Environmental Factors Linked to Reporting of Active Malaria Foci in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Malaria Data
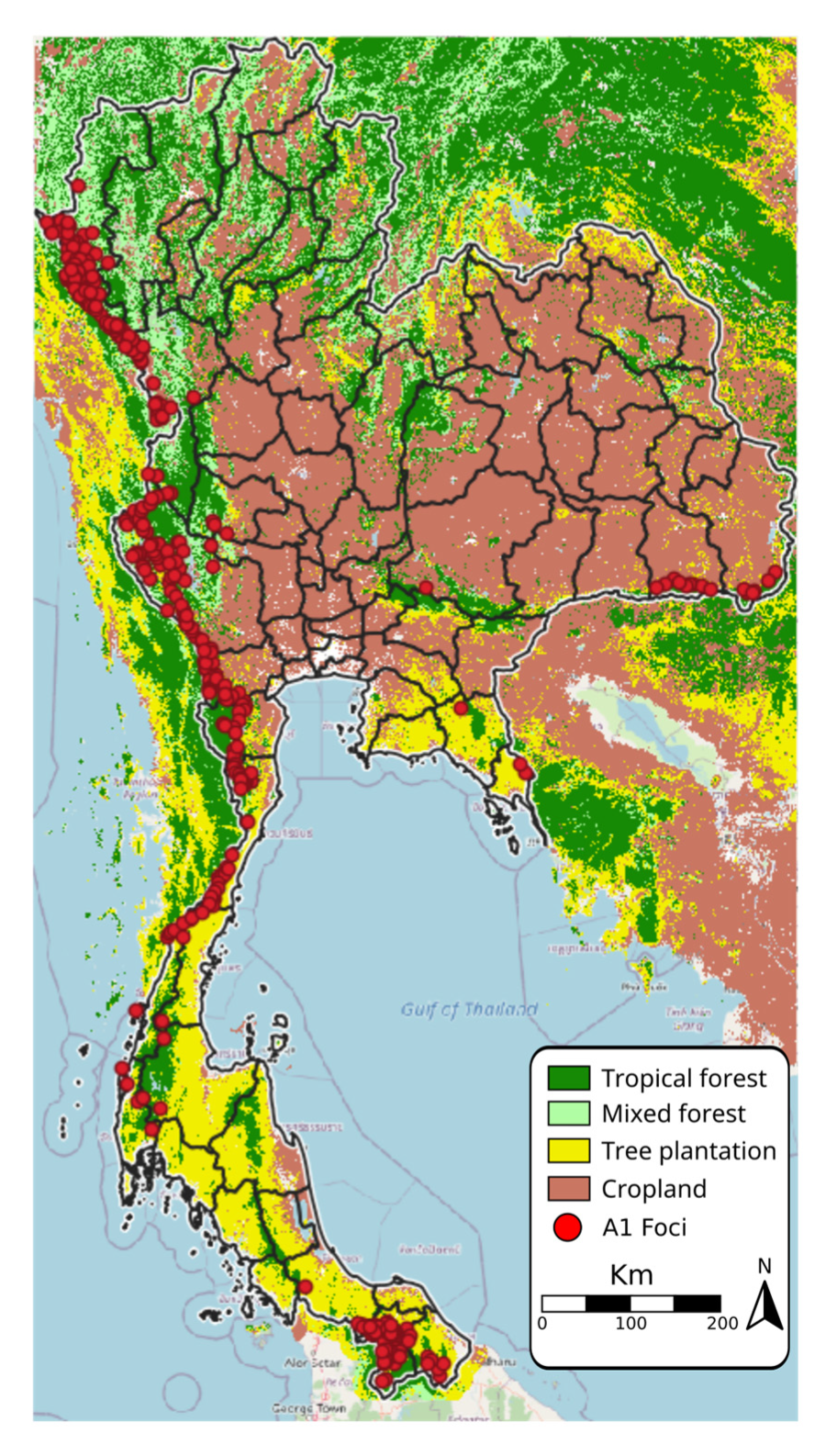
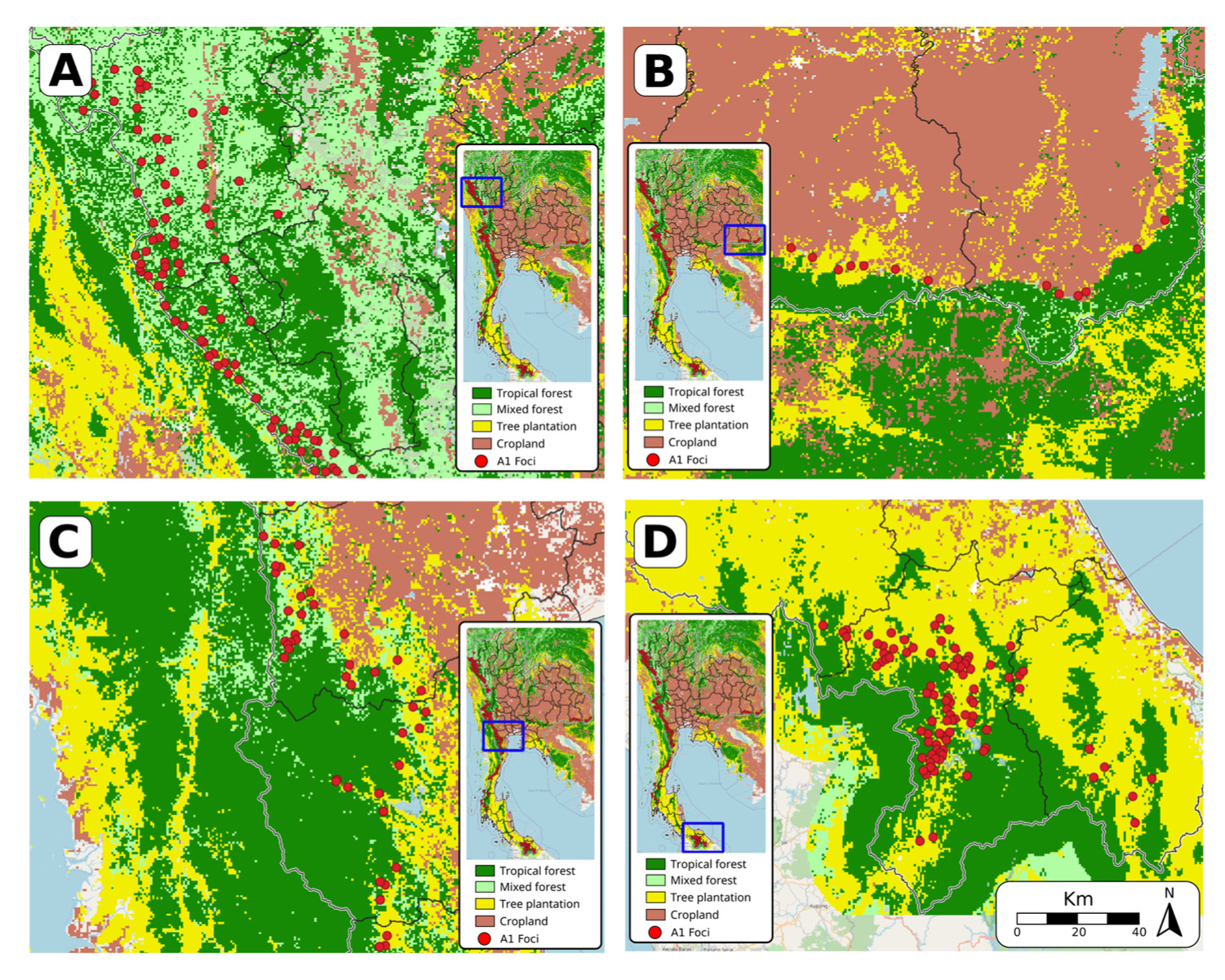
2.2. Land Use Data
- Cropland includes herbaceous and shrubby crops such as cereals, oils seeds, vegetables, root crops and forages, but excludes tea, coffee, and rice.
- Plantation includes perennial crops of >5 m such as rubber, palm oil, cashew, and coconut.
- Tropical forests have >60% canopy cover from trees at least 5 m tall, and the dominant tree species are evergreen broadleaf
- Mixed forests have >60% canopy cover from trees at least 5 m tall; the forest is considered mixed because no single forest type makes up >60% of the total tree cover.
