Detection of Crimean–Congo Haemorrhagic Fever Virus from Livestock Ticks in Northern, Central and Southern Senegal in 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection Sites
2.2. Tick Sampling
2.3. Identification and Processing of Tick Samples
2.4. Detection of CCHFV by RT-PCR
2.5. Data Analysis
3. Results
3.1. Tick Infestation Prevalence in Vertebrate Hosts
3.2. Specific Richness and Diversity
3.3. Relative Abundance of Tick Species by Host and Locality
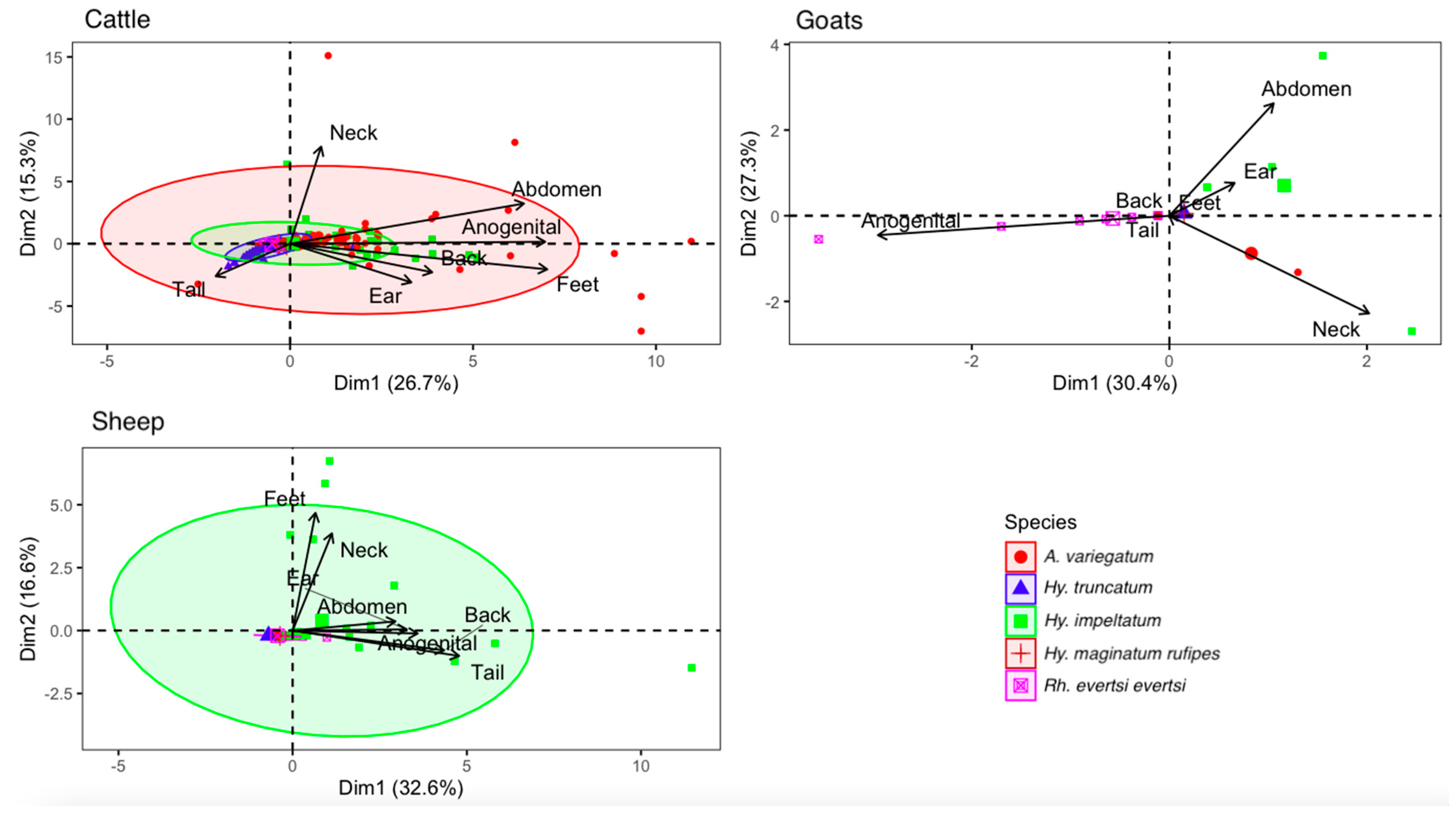
3.4. Tick Attachment Sites on Hosts
3.5. Detection of CCHFV in Ticks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duvallet, G.; Fontenille, D.; Robert, V. Entomologie Médicale et Vétérinaire; IRD Éditions: Marseille, France, 2018; Available online: http://books.openedition.org/irdeditions/21923 (accessed on 20 December 2022).
