Zika, Flavivirus and Malaria Antibody Cocirculation in Nigeria
Abstract
1. Background
2. Methods
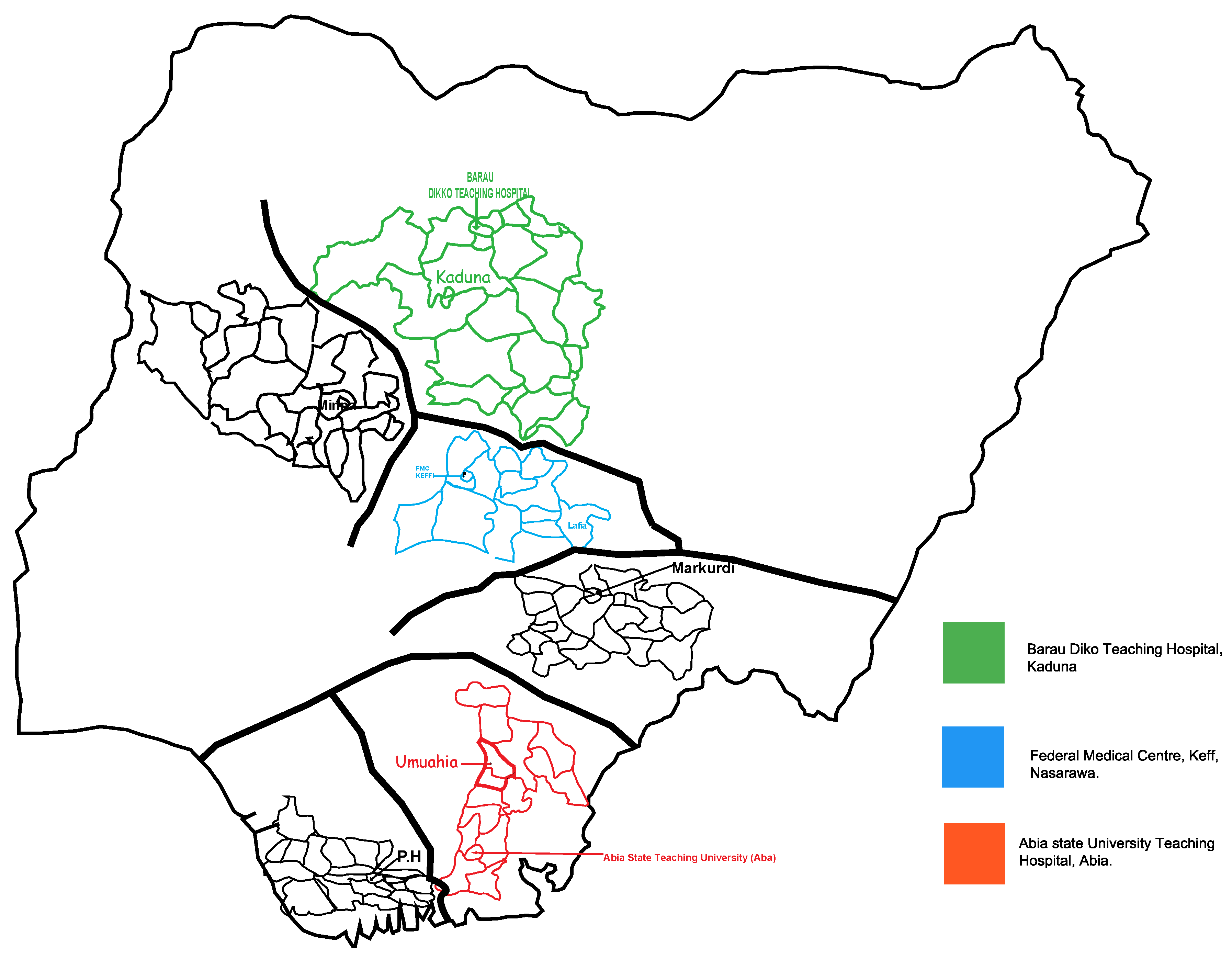
2.1. Study Design and Site
2.2. Study Population
2.3. Screening of the Study Participants
2.4. Total Number of Samples Collected
2.5. Laboratory Testing
2.6. Statistical Tests
3. Ethics Statement
4. Results
4.1. Signs and Symptoms Presented by ZIKV, FLAVI Malaria Monoinfected Patients
4.2. Arbovirus Serology
4.3. Demographic Characteristics of Participants and ZIKV, FLAVI, and Malaria Antibody Seropositivity
4.4. Sex-Specific Antibody Seropositivity of Arboviral Infection
4.5. Place-Specific Antibody Seropositivity of Arboviral Infection
4.6. ZIKV, FLAVI, and Malaria Antibody Seropositivity in Pregnant and Non-Pregnant Participants
4.7. HIV Status-Specific Antibody Seropositivity
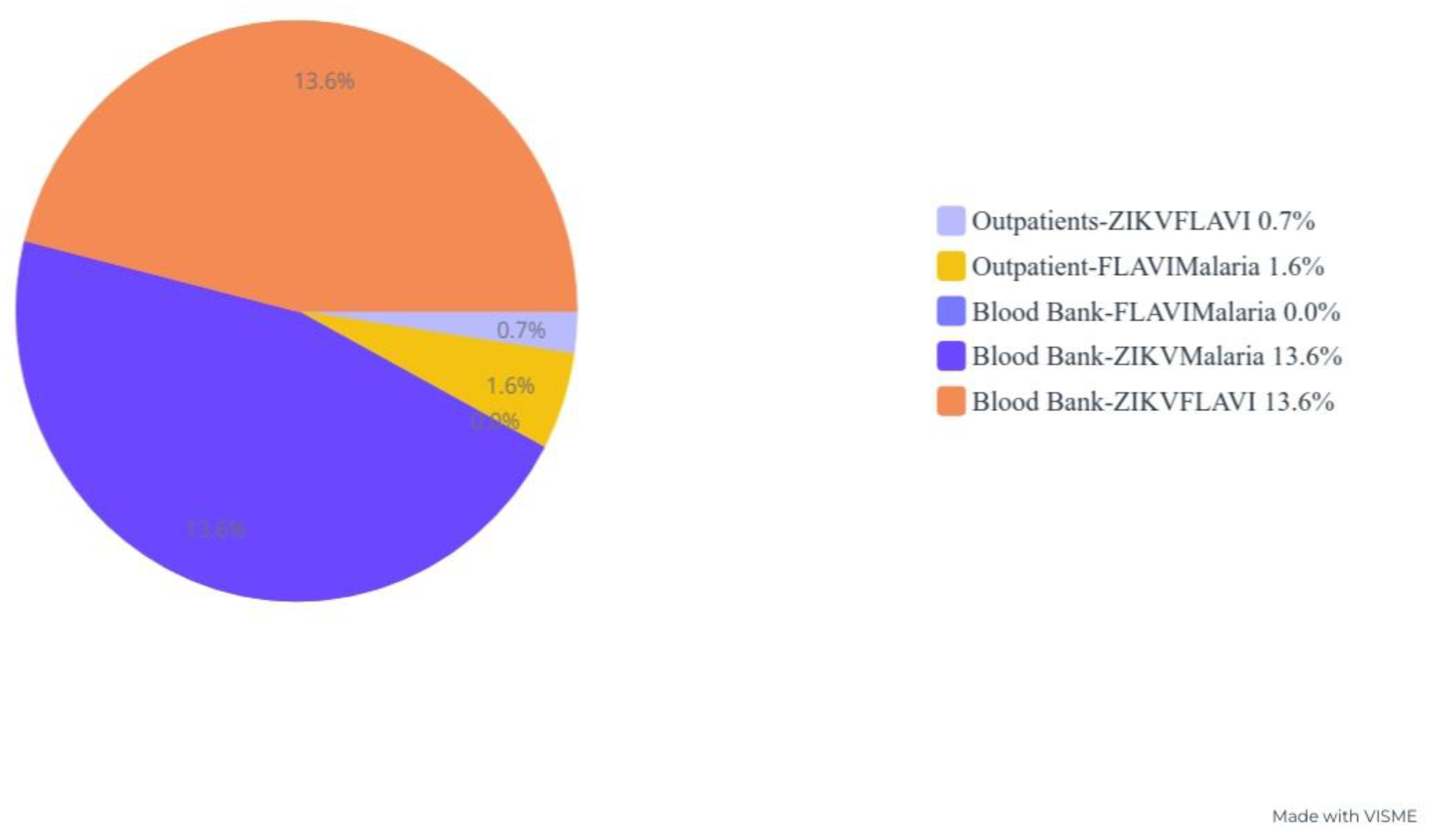
4.8. Blood Product Antibody Seropositivity
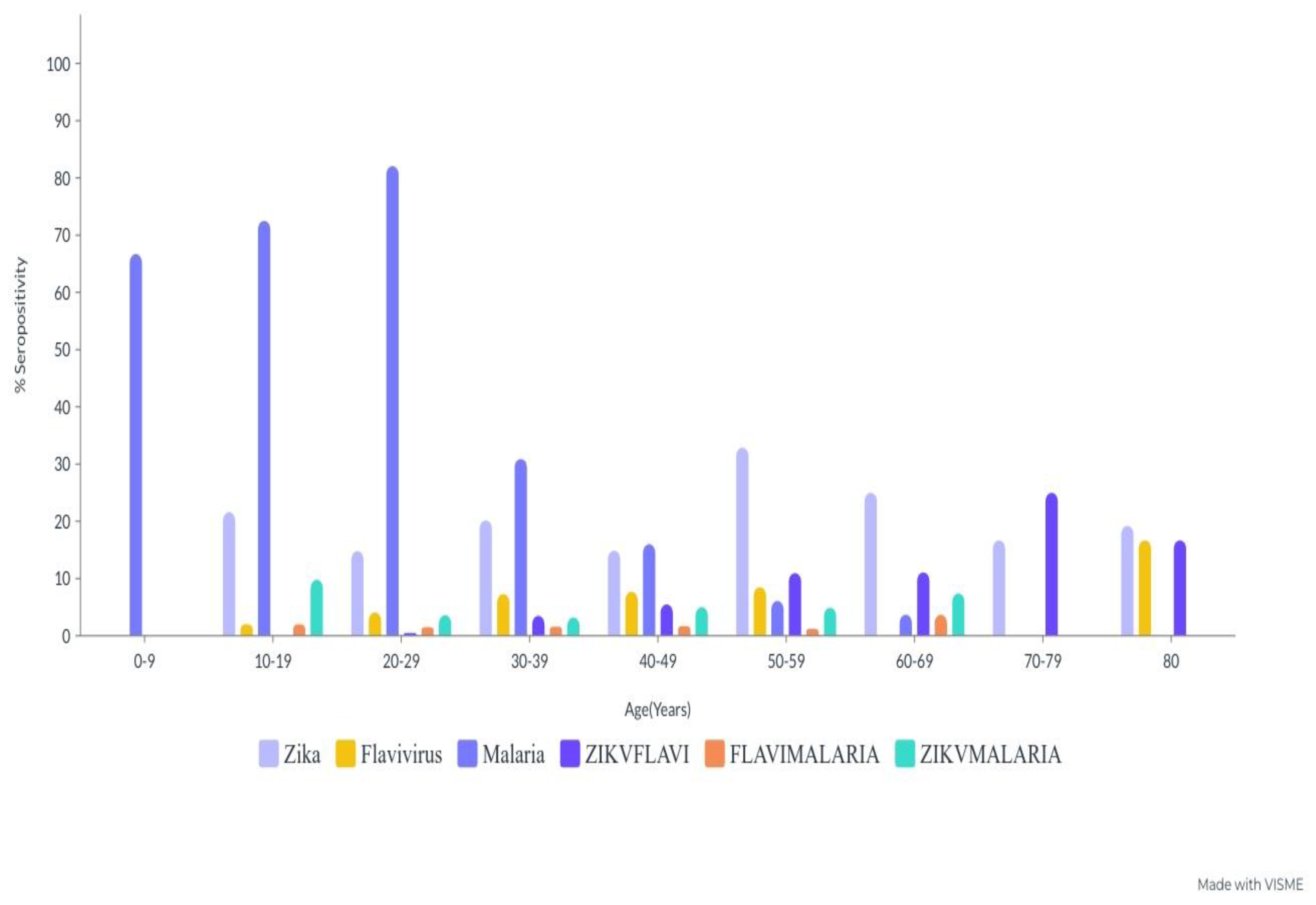
4.9. Age-Specific Antibody Seropositivity of ZIKV, FLAVI, and Malaria Co-Circulation
4.10. Sociodemographic Characteristics and Cocirculation Antibody Seropositivity of ZIKV, FLAVI and Malaria
4.11. Sex-Specific Cocirculation Antibody Seropositivity against ZIKV, FLAVI and Malaria
4.12. Place-Specific Cocirculation Antibody Seropositivity against ZIKV, FLAVI and Malaria
4.13. Pregnancy Status-Specific Cocirculation Antibody Seropositivity against ZIKV, FLAVI and Malaria
4.14. HIV-Status-Specific Cocirculation Antibody Seropositivity against ZIKV, FLAVI and Malaria
4.15. Blood Product-Specific Cocirculation Antibody Seropositivity against ZIKV, FLAVI and Malaria