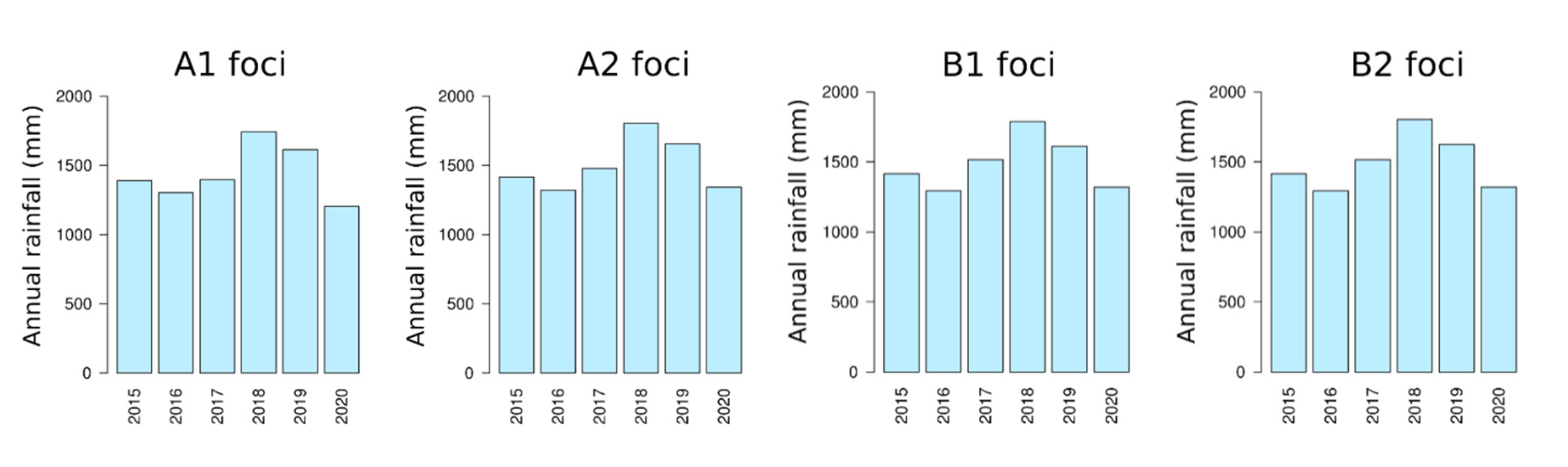
2.3. Rainfall Data
2.4. Forest Disturbance Data
2.5. Statistical Analysis
- RAINFALLfoci: annual monthly mean rainfall
- FOREST-Tropfoci: the percentage of land occupied by tropical forest
- FOREST-Mixfoci: the percentage of land occupied by mixed forest
- CROPfoci: the percentage of land occupied by crops
- BUILTfoci: the percentage of land occupied by built area
- PLANTATIONfoci: the percentage of land occupied by plantation area
- SEAS WATERfoci: the percentage of land occupied by seasonal water bodies
- PERM WATERfoci: the percentage of land occupied by permanent water bodies
- DISTURBANCEfoci: the level of forest disturbance
- DIST BORDERfoci: the distance in kilometers from an international border
- AGEcase: the median age of reported cases from FY2016 to FY2020
- MALEcase: the percentage of males among reported cases from FY2016 to FY2020