- Sylla, M.; Molez, J.-F.; Cornet, J.-P.; Camicas, J.-L.; Pourrut, X. Variabilité climatique et répartition de la fièvre hémorragique de Crimée-Congo et de la cowdriose, maladies à tiques au Sénégal. Acarologia 2008, 48, 155–161. [Google Scholar]
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- Fakoorziba, M.R.; Golmohammadi, P.; Moradzadeh, R.; Moemenbellah-Fard, M.D.; Azizi, K.; Davari, B.; Alipour, H.; Ahmadnia, S.; Chinikar, S. Reverse Transcription PCR-Based Detection of Crimean-Congo Hemorrhagic Fever Virus Isolated from Ticks of Domestic Ruminants in Kurdistan Province of Iran. Vector-Borne Zoonotic Dis. 2012, 12, 794–799. [Google Scholar] [CrossRef]
- Whitehouse, C.A. Crimean-Congo hemorrhagic fever. Antivir. Res. 2004, 64, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Ergönül, O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006, 6, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.; Velo, E.; Papadimitriou, E.; Cahani, G.; Kota, M.; Bino, S. Ecology of the Crimean-Congo hemorrhagic fever endemic area in Albania. Vector Borne Zoonotic Dis. 2009, 9, 713–716. [Google Scholar] [CrossRef]
- Nabeth, P.; Cheikh, D.O.; Lo, B.; Faye, O.; Vall, I.O.M.; Niang, M.; Wague, B.; Diop, D.; Diallo, M.; Diallo, B. Crimean-Congohemorrhagic fever, Mauritania. Emerg. Infect. Dis. 2004, 10, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 2013, 100, 159–189. [Google Scholar] [CrossRef]
- Chunikhin, S.; Chumakov, M.; Butenko, A.; Smirnova, S.E.; Taufflieb, R.; Camicas, J.L.; Robin, Y.; Cornet, M.; Shabon, Z. Results from investigating human and domestic and wild animal blood sera in the Senegal Republic (Western Africa) for antibodies to crimean hemorrhagic fever virus. Nauchn Sess Polio Virus Entsefalitov Mosc. 1969, 2, 158–160. [Google Scholar]
- Temur, A.I.; Kuhn, J.H.; Pecor, D.B.; Apanaskevich, D.A.; Keshtkar-Jahromi, M. Epidemiology of Crimean-Congo Hemorrhagic Fever (CCHF) in Africa—Underestimated for Decades. Am. J. Trop. Med. Hyg. 2021, 104, 1978–1990. [Google Scholar] [CrossRef]
- Wilson, M.L.; Gonzalez, J.-P.; LeGuenno, B.; Cornet, J.-P.; Guillaud, M.; Calvo, M.-A.; Digoutte, J.-P.; Camicas, J.-L. Epidemiology of Crimean-Congo hemorrhagic fever in Senegal: Temporal and spatial patterns. In Hemorrhagic Fever with Renal Syndrome Tick- and Mosquito-Borne Viruses; Calisher, C.H., Ed.; Springer: Vienna, Austria, 1990; pp. 323–340. Available online: http://link.springer.com/10.1007/978-3-7091-9091-3_35 (accessed on 10 November 2022).