4.16. Age-Specific Cocirculation Antibody Seropositivity against ZIKV, FLAVI and Malaria
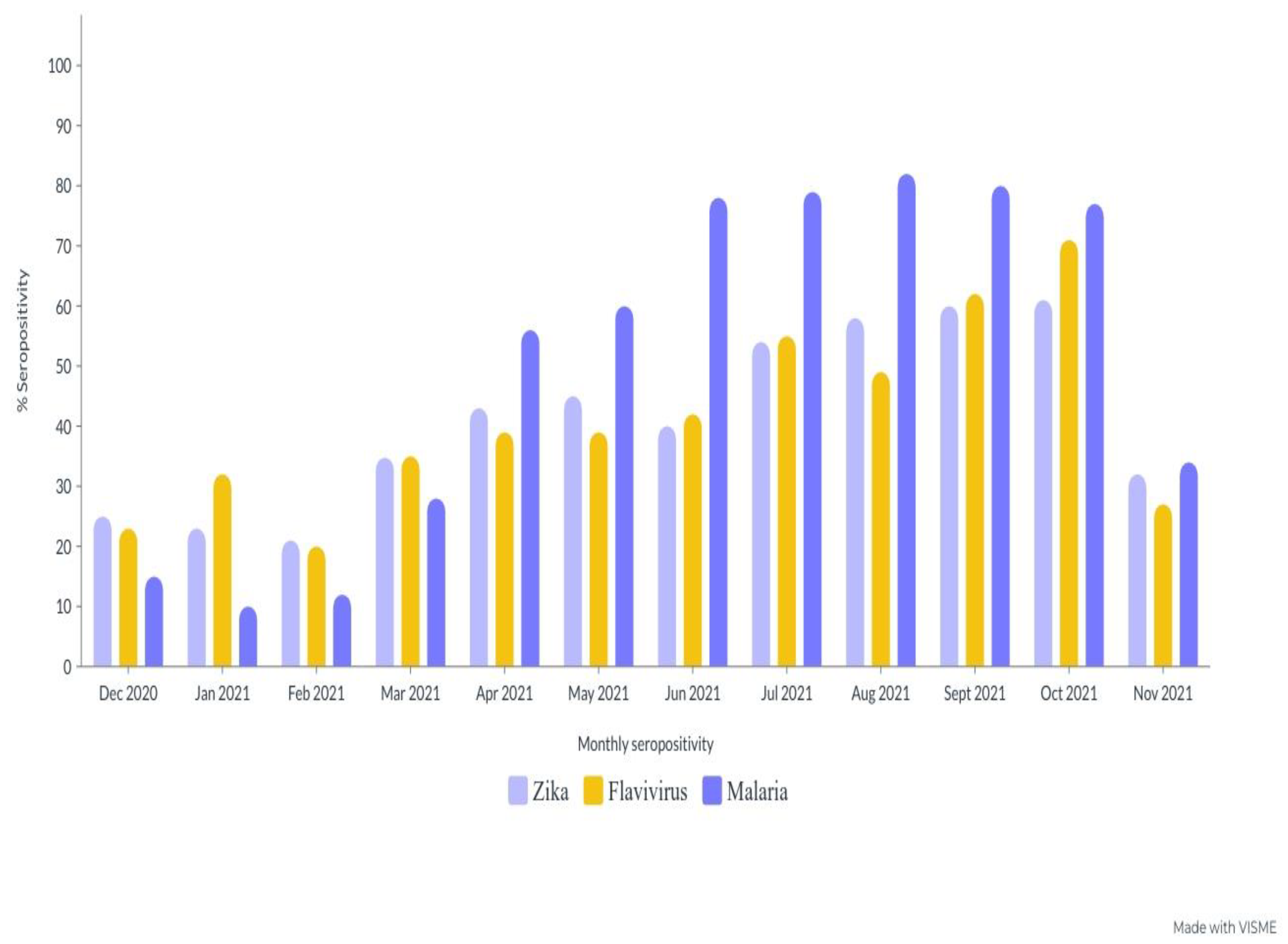
4.17. Monthly Antibody Seropositivity ZIKV, FLAVI and Malaria during the Sampling Period
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AFLs | Acute febrile illnesses |
| ZIKV | Zika virus |
| CI | Confidence interval |
| FLAVI | Flavivirus virus |
| ZIKVFLAVI | Zika and flavivirus |
| FLAVI-Malaria | Flavivirus and malaria |
| ZIKV-Malaria | Zika and Malaria |
| NS1 | Non-structural proteins; 1 |
| pLDH | Parasite lactate dehydrogenase |
| HRP | Histidine-rich protein 2 |
| IgG | Immunoglobulin G |
| RDT | Rapid Diagnostic Test |
| RT–PCR | Reverse transcription polymerase chain reaction |
| VLP | Viral Live Particle |
References
- Nunez-Avellaneda, D.; Tangudu, C.; Barrios-Palacios, J.; Machain-Williams, C.; Alarcón-Romero, L.D.C.; Zubillaga-Guerrero, M.I.; Nunez-Avellaneda, S.; McKeen, L.A.; Canche-Aguilar, I.; Loaeza-Díaz, L.; et al. Co-Circulation of All Four Dengue Viruses and Zika Virus in Guerrero, Mexico, 2019. Vector-Borne Zoonotic Dis. 2021, 21, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Fagbami, A.H. Zika Virus Infections in Nigeria: Virological and Seroepidemiological Investigations in Oyo State. J. Hyg. 1979, 83, 2. [Google Scholar] [CrossRef] [PubMed]
- WHO. Countries and Territories with Current or Previous Zika Virus Transmission. Updated February 2022. Available online: https://cdn.who.int/media/docs/default-source/documents/emergencies/zika/map-of-countries_with_zika_transmission_feb2022.pdf?sfvrsn=802a352a_5 (accessed on 1 February 2023).
