors of Cystoisospora belli and Cyclospora cayetanensis infection among Iranian patients with colorectal cancer. J. Parasit. Dis. 2019, 43, 402–405. [Google Scholar] [CrossRef]
- Opoku, Y.K.; Boampong, J.N.; Ayi, I.; Kwakye-Nuako, G.; Obiri-Yeboah, D.; Koranteng, H.; Ghartey-Kwansah, G.; Asare, K.K. Socio-Behavioral Risk Factors Associated with Cryptosporidiosis in HIV/AIDS Patients Visiting the HIV Referral Clinic at Cape Coast Teaching Hospital, Ghana. Open AIDS J. 2018, 12, 106. [Google Scholar] [CrossRef]
- Katiyar, M.; Gulati, R.; Pagal, S.; Rajkumari, N.; Singh, R. Molecular detection of Cystoisospora belli by single-run polymerase chain reaction in stool samples. Indian J. Gastroenterol. 2021, 40, 512–518. [Google Scholar] [CrossRef]



| Variables | Cyclospora cayetanensis | p Value | Cystoisospora belli | p Value | |||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||
| Gender | Female | 1 | 38 | 0.2501 | 2 | 37 | 0.1682 |
| Male | 1 | 97 | 2 | 96 | |||
| Age | <20 20–40 40–60 >60 | 0 1 1 0 | 2 79 53 1 | 0.3893 | 0 3 1 0 | 2 77 53 1 | 0.2926 |
| Residence | Urban | 2 | 128 | 0.3716 | 4 | 126 | 0.3203 |
| Rural | 0 | 7 | 0 | 7 | |||
| Economic status | Poor (<USD 400/month) Average (USD400–2000/month) Rich (>USD 2000/mounth) | 0 2 0 | 64 66 5 | 0.1401 | 2 2 0 | 62 66 5 | 0.4023 |
| Education | Illiterate Under high school degree High school degree University degree | 0 1 0 1 | 66 78 14 17 | 0.0939 | 1 2 0 1 | 25 77 14 17 | 0.4326 |
| Job | Student Freelance Employee Housekeeper Unemployed | 0 2 0 0 0 | 8 54 2 28 43 | 0.0958 | 0 1 0 2 1 | 8 55 2 26 42 | 0.2644 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramezanzadeh, S.; Barzegar, G.; Osquee, H.O.; Pirestani, M.; Mahami-Oskouei, M.; Hajizadeh, M.; Hosseini, S.A.; Rodrigues Oliveira, S.M.; Agholi, M.; de Lourdes Pereira, M.; et al. Microscopic and Molecular Identification of Cyclospora cayetanensis and Cystoisospora belli in HIV-Infected People in Tabriz, Northwest of Iran. Trop. Med. Infect. Dis. 2023, 8, 368. https://doi.org/10.3390/tropicalmed8070368
Ramezanzadeh S, Barzegar G, Osquee HO, Pirestani M, Mahami-Oskouei M, Hajizadeh M, Hosseini SA, Rodrigues Oliveira SM, Agholi M, de Lourdes Pereira M, et al. Microscopic and Molecular Identification of Cyclospora cayetanensis and Cystoisospora belli in HIV-Infected People in Tabriz, Northwest of Iran. Tropical Medicine and Infectious Disease. 2023; 8(7):368. https://doi.org/10.3390/tropicalmed8070368
Chicago/Turabian StyleRamezanzadeh, Saba, Gholamreza Barzegar, Hamid Owaysee Osquee, Majid Pirestani, Mahmoud Mahami-Oskouei, Maryam Hajizadeh, Seyed Abdollah Hosseini, Sonia M. Rodrigues Oliveira, Mahmoud Agholi, Maria de Lourdes Pereira, and et al. 2023. "Microscopic and Molecular Identification of Cyclospora cayetanensis and Cystoisospora belli in HIV-Infected People in Tabriz, Northwest of Iran" Tropical Medicine and Infectious Disease 8, no. 7: 368. https://doi.org/10.3390/tropicalmed8070368
APA StyleRamezanzadeh, S., Barzegar, G., Osquee, H. O., Pirestani, M., Mahami-Oskouei, M., Hajizadeh, M., Hosseini, S. A., Rodrigues Oliveira, S. M., Agholi, M., de Lourdes Pereira, M., & Ahmadpour, E. (2023). Microscopic and Molecular Identification of Cyclospora cayetanensis and Cystoisospora belli in HIV-Infected People in Tabriz, Northwest of Iran. Tropical Medicine and Infectious Disease, 8(7), 368. https://doi.org/10.3390/tropicalmed8070368