- Dieng, I.; Barry, M.A.; Diagne, M.M.; Diop, B.; Ndiaye, M.; Faye, M.; Ndione, M.H.D.; Dieng, M.M.; Bousso, A.; Fall, G.; et al. Detection of Crimean Congo haemorrhagic fever virus in North-eastern Senegal, Bokidiawé 2019. Emerg. Microbes Infect. 2020, 9, 2485–2487. [Google Scholar] [CrossRef] [PubMed]
- Mhamadi, M.; Badji, A.; Dieng, I.; Gaye, A.; Ndiaye, E.H.; Ndiaye, M.; Mhamadi, M.; Toure, C.T.; Barry, A.; Ndiaye, O.; et al. Multiple genotypes of Crimean-Congo hemorrhagic fever virus detected in ticks during a one health survey in Agnam, Northeastern Senegal. Emerg. Microbes Infect. 2022, 11, 2711–2714. [Google Scholar] [CrossRef]
- ANSD/RGPHAE-Sénégal. 2019. Agence National de la Statistique et de la Démographie. Available online: http://www.ansd.sn/index.php (accessed on 10 November 2022).
- Hoogstraal, H. African Ixodoidea. Ticks of le Soudan (avec Référence Spéciale à la Province d’Équatoria et avec Examens Préliminaires pf les Genres Boophilus, Margaropus, et Hyalomma); Report No, 005 050.29.07; Departement of Medical Zoology: Caire, Egypt, 1956; p. 1101. Available online: https://www.biodiversitylibrary.org/part/97784 (accessed on 18 October 2022).
- Walker, A.R.; Bouattour, A.; Camicas, J.-L.; Estrada-Pena, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, Scotland, 2003. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Nolan, K.; Callahan, J. Beachcomber Biology: The Shannon-Weiner Species Diversity Index. Proc. Workshop ABLE 2006, 27, 334–338. [Google Scholar]
- Biggerstaff, B. PooledInRate, Version 4.0: An Excel® Add-In to Compute Infection Rates from Pooled Data. Centers for Disease Control and Prevention Fort Collins: Colorado. 2016. Available online: https://github.com/CDCgov/PooledInfRate (accessed on 1 April 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Volume 145, Available online: https://www.r-project.org/ (accessed on 1 April 2023).
- Gueye, A.; Mbengue, M.; Diouf, A. Tiques et hémoparasitoses du bétail au Sénégal. VI. La zone soudano-sahélienne. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1994, 47, 39–46. [Google Scholar] [CrossRef]
- Ramzan, M.; Naeem-Ullah, U.; Abbas, H.; Adnan, M.; Rasheed, Z.; Khan, S. Diversity of hard ticks in goats and sheep in Multan, Punjab, Pakistan. Int. J. Agric. Biol. Res. 2019, 35, 7–9. [Google Scholar]
- Khan, S.S.; Ahmed, H.; Afzal, M.S.; Khan, M.R.; Birtles, R.J.; Oliver, J.D. Epidemiology, Distribution and Identification of Ticks on Livestock in Pakistan. Int. J. Environ. Res. Public Health 2022, 19, 3024. [Google Scholar] [CrossRef]
- Gueye, A.; Mbengue, M.; Diouf, A. Tiques et hémoparasitoses du bétail au Sénégal. IV. La zone Sud-soudanienne. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1989, 42, 517–528. [Google Scholar]
- Morel, P.-C. Contribution à la connaissance de la distribution des tiques (acariens, Ixodidae et Amblyomnisdae), en Afrique ethiopienne continentale. Ph.D Thesis, Université de Paris-Sud, Paris, France, 1969. Available online: https://agritrop.cirad.fr/355829/ (accessed on 10 November 2022).