- Giron, S.; Franke, F.; Decoppet, A.; Cadiou, B.; Travaglini, T.; Thirion, L.; Durand, G.; Jeannin, C.; L’ambert, G.; Grard, G.; et al. Vector-borne transmission of Zika virus in Europe, southern France, August 2019. Eurosurveillance 2019, 24, 1900655. [Google Scholar] [CrossRef] [PubMed]
- Otu, A.; Udoh, U.; Ita, O.; Hicks, J.P.; Ukpeh, I.; Walley, J. Prevalence of Zika and malaria in patients with fever in secondary healthcare facilities in south-eastern Nigeria. Trop. Dr. 2019, 50, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Oluwole, T.; Fowotade, A.; Mirchandani, D.; Almeida, S.; Plante, K.; Weaver, S.; Bakare, R. Seroprevalence of some Arboviruses among Pregnant Women in Ibadan, Southwestern, Nigeria. Int. J. Infect. Dis. 2022, 116, S130. [Google Scholar] [CrossRef]
- Mathé, P.; Egah, D.Z.; Müller, J.A.; Shehu, N.Y.; Obishakin, E.T.; Shwe, D.D.; Pam, V.C.; Okolo, M.O.; Yilgwan, C.; Gomerep, S.S.; et al. Low Zika virus seroprevalence among pregnant women in North Central Nigeria, 2016. J. Clin. Virol. 2018, 105, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Anejo-Okopi, J.; Gotom, D.Y.; Chiehiura, N.A.; Okojokwu, J.O.; Amanyi, D.O.; Egbere, J.O.; Adetunji, J.; Ujah, O.I.; Audu, O. The Seroprevalence of Zika Virus Infection among HIV Positive and HIV Negative Pregnant Women in Jos, Nigeria. Hosts Viruses 2020, 7, 129–136. [Google Scholar] [CrossRef]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.-M. Potential Sexual Transmission of Zika Virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Besnard, M.; Lastère, S.; Teissier, A.; Cao-Lormeau, V.M.; Musso, D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Eurosurveillance 2014, 19, 20751. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Carvalho, G.C.; Nascimento-Carvalho, E.C.; VanDuijn, M.M.; Ramos, C.L.; Vilas-Boas, A.-L.; Moreno-Carvalho, O.A.; Zeneyedpour, L.; Ferwerda, G.; de Groot, R.; Luider, T.M.; et al. Cerebrospinal fluid immunoglobulins are increased in neonates exposed to Zika virus during foetal life. J. Infect. 2020, 80, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika Virus in Gabon (Central Africa)—2007: A New Threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef] [PubMed]
- Shaibu, J.O.; Okwuraiwe, A.P.; Jakkari, A.; Dennis, A.; Akinyemi, K.O.; Li, J.; Audu, R.A.; Oyefolu, A.O.B. Sero-molecular Prevalence of Zika Virus among Pregnant Women Attending Some Public Hospitals in Lagos State, Nigeria. Eur. J. Med. Health Sci. 2021, 3, 77–82. [Google Scholar] [CrossRef]
- Fagbami, A.; Halstead, S.B.; Marchette, N.; Larsen, K. Heterologous Flavivirus Infection-Enhancing Antibodies in Sera of Nigerians. Am. J. Trop. Med. Hyg. 1988, 38, 205–207. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lang, X.; Yu, J.; Zhu, L.; Qin, Z.; Liu, X.; Chen, P.; Dai, C.; Chen, T.; Li, X.; et al. The effects of Japanese encephalitis virus antibodies on Zika virus infection. Med. Microbiol. Immunol. 2020, 209, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Melo, A.S.; Malinger, G.; Ximenes, R.; Szejnfeld, P.O.; Alves Sampaio, S.; Bispo de Filippis, A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obs. Gynecol 2016, 47, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.medcalc.org/calc/odds_ratio.php (accessed on 2 February 2023).