- SHORT-RESIDENTcase: the percentage of people among reported cases from FY2016 to FY2020 who have lived in Thailand for less than 6 months
- Pf RATIOcase: the percentage of P. falciparum among reported cases from FY2016 to FY2020
- 1-3-7 ADHERENCEfoci: percentage of malaria cases in each focus managed without delays (i.e., adherence to 1-3-7 surveillance protocols in full and on time)
- STATUSy-1: focus status of the previous year
- STATUSy-5: the number of years with reported indigenous cases (i.e., A1 focus classification), up to 5 years
- PROVINCERND: the province in which the focus is located, included as random effect
- FOCISPAT: spatial random effect at the foci level represented by a geospatial spline
3. Results
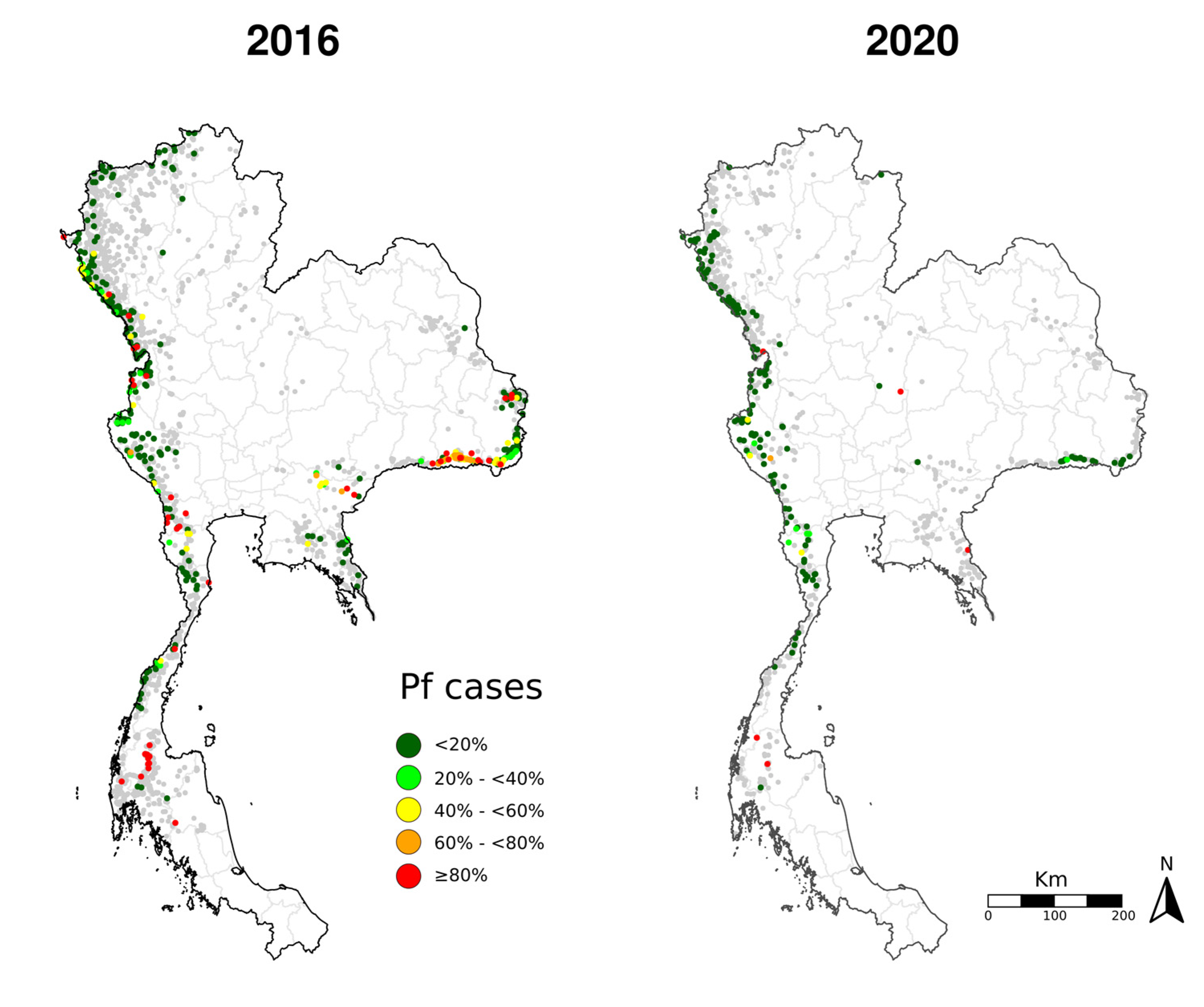
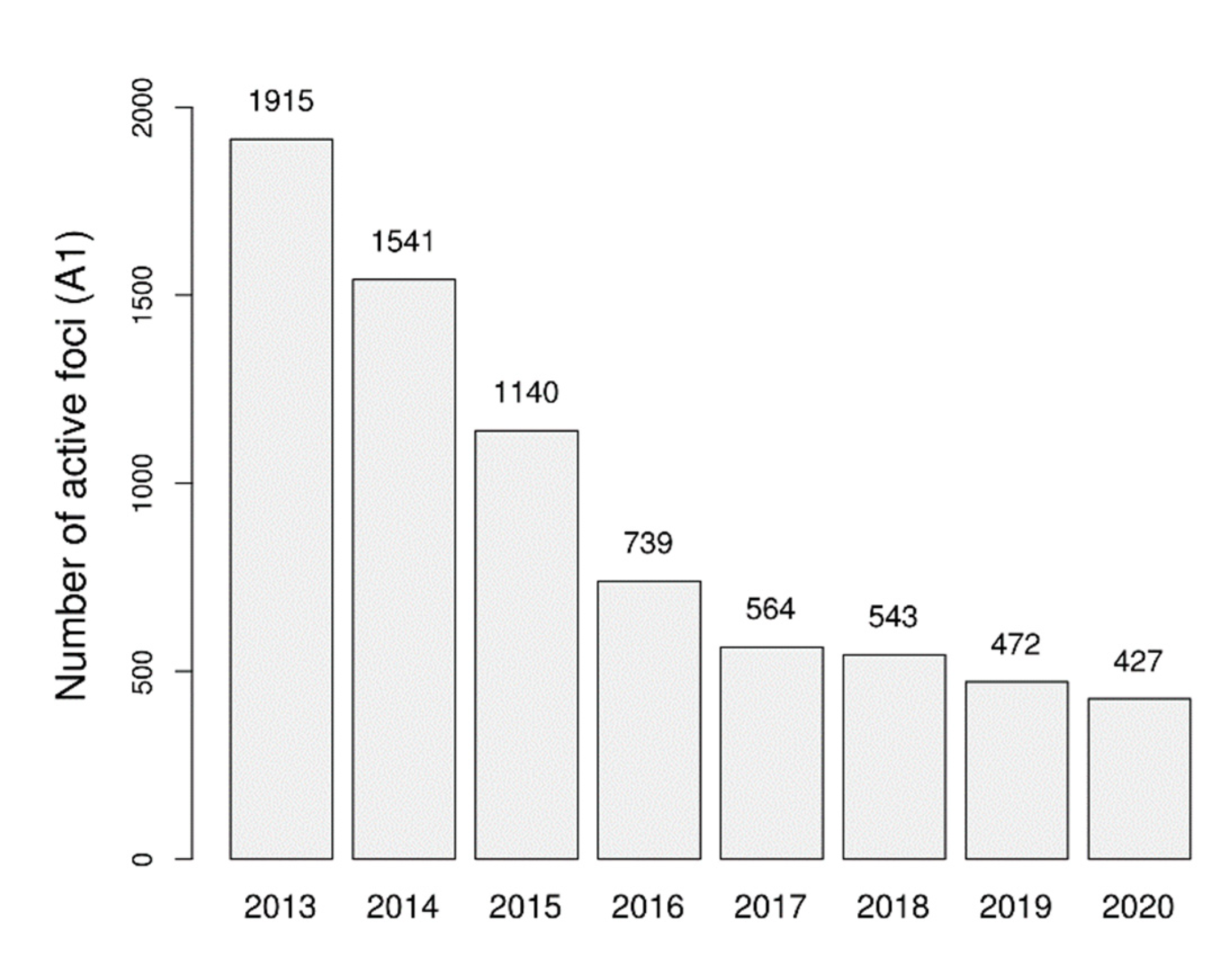
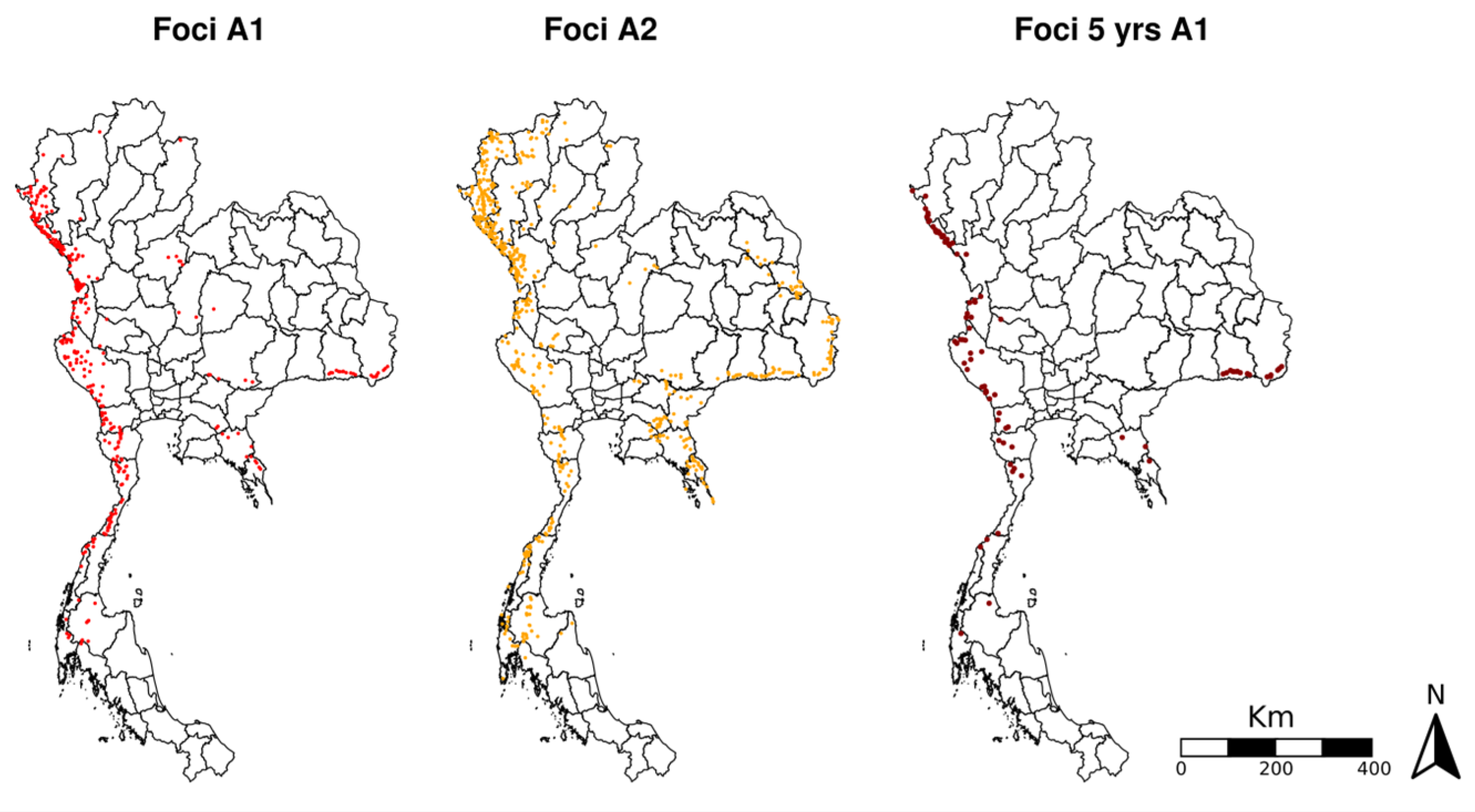
3.1. Changes over Time in Active Foci
3.2. Assessment of Environmental Composition
3.3. Results of Statistical Modelling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | coronavirus disease 2019 |
| DVBD | Division of Vector Borne Diseases |
| FY | fiscal year |
| GMS | Greater Mekong Subregion |
| Lao PDR | Lao People’s Democratic Republic |
| MIS | Malaria Information System |
| MOPH | Ministry of Public Health |
| NMES | National Malaria Elimination Strategy |
| PMI | President’s Malaria Initiative |
| POR | prevention of reestablishment |
| USAID | United States Agency for International Development |
| WHO | World Health Organization |
Appendix A
References
- World Health Organization (WHO). World Malaria Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- The Mekong Malaria Elimination Programme. Accelerating Malaria Elimination in the Greater Mekong. World Health Organization. Available online: https://www.who.int/publications-detail-redirect/WHO-UCN-GMP-MME-2022.01 (accessed on 3 June 2022).
