Silver(I) and Copper(II) 1,10-Phenanthroline-5,6-dione Complexes as Promising Antivirulence Strategy against Leishmania: Focus on Gp63 (Leishmanolysin)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compounds
2.2. In Silico Analyses
2.3. Parasite and Growth Conditions
2.4. Secretion Assay to Obtain the Gp63-Rich Supernatant
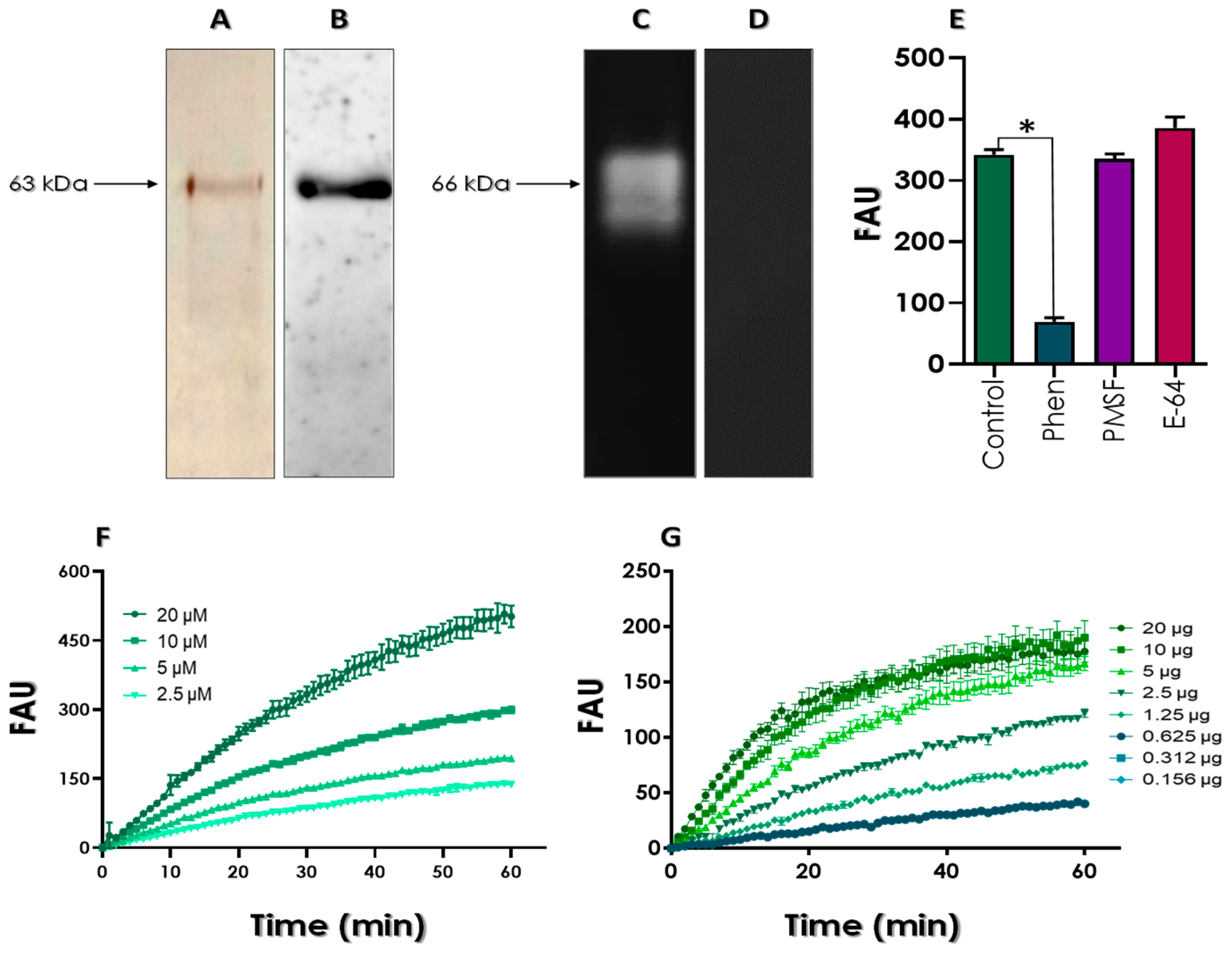
2.5. Secreted Protein Profile
2.6. Western Blotting for Detecting the Gp63 Protein
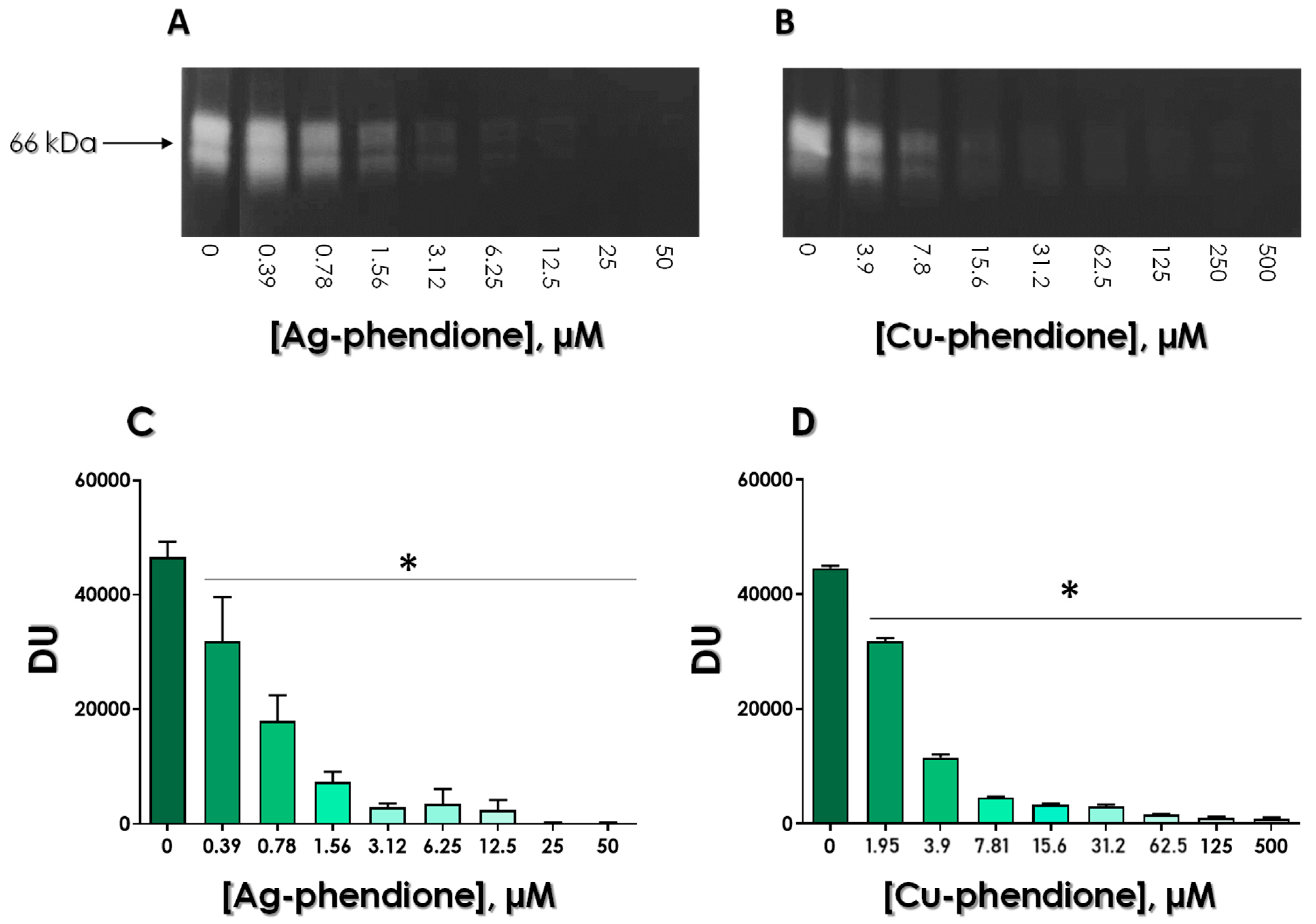
2.7. Zymography for Evidencing the Gp63 Proteolysis
2.8. Detection of Gp63 Activity Using Fluorogenic Substrate