- Gueye, A.; Mbengue, M.; Diouf, A.; Sonko, M.L. Tiques et hémoparasitoses du bétail au Sénégal. V. La zone Nord-guinéenne. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1993, 46, 551–561. [Google Scholar] [CrossRef]
- Gueye, A.; Camicas, J.-L.; Diouf, A.; Mbengue, M. Tiques et hémoparasitoses du bétail au Sénégal. II. La zone sahélienne. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1987, 40, 119–125. [Google Scholar]
- Boulanger, N.; McCoy, K.D. Tiques et Maladies à Tiques: Biologie, Ecologie Evolutive, Épidémiologie; IRD Éditions: Marseille, France, 2016; Volume 1, 669p. [Google Scholar]
- Stachurski, F. Invasion of West African cattle by the tick Amblyomma variegatum. Med. Vet. Èntomol. 2000, 14, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Muchenje, V.; Dzama, K.; Chimonyo, M.; Raats, J.G.; Strydom, P. Tick susceptibility and its effects on growth performance and carcass characteristics of Nguni, Bonsmara and Angus steers raised on natural pasture. Animal 2008, 2, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Camicas, J.L.; Cornet, J.-P.; Wilson, M.L.; Adam, F.; Zeller, H.G. La fièvre hémorragique de Crimée-Congo au Sénégal: Dernières données sur l’écologie du virus CCHF- fdi:41933-Horizon. Bull. Société Pathol. Exot. 1994, 87, 11–16. [Google Scholar]
- Faye, O.; Cornet, J.P.; Camicas, J.L.; Fontenille, D.; Gonzalez, J.P. Experimental transmission of Crimean-Congo hemorrhagic fever virus: Role of 3 vector species in the maintenance and transmission cycles in Senegal. Parasite 1999, 6, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Fontenille, D.; Thonnon, J.; Gonzalez, J.P.; Cornet, J.P.; Camicas, J.L. Experimental transmission of Crimean-Congo hemorrhagic fever virus by Rhipicephalus evertsi evertsi (Acarina: Ixodidae). Bull. Soc. Pathol. Exot. 1999, 92, 143–147. [Google Scholar] [PubMed]
- Dohm, D.J.; Logan, T.M.; Linthicum, K.J.; Rossi, C.A.; Turell, M.J. Transmission of Crimean-Congo Hemorrhagic Fever Virus by Hyalomma impeltatum (Acari: Ixodidae) after Experimental Infection. J. Med. Entomol. 1996, 33, 848–851. [Google Scholar] [CrossRef]
- Mhamadi, M.; Badji, A.; Dieng, I.; Gaye, A.; Ndiaye, E.H.; Ndiaye, M.; Mhamadi, M.; Touré, C.T.; Mbaye, M.R.; Barry, M.A.; et al. Crimean–Congo Hemorrhagic Fever Virus Survey in Humans, Ticks, and Livestock in Agnam (Northeastern Senegal) from February 2021 to March 2022. Trop. Med. Infect. Dis. 2022, 7, 324. [Google Scholar] [CrossRef]
- Boushab, B.M.; Kelly, M.; Kébé, H.; Bollahi, M.A.; Basco, L.K. Crimean-Congo Hemorrhagic Fever, Mauritania. Emerg. Infect. Dis. 2020, 26, 817–818. [Google Scholar] [CrossRef]
- Kasi, K.K.; von Arnim, F.; Schulz, A.; Rehman, A.; Chudhary, A.; Oneeb, M.; Sas, M.A.; Jamil, T.; Maksimov, P.; Sauter-Louis, C.; et al. Crimean-Congo haemorrhagic fever virus in ticks collected from livestock in Balochistan, Pakistan. Transbound. Emerg. Dis. 2020, 67, 1543–1552. [Google Scholar] [CrossRef]