- World Health Organization (WHO). Strategy for Malaria Elimination in the Greater Mekong Subregion (2015–2030); WHO Regional Office for the Western Pacific: Manila, Philippines, 2015. [Google Scholar]
- Bureau of Vector Borne Diseases, Department of Disease Control, Thailand Ministry of Public Health. Guide to Malaria Elimination for Thailand’s Local Administrative Organizations; Ministry of Public Health: Nonthaburi, Thailand, 2019. [Google Scholar]
- Sudathip, P.; Kitchakarn, S.; Shah, J.A.; Bisanzio, D.; Young, F.; Gopinath, D.; Pinyajeerapat, N.; Sintasath, D.; Lertpiriyasuwat, C. A foci cohort analysis to monitor successful and persistent foci under Thailand’s Malaria Elimination Strategy. Malar. J. 2021, 20, 118. [Google Scholar] [CrossRef]
- Lertpiriyasuwat, C.; Sudathip, P.; Kitchakarn, S.; Areechokchai, D.; Naowarat, S.; Shah, J.A.; Sintasath, D.; Pinyajeerapat, N.; Young, F.; Thimasarn, K.; et al. Implementation and success factors from Thailand’s 1-3-7 surveillance strategy for malaria elimination. Malar. J. 2021, 20, 201. [Google Scholar] [CrossRef]
- Feng, J.; Liu, J.; Feng, X.; Zhang, L.; Xiao, H.; Xia, Z. Towards Malaria Elimination: Monitoring and Evaluation of the “1-3-7” Approach at the China-Myanmar Border. Am. J. Trop. Med. Hyg. 2016, 95, 806. [Google Scholar] [PubMed]
- Nguitragool, W.; Karl, S.; White, M.; Koepfli, C.; Felger, I.; Singhasivanon, P.; Mueller, I.; Sattabongkot, J. Highly heterogeneous residual malaria risk in western Thailand. Int. J. Parasitol. 2019, 6, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.M.; Sriwichai, P.; Kirabittir, K.; Prachumsri, J.; Chavez, I.F.; Hii, J. Transmission risk beyond the village: Entomological and human factors contributing to residual malaria transmission in an area approaching malaria elimination on the Thailand–Myanmar border. Malar. J. 2019, 18, 221. [Google Scholar] [CrossRef]
- Parker, D.M.; Carrara, V.I.; Pukrittayakamee, S.; McGready, R.; Nosten, F.H. Malaria ecology along the Thailand-Myanmar border. Malar. J. 2015, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sudathip, P.; Saejeng, A.; Khantikul, N.; Thongrad, T.; Kitchakarn, S.; Sugaram, R.; Lertpiriyasuwat, C.; Areechokchai, D.; Gopinath, D.; Sintasath, D.; et al. Progress and challenges of integrated drug efficacy surveillance for uncomplicated malaria in Thailand. Malar. J. 2021, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.A. Learnings from Thailand in building strong surveillance for malaria elimination. Nat. Commun. 2022, 13, 2677. [Google Scholar]
- Kaehler, N.; Adhikari, B.; Cheah, P.Y.; von Seidlein, L.; Day, N.P.; Paris, D.H.; Tanner, M.; Pell, C. Prospects and strategies for malaria elimination in the Greater Mekong Sub-region: A qualitative study. Malar. J. 2019, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Saita, S.; Silawan, T.; Parker, D.M.; Sriwichai, P.; Phuanukoonnon, S.; Sudathip, P.; Maude, R.J.; White, L.J.; Pan-Ngum, W. Spatial Heterogeneity and Temporal Trends in Malaria on the Thai-Myanmar Border (2012–2017): A Retrospective Observational Study. Trop. Med. Infect. Dis. 2019, 4, 62. [Google Scholar] [CrossRef]
- Sudathip, P.; Naowarat, S.; Kitchakarn, S.; Gopinath, D.; Bisanzio, D.; Pinyajeerapat, N.; Sintasath, D.; Shah, J.A. Assessing Thailand’s 1-3-7 surveillance strategy in accelerating malaria elimination. Malar. J. 2022, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Sanann, N.; Peto, T.J.; Tripura, R.; Callery, J.J.; Nguon, C.; Bui, T.M.; Nofal, S.D.; von Seidlein, L.; Lek, D.; Dondorp, A.M. Forest work and its implications for malaria elimination: A qualitative study. Malar. J. 2019, 18, 376. [Google Scholar] [CrossRef]