2.9. Effects of Ag-Phendione and Cu-Phendione on Gp63 Proteolysis
2.10. Effects of Ag-Phendione and Cu-Phendione on Stability of Binding to Gp63
2.11. Effects of Ag-Phendione and Cu-Phendione on Parasite Cells: Looking for Gp63 Levels
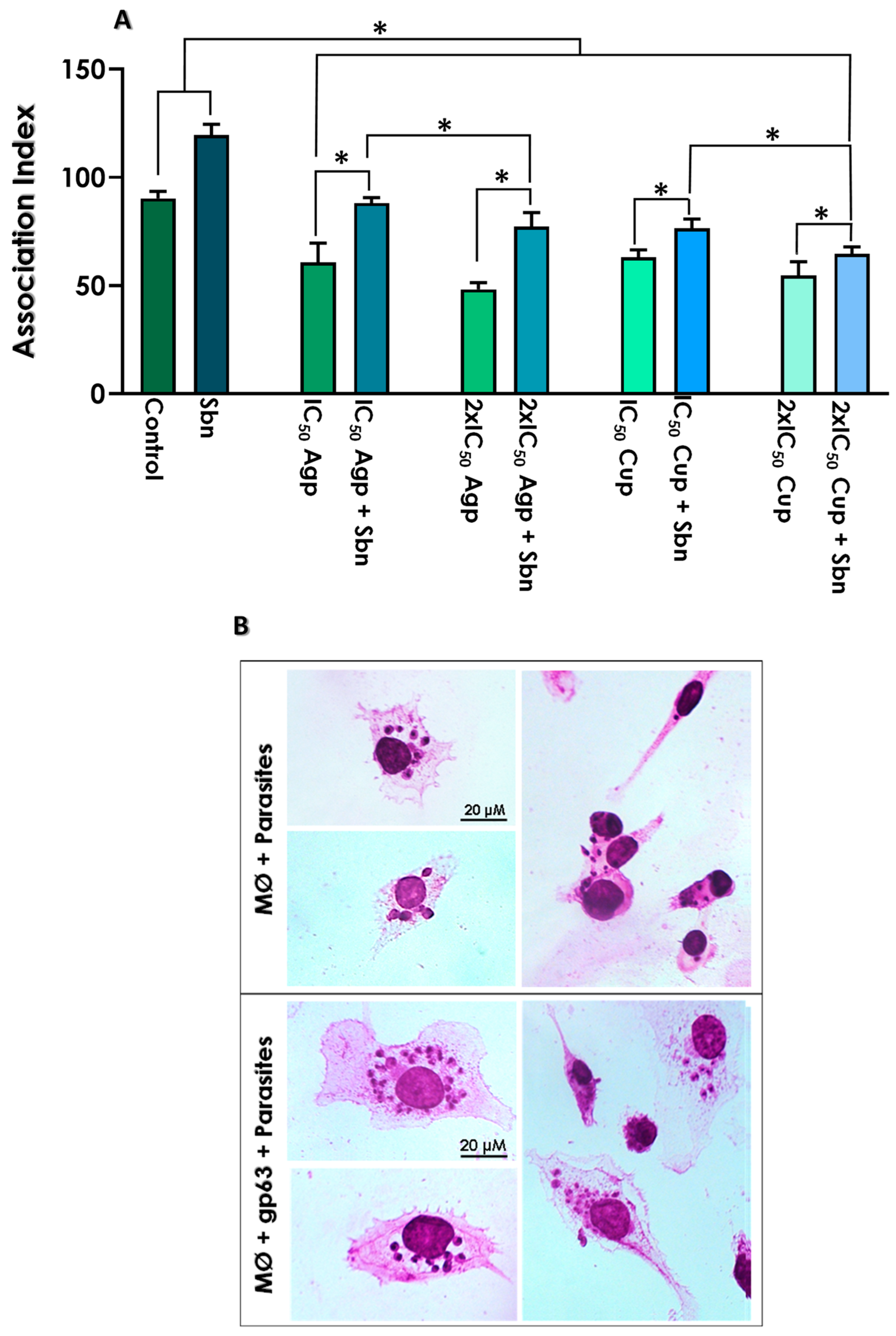
2.12. Effects of Ag-Phendione, Cu-Phendione and Soluble Gp63 on Promastigotes–Macrophage Interaction
2.13. Statistics
3. Results and Discussion
3.1. Ag-Phendione and Cu-Phendione Bind to the Gp63 Active Site: In Silico Approach
3.2. Secretion of Gp63 by L. amazonensis Promastigotes under Starvation Conditions
3.3. Effects of Test Compounds on the Enzymatic Activity of Gp63
3.4. Assessing the Stability of Gp63/Test Compounds
3.5. Effects of Test Compounds on Gp63 Expression by Living L. amazonensis Promastigotes
3.6. Effects of Test Compounds on the Interaction with THP-1 Macrophages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.D.; Coutinho-Abreu, I.V.; Dey, R.; Kissinger, R.; Valenzuela, J.G.; Oliveira, F.; Kamhawi, S. Leishmaniasis: The act of transmission. Trends Parasitol. 2021, 37, 976–987. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO) March 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 15 March 2023).