- Available online: https://www.mikrogen.de/english/products/product-overview/testsystem/tropical-fever-igg.html (accessed on 12 June 2022).
- Kisuya, B.; Masika, M.M.; Bahizire, E.; Oyugi, J. Seroprevalence of Zika virus in selected regions in Kenya. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Asebe, G.; Michlmayr, D.; Mamo, G.; Abegaz, W.E.; Endale, A.; Medhin, G.; Larrick, J.W.; Legesse, M. Seroprevalence of Yellow fever, Chikungunya, and Zika virus at a community level in the Gambella Region, South West Ethiopia. PLoS ONE 2021, 16, e0253953. [Google Scholar] [CrossRef] [PubMed]
- Fagbami, A.H.; Halstead, S.B.; Marchette, N.J.; Larsen, K. Cross-infection enhancement among African flaviviruses by immune mouse ascitic fluids. Cytobios 1987, 49, 49–55. [Google Scholar] [PubMed]
- Marchette, N.J.; Garcia, R.; Rudnick, A. Isolation of Zika Virus from Aedes Aegypti Mosquitoes in Malaysia. Am. J. Trop. Med. Hyg. 1969, 18, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B. Zika virus outside Africa. Emerg. Infect. Dis. 2009, 15, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
| Sign and Symptoms | Mono-Infection (% Sign & Symptoms) | ||
|---|---|---|---|
| Anti-Zika Positive (N = 167) | Anti-Flavivirus Positive (N = 54) | Anti-Malaria Positive (N = 362) | |
| Headaches | 43.7% (73/167) | 79.6% (43/54) | 83.4% (302/362) |
| Exanthema | 33.5% (56/167) | 18.5% (10/54) | 5.5% (20/362) |
| Fever | 61.1% (102/167) | 72.2% (39/54) | 88.4% (320/362) |
| Abdominal pain | 21.0% (35/167) | 62.9% (34/54) | 55.5% (201/362) |
| Diarrhoea | 13.8% (23/167) | 7.4% (4/54) | 2.8% (10/362) |
| Myalgia | 38.3% (64/167) | 74.1% (40/54) | 27.1% (98/362) |
| Vomiting | 32.9% (55/167) | 22.2% (12/54) | 18.5% (67/362) |
| Generalized body pains | 83.8% (140/167) | 72.2% (39/54) | 58.6% (212/362) |
| Arthralgia | 36.5% (61/167) | 38.9% (21/54) | 21.5% (78/362) |
| Edema | 1.2% (2/167) | 12.9% (7/54) | 3.8% (14/362) |
| Hemorrhagic manifestation | 0.0% (0/167) | 3.7% (2/54) | 2.5% (9/362) |
| Retro-orbital pain | 12.6% (21/167) | 24.1% (13/54) | 16.3% (59/362) |
| Nausea | 7.2% (12/167) | 59.3% (32/54) | 13.5% (49/362) |
| Non-purulent conjunctivitis | 19.2% (32/167) | 22.2% (12/54) | 0.6% (2/362) |
| Leukopenia | 25.7% (43/167) | 50.0% (27/54) | 4.7% (17/362) |
| Region | Zika Virus (ZIKV) | Flavivirus (FLAVI) | Malaria | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Negative | Positive | Total Examined (N) | 95% CI | OR | p-Value | Negative | Positive | Total Examined (N) | 95% CI | OR | p-Value | Negative | Positive | Total Examined (N) | 95% CI | OR | p-Value | |
| South | 79 (52.0%) | 119 (78.3%) | 33 (21.7%) | 152 (100%) | 1 | 0.85 | 139 (91.4%) | 13 (8.6%) | 152 (100%) | 1 | 0.18 | 50 (32.9%) | 102 (67.1%) | 152 (100%) | 1 | 0.67 | |||
| North | 99 (33.0%) | 245 (81.7%) | 55 (18.3%) | 300 (100%) | 0.65–1.41 | 1.0 | 287 (95.7%) | 13 (4.3%) | 300 (100%) | 0.32–1.24 | 0.6 | 99 (33.0%) | 201 (67.0%) | 300 (100%) | 0.68–1.28 | 0.9 | |||
| Central | 128 (30.5%) | 340 (81.1%) | 79 (18.9%) | 419 (100%) | 0.70–1.51 | 1.0 | 391 (93.3%) | 28 (6.7%) | 419 (100%) | 0.80–3.10 | 1.5 | 132 (31.5%) | 287 (68.5%) | 419 (100%) | 0.78–1.47 | 1.0 | |||
| Sex | |||||||||||||||||||