- Shahid, M.F.; Yaqub, T.; Ali, M.; Ul-Rahman, A.; Bente, A.D. Prevalence and phylogenetic analysis of Crimean-Congo hemorrhagic fever virus in ticks collected from Punjab province of Pakistan. Acta Trop. 2021, 218, 105892. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Camicas, J.L.; Cornet, J.P.; Faye, O.; Wilson, M.L. Sexual and transovarian transmission of Crimean-Congo haemorrhagic fever virus in Hyalomma truncatum ticks. Res. Virol. 1992, 143, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Logan, T.M.; Watts, D.M.; Linthicum, K.J.; Moulton, J.R.; Bailey, C.L. Experimental Transmission of Crimean-Congo Hemorrhagic Fever Virus by Hyalomma Truncatum Koch. Am. J. Trop. Med. Hyg. 1989, 40, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef] [PubMed]


| Localities | Latitude N | Longitude W |
|---|---|---|
| Nabadji | 15°43′44.78″ | 13°24′09.76″ |
| Haere Lao | 16°23′59.71″ | 14°19′26.91″ |
| Tatki | 16°06′49.36″ | 15°16′19.28″ |
| Tessekere | 15°48′55.50″ | 15°08′09.88″ |
| Kassack | 16°25′29.52″ | 15°59′03.81″ |
| Saint-Louis | 15°51′58.27″ | 16°29′15.26″ |
| Bandia | 14°35′19.13″ | 17°01′06.43″ |
| Kolda | 12°53′02.54″ | 14°56′19.88″ |
| Localities | Host | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cattle | Goats | Sheep | ||||||||||
| P | I | IP (%) | P | I | IP (%) | P | I | IP (%) | P | I | IP (%) | |
| Nabadji | 30 | 23 | 77 | 30 | 4 | 13 | 30 | 5 | 17 | 90 | 32 | 35 |
| Haere Lao | 30 | 30 | 100 | 30 | 4 | 13 | 30 | 21 | 70 | 90 | 55 | 61 |
| Tatki | 30 | 28 | 93 | 30 | 5 | 17 | 30 | 30 | 100 | 90 | 63 | 70 |
| Tessekere | 30 | 28 | 93 | 30 | 0 | 0 | 30 | 19 | 63 | 90 | 47 | 52 |
| Kassack | 30 | 22 | 73 | 30 | 4 | 13 | 30 | 17 | 57 | 90 | 43 | 48 |
| Saint-louis | 30 | 29 | 97 | 30 | 13 | 43 | 30 | 12 | 40 | 90 | 54 | 60 |
| Bandia | 30 | 30 | 100 | 30 | 2 | 7 | 30 | 14 | 47 | 90 | 46 | 51 |
| Kolda | 30 | 30 | 100 | 30 | 0 | 0 | 30 | 13 | 43 | 90 | 43 | 48 |
| Total | 240 | 220 | 92 | 240 | 32 | 13 | 240 | 131 | 55 | 720 | 383 | 53 |
| Locality | Female | Male | Nymph | Abundance Total | Relative Abundance (RA%) | Shannon (H′) | Specific Richness (S) |
|---|---|---|---|---|---|---|---|
| Bandia | 411 | 1219 | 3 | 1633 | 26.6 | 1.05 | 07 |
| Haere Lao | 149 | 336 | 0 | 485 | 7.91 | 1.24 | 06 |
| Kassack | 74 | 150 | 9 | 233 | 3.80 | 1.57 | 08 |
| Kolda | 373 | 1003 | 13 | 1389 | 22.6 | 0.74 | 10 |
| Nabadji | 108 | 183 | 0 | 291 | 4.74 | 0.68 | 05 |
| Saint-Louis | 152 | 384 | 0 | 536 | 8.74 | 1.68 | 08 |
| Tatki | 460 | 890 | 1 | 1351 | 22.0 | 0.49 | 04 |
| Tessekere | 65 | 152 | 0 | 217 | 3.54 | 1.37 | 07 |
| Localities | Species | Host | IR | 95% CI | TOTAL (95% CI) |
|---|---|---|---|---|---|
| Bandia | A. variegatum | Cattle | 0.09 | (0.05–0.17) | 0.14 (0.09–0.22) |
| Hy.m rufipes | Cattle | 0.18 | (0.03–0.58) | ||
| Hy. truncatum | Cattle | 0.25 | (0.01–1.21) | ||
| Rh. evertsi evertsi | Cattle | 16.3 | (3.04–52.91) | ||