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.; Tomiotto-Pellissier, F.; Pasquali, A.K.S.; Pinto-Ferreira, F.; Pavanelli, W.R.; Conchon-Costa, I.; Navarro, I.T.; Caldart, E.T. Phenotypical and genotypical differences among Leishmania (Leishmania) amazonensis isolates that caused different clinical frames in humans and dogs: A systematic review. Acta Trop. 2021, 221, 106018. [Google Scholar] [CrossRef] [PubMed]
- Porto, V.B.G.; Carvalho, L.B.; Buzo, B.F.; Litvoc, M.N.; Santos, A.C.S.; Rocci, R.A.; Soares, S.R.C.; Zampieri, R.A.; Duarte, M.I.S.; Lindoso, J.A.L. Visceral leishmaniasis caused by Leishmania (Leishmania) amazonensis associated with Hodgkin’s lymphoma. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e51. [Google Scholar] [CrossRef]
- Roatt, B.M.; de Oliveira Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Kammona, O.; Tsanaktsidou, E. Nanotechnology-aided diagnosis, treatment and prevention of leishmaniasis. Int. J. Pharm. 2021, 10, 120761. [Google Scholar] [CrossRef]
- Singh, R.; Kashif, M.; Srivastava, P.; Manna, P.P. Recent advances in chemotherapeutics for leishmaniasis: Importance of the cellular biochemistry of the parasite and its molecular interaction with the host. Pathogens 2023, 12, 706. [Google Scholar] [CrossRef]
- Khalid, S.; Salman, S.; Iqbal, K.; Rehman, F.U.; Ullah, I.; Satoskar, A.R.; Khan, G.M.; Dar, M.J. Surfactant free synthesis of cationic nano-vesicles: A safe triple drug loaded vehicle for the topical treatment of cutaneous leishmaniasis. Nanomedicine 2022, 40, 102490. [Google Scholar] [CrossRef]
- Iqbal, K.; Khalid, S.; McElroy, C.A.; Adnan, M.; Khan, G.M.; Dar, M.J. Triple-combination therapy for cutaneous leishmaniasis using detergent-free, hyaluronate-coated elastic nanovesicles. Nanomedicine 2022, 17, 1429–1447. [Google Scholar] [CrossRef]
- Dar, M.J.; McElroy, C.A.; Khan, M.I.; Satoskar, A.R.; Khan, G.M. Development and evaluation of novel miltefosine-polyphenol co-loaded second-generation nano-transfersomes for the topical treatment of cutaneous leishmaniasis. Expert. Opin. Drug. Deliv. 2020, 17, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug. Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Kahler, C.M.; Sarkar-Tyson, M.; Kibble, E.A.; Stubbs, K.A.; Vrielink, A. Enzyme targets for drug design of new anti-virulence therapeutics. Curr. Opin. Struct. Biol. 2018, 53, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Virulence factors and their mechanisms of action: The view from a damage-response framework. J. Water Health 2009, 1, S2–S18. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Donelson, J.E.; Wilson, M.E. The major surface protease (MSP or GP63) of Leishmania sp. Biosynthesis, regulation of expression, and function. Mol. Biochem. Parasitol. 2003, 132, 1–16. [Google Scholar] [CrossRef]
- Isnard, A.; Shio, M.T.; Olivier, M. Impact of Leishmania metalloprotease GP63 on macrophage signaling. Front. Cell Infect. Microbiol. 2012, 2, 72. [Google Scholar] [CrossRef]
- Guay-Vincent, M.M.; Matte, C.; Berthiaume, A.M.; Olivier, M.; Jaramillo, M.; Descoteaux, A. Revisiting Leishmania GP63 host cell targets reveal a limited spectrum of substrates. PLoS Pathog. 2022, 18, e1010640. [Google Scholar] [CrossRef]
- Atayde, V.D.; Hassani, K.; da Silva Lira Filho, A.; Borges, A.R.; Adhikari, A.; Martel, C.; Olivier, M. Leishmania exosomes and other virulence factors: Impact on innate immune response and macrophage functions. Cell Immunol. 2016, 309, 7–18. [Google Scholar] [CrossRef]