| Male | 90 (35.7%) | 200 (79.4%) | 52 (20.6%) | 252 (100%) | 1 | 0.48 | 240 (95.2%) | 12 (4.8%) | 252 (100%) | 1 | 0.00 | 147 (58.3%) | 105 (41.7%) | 252 (100%) | 1 | 0.00 | |||
| Female | 216 (34.9%) | 504 (81.4%) | 115 (18.6%) | 619 (100%) | 0.79–1.64 | 1.1 | 577 (93.2%) | 42 (6.8%) | 619 (100%) | 0.35–1.32 | 0.6 | 231 (37.3%) | 388 (62.7%) | 619 (100%) | 0.31–0.57 | 0.4 | |||
| Domicile | |||||||||||||||||||
| Urban | 197 (38.9%) | 412 (81.3%) | 95 (18.7%) | 507 (100%) | 1 | 0.99 | 483 (95.3%) | 24 (4.7%) | 507 (100%) | 1 | 0.26 | 60 (23.3%) | 198 (76.7%) | 258 (100%) | 1 | 0.00 | |||
| Rural | 78 (30.2%) | 207 (80.2%) | 51 (19.8%) | 258 (100%) | 0.56–1.75 | 1.0 | 239 (92.6%) | 19 (7.4%) | 258 (100%) | 0.57–2.79 | 1.3 | 203 (40.1%) | 304 (59.9%) | 507 (100%) | 0.01–0.13 | 0.4 | |||
| Slum | 31 (29.2%) | 85 (80.2%) | 21 (19.8%) | 106 (100%) | 0.56–1.76 | 1.0 | 95 (89.6%) | 11 (10.4%) | 106 (100%) | 0.35–1.72 | 0.8 | 3 (2.8%) | 103 (97.2%) | 106 (100%) | 7.17–73.2 | 22.9 | |||
| Pregnancy status | |||||||||||||||||||
| Pregnant | 88 (37.8%) | 191 (82.0%) | 42 (18.0%) | 233 (100%) | 1 | 0.60 | 220 (94.4%) | 13 (5.6%) | 233 (100%) | 1 | 0.64 | 135 (57.9%) | 98 (42.1%) | 233 (100%) | 1 | 0.14 | |||
| Nonpregnant | 218 (34.2%) | 513 (81.2%) | 125 (18.8%) | 638 (100%) | 0.61–1.32 | 0.9 | 597 (93.6%) | 41 (6.4%) | 638 (100%) | 0.45–1.63 | 0.8 | 334 (52.4%) | 304 (47.6%) | 638 (100%) | 0.58–1.07 | 0.8 | |||
| HIV status | |||||||||||||||||||
| HIV positive | 282 (56.5%) | 429 (86.0%) | 70 (14.0%) | 499 (100%) | 1 | 0.00 | 464 (93.0%) | 35 (7.0%) | 499 (100%) | 1 | 0.00 | 376 (75.4%) | 123 (24.6%) | 499 (100%) | 1 | 0.00 | |||
| HIV negative | 324 (87.1%) | 275 (73.9%) | 97 (26.1%) | 372 (100%) | 0.32–0.65 | 0.5 | 369 (99.2%) | 3 (0.8%) | 372 (100%) | 2.83–30.4 | 9.2 | 172 (46.2%) | 200 (53.8%) | 372 (100%) | 0.21–0.37 | 0.3 | |||
| Blood products | |||||||||||||||||||
| Outpatient serum | 287 (37.7%) | 663 (87.1%) | 98 (13.9%) | 761 (100%) | 1 | 0.00 | 606 (79.6%) | 155 (20.4%) | 761 (100%) | 1 | 0.00 | 372 (48.9%) | 389 (51.1%) | 761 (100%) | 1 | 0.00 | |||
| Blood bank serum | 19 (17.3%) | 41 (37.3%) | 69 (62.7%) | 110 (100%) | 0.05–0.13 | 0.1 | 68 (61.8%) | 42 (38.2%) | 110 (100%) | 0.27–0.63 | 0.4 | 110 (100%) | 0 (0.0%) | 110 (100%) | 14.3–373 | 231 | |||
| Grand Total (N) | 306 (35.1%) | 704 (80.8%) | 167 (19.2%) | 871 (100%) | 0.17–0.22 | 817 (93.8%) | 54 (6.2%) | 871 (100%) | 0.5–0.7 | 523 (60.0%) | 348 (40.0%) | 871 (100%) | 0.39–0.41 | ||||||
| Age (Years) | Zika Virus | Malaria | Flavivirus | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positives | Total Examined (N) | 95% CI | p-Value | Negative | Positive | Total Examined (N) | 95% CI | p-Value | Negative | Positive | Total Examined (N) | 95% CI | p-Value | |
| 0–9 | 3 (100%) | 0 (0.0%) | 3 (100%) | 0 | 0.00 | 1 (33.3%) | 2 (66.7%) | 3 (100%) | 0.65–0.67 | 0.03 | 3 (100%) | 0 (0.0%) | 3 (100%) | 0 | 0.05 |
| 10–19 | 40 (78.4%) | 11 (21.6%) | 51 (100%) | 0.20–0.22 | 14 (27.5%) | 37 (72.5%) | 51 (100%) | 0.71–0.73 | 50 (98.0%) | 1 (2.0%) | 51 (100%) | 0.1–0.3 | |||
| 20–29 | 167 (85.2%) | 29 (14.8%) | 196 (100%) | 0.13–0.15 | 35 (17.9%) | 161 (82.1%) | 196 (100%) | 0.81–0.83 | 188 (95.9%) | 8 (4.1%) | 196 (100%) | 0.3–0.5 | |||