- Zhang, S.S.; Feng, J.; Zhang, L.; Ren, X.; Geoffroy, E.; Manguin, S.; Frutos, R.; Zhou, S.S. Imported malaria cases in former endemic and non-malaria endemic areas in China: Are there differences in case profile and time to response? Infect. Dis. Poverty 2019, 8, 61. [Google Scholar] [CrossRef]
- Win, A.Y.N.; Maung, T.M.; Wai, K.T.; Oo, T.; Thi, A.; Tipmontree, R.; Soonthornworasiri, N.; Kengganpanich, M.; Kaewkungwal, J. Understanding malaria treatment-seeking preferences within the public sector amongst mobile/migrant workers in a malaria elimination scenario: A mixed-methods study. Malar. J. 2017, 16, 462. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Chen, X.; Gao, Y.; Mi, J. GLC_FCS30-2020: Global Land Cover with Fine Classification System at 30m in 2020. Earth Syst. Sci. Data. 2020, 13, 2753–2776. [Google Scholar]
- Saah, D.; Tenneson, K.; Poortinga, A.; Nguyen, Q.; Chishtie, F.; San Aung, K.; Markert, K.N.; Clinton, N.; Anderson, E.R.; Cutter, P.; et al. Primitives as building blocks for constructing land cover maps. Int. J. Appl. Earth Obs. Geoinf. 2020, 85, 101979. [Google Scholar] [CrossRef]
- GRASS Development Team. Geographic Resources Analysis Support System (GRASS) Software, Version 7.2. Open Source Geospatial Foundation. Electronic Document. 2017. Available online: http://grass.osgeo.org (accessed on 12 November 2021).
- National Center for Atmospheric Research Staff (Ed.) Last Modified 27 February 2020. The Climate Data Guide: GPCC: Global Precipitation Climatology Centre. Available online: https://climatedataguide.ucar.edu/climate-data/gpcc-global-precipitation-climatology-centre (accessed on 18 February 2022).
- Schneider, U.; Becker, A.; Finger, P.; Meyer-Christoffer, A.; Ziese, M.; Rudolf, B. GPCC’s new land surface precipitation climatology based on quality-controlled in situ data and its role in quantifying the global water cycle. Theor. Appl. Climatol. 2014, 115, 15–40. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Umlauf, N.; Adler, D.; Kneib, T.; Lang, S.; Zeileis, A. Structured additive regression models: An R interface to BayesX. Work. Pap. Econ. Stat. 2012, 10, 73863. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 9 September 2021).
- Perera, R.; Caldera, A.; Wickremasinghe, A.R. Reactive Case Detection (RACD) and foci investigation strategies in malaria control and elimination: A review. Malar. J. 2020, 19, 401. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Atkinson, P.M.; Snow, R.W. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005, 3, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.W.; Campbell, H.; Adiamah, J.H.; Greenwood, A.M.; Bangali, J.E.; Greenwood, B.M. Malaria in a peri-urban area of The Gambia. Ann. Trop. Med. Parasitol. 1990, 84, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Biggar, R.J.; Collins, W.E.; Nkrumah, F.K. Malaria in urban and rural areas of southern Ghana: A survey of parasitaemia, antibodies, and antimalarial practices. Bull. World Health Organ. 1984, 62, 607–613. [Google Scholar] [PubMed]
- Mathanga, D.P.; Tembo, A.K.; Mzilahowa, T.; Bauleni, A.; Mtimaukenena, K.; Taylor, T.E.; Valim, C.; Walker, E.D.; Wilson, M.L. Patterns and determinants of malaria risk in urban and peri-urban areas of Blantyre, Malawi. Malar. J. 2016, 15, 509. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Correlation between rainfall and the prevalence of malaria in Thailand. J. Infect. 2006, 52, 227–230. [Google Scholar] [CrossRef]
- World Malaria Report 2021. World Health Organization. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 3 June 2022).