- de Castro Neto, A.L.; da Silveira, J.F.; Mortara, R.A. Comparative analysis of virulence mechanisms of trypanosomatids pathogenic to humans. Front. Cell Infect. Microbiol. 2021, 11, 669079. [Google Scholar] [CrossRef]
- Gupta, A.K.; Das, S.; Kamran, M.; Ejazi, S.A.; Ali, N. The pathogenicity and virulence of Leishmania—Interplay of virulence factors with host defenses. Virulence 2022, 13, 903–935. [Google Scholar] [CrossRef]
- Olivier, M.; Atayde, V.D.; Isnard, A.; Hassani, K.; Shio, M.T. Leishmania virulence factors: Focus on the metalloprotease GP63. Microbes Infect. 2012, 14, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.K.; Elias, C.G.; Souza, J.E.; Santos, A.L.S.; Dutra, P.M. Dissimilar peptidase production by avirulent and virulent promastigotes of Leishmania braziliensis: Inference on the parasite proliferation and interaction with macrophages. Parasitology 2009, 136, 1179–1191. [Google Scholar] [CrossRef]
- Santos, A.L.S.; Lima, A.K.; Oliveira, S.S.C.; dos Santos, R.F.; Devereux, M.; McCann, M.; Branquinha, M.H.; Dutra, P.M.L. Decoding the anti-Leishmania braziliensis activity of 1,10-phenanthroline-5,6-dione and its silver- and copper-based complexes: In vitro and in vivo approaches. Eur. J. Med. Chem. 2022, 6, 100093. [Google Scholar] [CrossRef]
- Oliveira, S.S.C.; Santos, V.S.; Devereux, M.; McCann, M.; Santos, A.L.S.; Branquinha, M.H. The Anti-Leishmania amazonensis and anti-Leishmania chagasi action of copper(II) and silver(I) 1,10-phenanthroline-5,6-dione coordination compounds. Pathogens 2023, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.P.; Altoé, E.C.F.; Ennes-Vidal, V.; da Costa, S.M.; Rangel, E.F.; de Souza, N.A.; da Silva, V.C.; Volf, P.; d’Avila-Levy, C.M. In vitro inhibition of Leishmania attachment to sandfly midguts and LL-5 cells by divalent metal chelators, anti-gp63 and phosphoglycans. Protist 2017, 168, 326–334. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Kellett, A.; Kavanagh, K.; Devereux, M.; Santos, A.L.S. Deciphering the antimicrobial activity of phenanthroline chelators. Curr. Med. Chem. 2012, 19, 2703–2714. [Google Scholar] [CrossRef]
- McCann, M.; Santos, A.L.S.; Silva, B.A.; Romanos, M.T.V.; Pyrrho, A.S.; Devereux, M.; Kavanagh, K.; Fichtner, I.; Kellett, A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(II) and silver(I) complexes. Toxicology Res. 2012, 1, 47–54. [Google Scholar] [CrossRef]
- McCann, M.; Coyle, B.; McKay, S.; McCormack, P.; Kavanagh, K.; Devereux, M.; McKee, V.; Kinsella, P.; O’Connor, R.; Clynes, M. Synthesis and X-ray crystal structure of [Ag(phendio)2]ClO4 (phendio = 1,10-phenanthroline-5,6-dione) and its effects on fungal and mammalian cells. Biometals 2004, 17, 635–645. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Schlagenhauf, E.; Etges, R.; Metcalf, P. The crystal structure of the Leishmania major surface proteinase leishmanolysin (gp63). Structure. 1998, 6, 1035–1046. [Google Scholar] [CrossRef]
- Niwa, T.; Tanaka, S.; Mizutani, M. On the law of entropy increasing of a one-dimensional infinite system II. J. Math. Kyoto Univ. 1994, 34, 699–708. [Google Scholar] [CrossRef]
- Gontijo, T.B.; Lima, P.S.; Icimoto, M.Y.; Neves, R.L.; de Alvarenga, É.C.; Carmona, A.K.; de Castro, A.A.; Ramalho, T.C.; da Silva Júnior, E.N.; de Freitas, R.P. Cathepsin K inhibitors based on 2-amino-1,3,4-oxadiazole derivatives. Bioorg. Chem. 2021, 109, 104662. [Google Scholar] [CrossRef] [PubMed]