| 30–39 | 253 (79.8%) | 64 (20.2%) | 317 (100%) | 0.19–0.21 | 219 (69.1%) | 98 (30.9%) | 317 (100%) | 0.29–0.31 | 294 (92.7%) | 23 (7.3%) | 317 (100%) | 0.6–0.8 | |||
| 40–49 | 154 (85.1%) | 27 (14.9%) | 181 (100%) | 0.13–0.15 | 152 (84.0%) | 29 (16.0%) | 181 (100%) | 0.15–0.17 | 167 (92.3%) | 14 (7.7%) | 181 (100%) | 0.6–0.8 | |||
| 50–59 | 55 (67.1%) | 27 (32.9%) | 82 (100%) | 0.31–0.33 | 77 (93.9%) | 5 (6.1%) | 82 (100%) 0 | 0.5–0.7 | 75 (91.5%) | 7 (8.5%) | 82 (100%) | 0.7–0.9 | |||
| 60–69 | 21 (77.8%) | 6 (22.2%) | 27 (100%) | 0.21–0.23 | 26 (96.3%) | 1 (3.7%) | 27 (100%) | 0.2–0.4 | 27 (100%) | 0 (0.0%) | 27 (100%) | 0 | |||
| 70–79 | 6 (75.0%) | 2 (25.0%) | 8 (100%) | 0.24–0.26 | 8 (100%) | 0 (0.0%) | 8 (100%) | 0 | 8 (100%) | 0 (0.0%) | 8 (100%) | 0 | |||
| 80+ | 5 (83.3%) | 1 (16.7%) | 6 (100%) | 0.15–0.17 | 6 (100%) | 0 (0.0%) | 6 (100%) | 0 | 5 (83.3%) | 1 (16.7%) | 6 (100%) | 0.15–0.17 | |||
| Grand Total (N) | 704 (80.8%) | 167 (19.2%) | 871 (100%) | 0.18–0.20 | 538 (61.8%) | 333 (38.2%) | 871 (100%) | 0.37–0.40 | 817 (93.8%) | 54 (6.2%) | 871 (100%) | 0.5–0.7 | |||
| Region | Zika and Flavivirus Cocirculation | Flavivirus-Malaria Cocirculation | Zika-Malaria Cocirculation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Total Examined (N) | 95% CI | OR | p-Value | Negative | Positive | Total Examined (N) | 95% CI | OR | p-Value | Negative | Positive | Total Examined (N) | 95% CI | OR | p-Value | |
| South | 141 (92.8%) | 11 (7.2%) | 152 (100%) | 1 | 0.14 | 146 (96.1%) | 6 (3.9%) | 152 (100%) | 1 | 0.24 | 141 (92.8%) | 11 (7.2%) | 152 (100%) | 1 | 0.14 | |||
| North | 294 (98.0%) | 6 (2.0%) | 300 (100%) | 0.70–11.4 | 2.8 | 297 (99.0%) | 3 (1.0%) | 300 (100%) | 0.12–1.71 | 0.3 | 294 (98.0%) | 6 (2.0%) | 300 (100%) | 0.70–11.4 | 2.8 | |||
| Central | 416 (99.3%) | 3 (0.7%) | 419 (100%) | 0.08–1.42 | 0.3 | 410 (97.9%) | 9 (2.1%) | 419 (100%) | 0.58–8.09 | 2.2 | 416 (99.3%) | 3 (0.7%) | 419 (100%) | 0.08–1.42 | 0.3 | |||
| Sex | ||||||||||||||||||
| Male | 243 (96.4%) | 9 (3.6%) | 252 (100%) | 1 | 0.27 | 246 (97.6%) | 6 (2.4%) | 252 (100%) | 1 | 0.01 | 243 (96.4%) | 9 (3.6%) | 252 (100%) | 1 | 0.28 | |||
| Female | 605 (97.7%) | 14 (2.3%) | 619 (100%) | 0.68–3.74 | 1.6 | 617 (99.7%) | 2 (0.3%) | 619 (100%) | 1.50–37.5 | 7.5 | 605 (97.7%) | 14 (2.3%) | 619 (100%) | 0.67–3.72 | 1.5 | |||
| Domicile | ||||||||||||||||||
| Urban | 495 (97.6%) | 12 (2.4%) | 507 (100%) | 1 | 0.00 | 499 (98.4%) | 8 (1.6%) | 507 (100%) | 1 | 0.00 | 495 (97.6%) | 12 (2.4%) | 507 (100%) | 1 | 0.00 | |||
| Rural | 254 (98.4%) | 4 (1.6%) | 258 (100%) | 0.03–0.32 | 0.1 | 254 (98.4%) | 4 (1.6%) | 258 (100%) | 0.03–0.39 | 0.1 | 254 (98.4%) | 4 (1.6%) | 258 (100%) | 0.03–0.32 | 0.1 | |||
| Slum | 92 (86.8%) | 14 (13.2%) | 106 (100%) | 3.10–30.1 | 9.6 | 94 (88.7%) | 12 (11.3%) | 106 (100%) | 2.55–25.7 | 8.1 | 92 (86.8%) | 14 (13.2%) | 106 (100%) | 3.10–30.1 | 9.6 | |||
| Pregnancy status | ||||||||||||||||||
| Pregnant | 217 (93.1%) | 16 (6.9%) | 233 (100%) | 1 | 0.00 | 230 (98.7%) | 3 (1.3%) | 233 (100%) | 1 | 0.49 | 217 (93.1%) | 16 (6.9%) | 233 (100%) | 1 | 0.00 | |||
| Nonpregnant | 634 (99.4%) | 4 (0.6%) | 638 (100%) | 3.86–35.3 | 11.6 | 633 (99.2%) | 5 (0.8%) | 638 (100%) | 0.39–6.96 | 1.7 | 634 (99.4%) | 4 (0.6%) | 638 (100%) | 3.86–35.3 | 11.6 | |||
| HIV status | ||||||||||||||||||