| Rh. evertsi evertsi | sheep | 7.46 | (1.36–24.41) | ||
| Kolda | A. variegatum | Cattle | 0.03 | (0.01–0.08) | 0.04 (0.01–0.09) |
| Hy. truncatum | Cattle | 0.21 | (0.01–0.99) | ||
| Haere Lao | Hy. impeltatum | Cattle | 0.36 | (0.09–0.98) | 0.48 (0.19–1.00) |
| Hy. impeltatum | Sheep | 107 | (8.20–422.82) | ||
| Hy.m rufipes | Cattle | 0.49 | (0.09–1.62) | ||
| Kassack | B. decoloratus | Cattle | 35.2 | (2.19–158.44) | 1.03 (0.19–3.33) |
| Hy. impeltatum | Sheep | 1000 | (206.55–1000) | ||
| Saint-Louis | Hy. impeltatum | Cattle | 0.50 | (0.09–1.65) | 0.35 (0.02–1.94) |
| Hy.m rufipes | Sheep | 1000 | (206.55–1000) | ||
| Hy. truncatum | Cattle | 0.39 | (0.02–1.94) | ||
| Tatki | Hy. impeltatum | Cattle | 0.22 | (0.12–0.37) | 0.22 (0.14–0.34) |
| Hy. impeltatum | Sheep | 1.16 | (0.05–0.39) | ||
| Rh. evertsi evertsi | Sheep | 6.17 | (1.13–20.17) | ||
| Tessekere | Hy. impeltatum | Cattle | 0.42 | (0.02–2.03) | 0.63 (0.11–2.08) |
| Hy.m rufipes | Cattle | 1.49 | (0.09–7.28) | ||
| Total species | B. decoloratus | 6.46 | (0.38–30.72) | 0.15 (0.11–0.19) | |
| A. variegatum | 0.06 | (0.03–0.10) | |||
| Hy. impeltatum | 0.24 | (0.16–0.35) | |||
| Hy. m rufipes | 0.25 | (0.10–0.53) | |||
| Hy. truncatum | 0.26 | 0.07–0.71) | |||
| Rh. evertsi evertsi | 3.47 | (1.44–7.15) | |||
| Total host | Cattle | 0.13 | (0.09–0.17) | ||
| Sheep | 0.42 | (0.22–0.72) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badji, A.; Ndiaye, M.; Gaye, A.; Dieng, I.; Ndiaye, E.H.; Dolgova, A.S.; Mhamadi, M.; Diouf, B.; Dia, I.; Dedkov, V.G.; et al. Detection of Crimean–Congo Haemorrhagic Fever Virus from Livestock Ticks in Northern, Central and Southern Senegal in 2021. Trop. Med. Infect. Dis. 2023, 8, 317. https://doi.org/10.3390/tropicalmed8060317
Badji A, Ndiaye M, Gaye A, Dieng I, Ndiaye EH, Dolgova AS, Mhamadi M, Diouf B, Dia I, Dedkov VG, et al. Detection of Crimean–Congo Haemorrhagic Fever Virus from Livestock Ticks in Northern, Central and Southern Senegal in 2021. Tropical Medicine and Infectious Disease. 2023; 8(6):317. https://doi.org/10.3390/tropicalmed8060317
Chicago/Turabian StyleBadji, Aminata, Mignane Ndiaye, Alioune Gaye, Idrissa Dieng, El Hadji Ndiaye, Anna S. Dolgova, Moufid Mhamadi, Babacar Diouf, Ibrahima Dia, Vladimir G. Dedkov, and et al. 2023. "Detection of Crimean–Congo Haemorrhagic Fever Virus from Livestock Ticks in Northern, Central and Southern Senegal in 2021" Tropical Medicine and Infectious Disease 8, no. 6: 317. https://doi.org/10.3390/tropicalmed8060317
APA StyleBadji, A., Ndiaye, M., Gaye, A., Dieng, I., Ndiaye, E. H., Dolgova, A. S., Mhamadi, M., Diouf, B., Dia, I., Dedkov, V. G., Faye, O., & Diallo, M. (2023). Detection of Crimean–Congo Haemorrhagic Fever Virus from Livestock Ticks in Northern, Central and Southern Senegal in 2021. Tropical Medicine and Infectious Disease, 8(6), 317. https://doi.org/10.3390/tropicalmed8060317