- Galdino, A.C.M.; Viganor, L.; de Castro, A.A.; da Cunha, E.F.F.; Mello, T.P.; Mattos, L.M.; Pereira, M.D.; Hunt, M.C.; O’Shaughnessy, M.; Howe, O.; et al. Disarming Pseudomonas aeruginosa virulence by the inhibitory action of 1,10-phenanthroline-5,6-dione-based compounds: Elastase B (Lasb) as a chemotherapeutic target. Front. Microbiol. 2019, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Heussen, C.; Dowdle, E.B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 1980, 102, 196–202. [Google Scholar] [CrossRef]
- Oliveira, S.S.C.; Elias, C.G.R.; Dias, F.A.; Lopes, A.H.; d’Avila-Levy, C.M.; Santos, A.L.S.; Branquinha, M.H. The enhanced expression of cruzipain-like molecules in the phytoflagellate Phytomonas serpens recovered from the invertebrate and plant hosts. Front. Cell Infect. Microbiol. 2022, 11, 819133. [Google Scholar] [CrossRef]
- Gaussem, P.; Grailhe, P.; Anglés-Cano, E. Sodium dodecyl sulfate-induced dissociation of complexes between human tissue plasminogen activator and its specific inhibitor. J. Biol. Chem. 1993, 268, 12150–12155. [Google Scholar] [CrossRef]
- Michaud, D.; Cantin, L.; Raworth, D.A.; Vrain, T.C. Assessing the stability of cystatin/cysteine proteinase complexes using mildly-denaturing gelatin-polyacrylamide gel electrophoresis. Electrophoresis 1996, 17, 74–79. [Google Scholar] [CrossRef]
- Santos, A.L.S.; Branquinha, M.H.; d’Avila-Levy, C.M. The ubiquitous gp63-like metalloprotease from lower trypanosomatids: In the search for a function. An. Acad. Bras. Cienc. 2006, 78, 687–714. [Google Scholar] [CrossRef]
- Kühne, V.; Rezaei, Z.; Pitzinger, P.; Büscher, P. Systematic review on antigens for serodiagnosis of visceral leishmaniasis, with a focus on East Africa. PLoS Negl. Trop. Dis. 2019, 15, e0007658. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Liao, X.; Xiao, Y.; Liang, C.; Zhou, Q.; Chen, H.; Zheng, Z.; Qin, H.; Chen, D.; et al. Development of dominant epitope-based vaccines encoding Gp63, Kmp-11 and Amastin against visceral leishmaniasis. Immunobiology 2021, 226, 152085. [Google Scholar] [CrossRef]
- Lima, A.K.C.; Elias, C.G.R.; Oliveira, S.S.C.; Santos-Mallet, J.R.; McCann, M.; Devereux, M.; Branquinha, M.H.; Dutra, P.M.L.; Santos, A.L.S. Anti-Leishmania braziliensis activity of 1,10-phenanthroline-5,6-dione and its Cu(II) and Ag(I) complexes. Parasitol. Res. 2021, 120, 3273–3285. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.S.; Sodre, C.L.; Valle, R.S.; Silva, B.A.; Abi-Chacra, E.A.; Silva, L.V.; Souza-Goncalves, A.L.; Sangenito, L.S.; Goncalves, D.S.; Souza, L.O.; et al. Antimicrobial action of chelating agents: Repercussions on the microorganism development, virulence and pathogenesis. Curr. Med. Chem. 2012, 19, 2715–2737. [Google Scholar] [CrossRef] [PubMed]
- Deegan, C.; Coyle, B.; McCann, M.; Devereux, M.; Egan, D.A. In vitro anti-tumour effect of 1,10-phenanthroline-5,6-dione (phendione), [Cu(phendione)3](ClO4)2.4H2O and [Ag(phendione)2]ClO4 using human epithelial cell lines. Chem. Biol. Interact. 2006, 164, 115–125. [Google Scholar] [CrossRef]
- Hou, H.; He, H.; Wang, Y. Effects of SDS on the activity and conformation of protein tyrosine phosphatase from Thermus thermophilus HB27. Sci. Rep. 2020, 21, 3195. [Google Scholar] [CrossRef]