- Jongdeepaisal, M.; Khonputsa, P.; Prasert, O.; Maneenet, S.; Pongsoipetch, K.; Jatapai, A.; Rotejanaprasert, C.; Sudathip, P.; Maude, R.J.; Pell, C. Forest malaria and prospects for anti-malarial chemoprophylaxis among forest goers: Findings from a qualitative study in Thailand. Malar. J. 2022, 21, 47. [Google Scholar] [CrossRef]
- Kheang, S.T.; Lin, M.A.; Lwin, S.; Naing, Y.H.; Yarzar, P.; Kak, N.; Price, T. Malaria Case Detection Among Mobile Populations and Migrant Workers in Myanmar: Comparison of 3 Service Delivery Approaches. Glob. Health Sci Pract. 2018, 6, 384–398, correction in Glob. Health Sci Pract. 2018, 6, 612.. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U. Impact of Weekly Climatic Variables on Weekly Malaria Incidence throughout Thailand: A Country-Based Six-Year Retrospective Study. J. Environ. Public Health. 2018, 2018, 8397815. [Google Scholar] [CrossRef]
| Focus Classification | Pre-2016 Definition | Current Definition |
|---|---|---|
| A1 | Perennial transmission village or hamlet where indigenous cases are reported at least 6 months out of the year | Active focus: Reported indigenous transmission in the current year |
| A2 | Periodic transmission area village or hamlet where indigenous cases are reported fewer than 6 months out of the year | Residual non-active focus: No indigenous cases in the current year but with indigenous cases in the previous 3 years |
| B1 | High-risk village or hamlet without transmission for a minimum of 3 years, but adult vectors or larvae are present or conditions are favorable for breeding | Cleared focus but receptive: No indigenous transmission in at least 3 years, but suitable environment for vector Anopheles spp. mosquitoes |
| B2 | Low-risk village or hamlet without transmission for a minimum of 3 years, and no presence of adult vectors or larvae and unfavorable conditions for breeding | Cleared focus but not receptive: No indigenous transmission in at least 3 years, but unsuitable environment for vector Anopheles spp. mosquitoes |
| Focus Classification in FY2020 | Distance from Border (Mean) | Forest Loss Score (Mean) | Fraction Plantation (Mean) | Fraction Tropical Forest (Mean) | Fraction Mixed Forest (Mean) | Fraction Evergreen Forest (Mean) | Fraction Urban Area (Mean) | Fraction Rice Field (Mean) |
|---|---|---|---|---|---|---|---|---|
| A1 | 54.8 km | 14.1 | 20.9% | 10.8% | 14.1% | 1.53% | 0.07% | 0% |
| A2 | 77.85 km | 9.8 | 18.09% | 8.45% | 13.36% | 1.39% | 0.19% | 0% |
| B1 | 119.56 km | 12.39 | 17.33% | 7.22% | 7.40% | 2.30% | 0.61% | 0.32% |
| B2 | 188.69 km | 12.54 | 6.70% | 1.84% | 0.89% | 0.09% | 1.00% | 3.37% |
| Variable | Type of Variable | Odds Ratio |
|---|---|---|
| STATUSy-1 (Ref: A1) | String | |
| A2 | 0.16 (0.09–0.28) ** | |
| B1 | 0.12 (0.05–0.25) ** | |
| B2 | 0.02 (0.01–0.28) * | |
| STATUSy-5 | Discrete | 1.20 (1.05–1.34) * |
| FOREST-Tropfoci | Discrete | 1.02 (1.01–1.03) ** |
| DISTURBANCEfoci | Discrete | 1.16 (1.01–1.73) * |
| DIST BORDERfoci | Discrete | 0.98 (0.97–0.99) * |
| MALEcase | Discrete | 1.66 (1.23–1.87) * |
| SHORT-RESIDENTcase | Discrete | 1.10 (1.03–1.16) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prempree, P.; Bisanzio, D.; Sudathip, P.; Kanjanasuwan, J.; Powell, I.; Gopinath, D.; Suttiwong, C.; Pinyajeerapat, N.; Poortinga, A.; Sintasath, D.; et al. Environmental Factors Linked to Reporting of Active Malaria Foci in Thailand. Trop. Med. Infect. Dis. 2023, 8, 179. https://doi.org/10.3390/tropicalmed8030179
Prempree P, Bisanzio D, Sudathip P, Kanjanasuwan J, Powell I, Gopinath D, Suttiwong C, Pinyajeerapat N, Poortinga A, Sintasath D, et al. Environmental Factors Linked to Reporting of Active Malaria Foci in Thailand. Tropical Medicine and Infectious Disease. 2023; 8(3):179. https://doi.org/10.3390/tropicalmed8030179
Chicago/Turabian StylePrempree, Preecha, Donal Bisanzio, Prayuth Sudathip, Jerdsuda Kanjanasuwan, Isabel Powell, Deyer Gopinath, Chalita Suttiwong, Niparueradee Pinyajeerapat, Ate Poortinga, David Sintasath, and et al. 2023. "Environmental Factors Linked to Reporting of Active Malaria Foci in Thailand" Tropical Medicine and Infectious Disease 8, no. 3: 179. https://doi.org/10.3390/tropicalmed8030179
APA StylePrempree, P., Bisanzio, D., Sudathip, P., Kanjanasuwan, J., Powell, I., Gopinath, D., Suttiwong, C., Pinyajeerapat, N., Poortinga, A., Sintasath, D., & Shah, J. A. (2023). Environmental Factors Linked to Reporting of Active Malaria Foci in Thailand. Tropical Medicine and Infectious Disease, 8(3), 179. https://doi.org/10.3390/tropicalmed8030179