- Vargas Rigo, G.; Gomes Cardoso, F.; Bongiorni Galego, G.; da Rosa, D.F.; Santos, A.L.S.; Tasca, T. Metallopeptidases as key virulence attributes of clinically relevant protozoa: New discoveries, perspectives, and frontiers of knowledge. Curr. Protein Pept. Sci. 2023, 24, 307–328. [Google Scholar] [CrossRef]
- Brittingham, A.; Chen, G.; McGwire, B.S.; Chang, K.P.; Mosser, D.M. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect. Immun. 1999, 67, 4477–4484. [Google Scholar] [CrossRef] [PubMed]
- Murase, L.S.; de Souza, J.V.P.; de Lima Neto, Q.A.; de Mello, T.F.P.; Cardoso, B.M.; Lera-Nonose, D.S.S.L.; Teixeira, J.J.V.; Lonardoni, M.V.C.; Demarchi, I.G. The role of metalloproteases in Leishmania species infection in the New World: A systematic review. Parasitology 2018, 145, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Forget, G.; Gregory, D.J.; Whitcombe, L.A.; Olivier, M. Role of host protein tyrosine phosphatase SHP-1 in Leishmania donovani-induced inhibition of nitric oxide production. Infect. Immun. 2006, 74, 6272–6279. [Google Scholar] [CrossRef]
- Jaramillo, M.; Gomez, M.A.; Larsson, O.; Shio, M.T.; Topisirovic, I.; Contreras, I.; Luxenburg, R.; Rosenfeld, A.; Colina, R.; McMaster, R.W.; et al. Leishmania repression of host translation through mTOR cleavage is required for parasite survival and infection. Cell Host Microbe. 2011, 9, 331–341. [Google Scholar] [CrossRef] [PubMed]





| Test Compounds | Total Interaction Energy (kcal/mol) | Hydrogen Bonds |
|---|---|---|
| Phen | −45.83 | Ser465 Thr467 2 H2O |
| Phendione | −39.75 | 4 H2O |
| Ag-phendione | −74.82 | Ser465 Thr467 6 H2O |
| Cu-phendione | −68.16 | Ser252 Ser465 5 H2O |
| Test Compounds | IC50 (µM) | Ki (µM) |
|---|---|---|
| Phendione | 1494 | 744.8 |
| Ag-Phendione | 2.16 | 5.13 |
| Cu-Phendione | 163 | 27.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, S.S.C.; Correia, C.A.; Santos, V.S.; da Cunha, E.F.F.; de Castro, A.A.; Ramalho, T.C.; Devereux, M.; McCann, M.; Branquinha, M.H.; Santos, A.L.S. Silver(I) and Copper(II) 1,10-Phenanthroline-5,6-dione Complexes as Promising Antivirulence Strategy against Leishmania: Focus on Gp63 (Leishmanolysin). Trop. Med. Infect. Dis. 2023, 8, 348. https://doi.org/10.3390/tropicalmed8070348
Oliveira SSC, Correia CA, Santos VS, da Cunha EFF, de Castro AA, Ramalho TC, Devereux M, McCann M, Branquinha MH, Santos ALS. Silver(I) and Copper(II) 1,10-Phenanthroline-5,6-dione Complexes as Promising Antivirulence Strategy against Leishmania: Focus on Gp63 (Leishmanolysin). Tropical Medicine and Infectious Disease. 2023; 8(7):348. https://doi.org/10.3390/tropicalmed8070348
Chicago/Turabian StyleOliveira, Simone S. C., Claudyane A. Correia, Vanessa S. Santos, Elaine F. F. da Cunha, Alexandre A. de Castro, Teodorico C. Ramalho, Michael Devereux, Malachy McCann, Marta H. Branquinha, and André L. S. Santos. 2023. "Silver(I) and Copper(II) 1,10-Phenanthroline-5,6-dione Complexes as Promising Antivirulence Strategy against Leishmania: Focus on Gp63 (Leishmanolysin)" Tropical Medicine and Infectious Disease 8, no. 7: 348. https://doi.org/10.3390/tropicalmed8070348
APA StyleOliveira, S. S. C., Correia, C. A., Santos, V. S., da Cunha, E. F. F., de Castro, A. A., Ramalho, T. C., Devereux, M., McCann, M., Branquinha, M. H., & Santos, A. L. S. (2023). Silver(I) and Copper(II) 1,10-Phenanthroline-5,6-dione Complexes as Promising Antivirulence Strategy against Leishmania: Focus on Gp63 (Leishmanolysin). Tropical Medicine and Infectious Disease, 8(7), 348. https://doi.org/10.3390/tropicalmed8070348