| HIV positive | 484 (97.0%) | 15 (3.0%) | 499 (100%) | 1 | 0.19 | 492 (98.6%) | 7 (1.4%) | 499 (100%) | 1 | 0.11 | 484 (97.0%) | 15 (3.0%) | 499 (100%) | 1 | 0.19 | |||
| HIV negative | 366 (98.4%) | 6 (1.6%) | 372 (100%) | 0.72–4.91 | 1.9 | 361 (97.0%) | 11 (3.0%) | 372 (100%) | 0.17–1.21 | 0.5 | 366 (98.4%) | 6 (1.6%) | 372 (100%) | 0.72–4.91 | 1.9 | |||
| Blood product source | ||||||||||||||||||
| Outpatient serum | 756 (99.3%) | 5 (0.7%) | 761 (100%) | 1 | 0.00 | 749 (98.4%) | 12 (1.6%) | 761 (100%) | 1 | 0.36 | 756 (99.3%) | 5 (0.7%) | 761 (100%) | 1 | 0.00 | |||
| Blood bank serum | 95 (86.4%) | 15 (13.6%) | 110 (100%) | 0.01–0.11 | 0.4 | 110 (100%) | 0 (0.0%) | 110 (100%) | 0.21–62.6 | 3.6 | 95 (86.4%) | 15 (13.6%) | 110 (100%) | 0.01–0.11 | 0.0 | |||
| Grand Total (N) | 718 (82.5%) | 153 (17.5%) | 871 (100%) | 0.16–0.18 | 852 (97.8(%) | 19 (2.2%) | 871 (100%) | 834 (95.7%) | 37 (4.3%) | 871 (100%) | 0.3–0.5 | |||||||
| Age (Years) | Zika-Flavivirus | Flavivirus-Malaria | Zika-Malaria | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Total Examined (N) | 95% CI | p-Value | Negative | Positive | Total Examined (N) | 95% CI | p-Value | Negative | Positive | Total Examined (N) | 95% CI | p-Value | ||
| 0–9 | 3 (100%) | 0 (0.0%) | 3 (100%) | 0 | 0.15 | 3 (100%) | 0 (0.0%) | 3 (100%) | 0 | 0.02 | 3 (%) | 1 (0.0%) | 3 (100%) | 0 | 0.05 | |
| 10–19 | 51 (100%) | 0 (0.0%) | 51 (100%) | 0 | 50 (98.0%) | 1 (2.0%) | 51 (100%) | 0.1–0.3 | 46 (90.2%) | 5 (9.8%) | 51 (100%) | 0.8–0.10 | ||||
| 20–29 | 195 (99.5%) | 1 (0.5%) | 196 (100%) | -0–0.1 | 193 (98.5%) | 3 (1.5%) | 196 (100%) | 0.0–0.2 | 195 (96.4%) | 7 (3.6%) | 196 (100%) | 0.2–0.4 | ||||
| 30–39 | 306 (96.5%) | 11 (3.5%) | 317 (100%) | 0.2–0.4 | 312 (98.4%) | 5 (1.6%) | 317 (100%) | 0.0–0.2 | 315 (96.8%) | 10 (3.2%) | 317 (100%) | 0.2–0.4 | ||||
| 40–49 | 171 (94.5%) | 10 (5.5%) | 181 (100%) | 0.4–0.6 | 178 (98.3%) | 3 (1.7%) | 181 (100%) | 0.0–0.2 | 180 (95.0%) | 9 (5.0%) | 181 (100%) | 0.4–0.6 | ||||
| 50–59 | 73 (89.0%) | 9 (11.0%) | 82 (100%) | 0.10–0.12 | 80 (98.8%) | 1 (1.2%) | 82 (100%) | 0.0–0.2 | 81 (95.1%) | 4 (4.9%) | 82 (100%) | 0.3–0.5 | ||||
| 60–69 | 24 (88.9%) | 3 (11.1%) | 27 (100%) | 0.10–0.12 | 26 (96.3%) | 1 (3.7%) | 27 (100%) | 0.2–0.4 | 25 (%) | 2 (7.4%) | 27 (100%) | 0.6–0.8 | ||||
| 70–79 | 6 (75.0%) | 2 (25.0%) | 8 (100%) | 0.24–0.26 | 8 (100%) | 0 (0.0%) | 8 (100%) | 0 | 0 (%) | 0 (0.0%) | 8 (100%) | 0 | ||||
| 80+ | 5 (83.3%) | 1 (16.7%) | 6 (100%) | 0.15–0.17 | 6 (100%) | 0 (0.0%) | 6 (100%) | 0 | 0 (%) | 0 (0.0%) | 6 (100%) | 0 | ||||
| Grand Total (N) | 834 (95.8%) | 37 (4.2%) | 871 (100%) | 0.2–0.5 | 857 (98.4%) | 14 (1.6%) | 871 (100%) | 0.0–0.2 | 833 (95.6%) | 38 (4.4%) | 871 (100%) | 0.3–0.5 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mac, P.A.; Kroeger, A.; Daehne, T.; Anyaike, C.; Velayudhan, R.; Panning, M. Zika, Flavivirus and Malaria Antibody Cocirculation in Nigeria. Trop. Med. Infect. Dis. 2023, 8, 171. https://doi.org/10.3390/tropicalmed8030171
Mac PA, Kroeger A, Daehne T, Anyaike C, Velayudhan R, Panning M. Zika, Flavivirus and Malaria Antibody Cocirculation in Nigeria. Tropical Medicine and Infectious Disease. 2023; 8(3):171. https://doi.org/10.3390/tropicalmed8030171
Chicago/Turabian StyleMac, Peter Asaga, Axel Kroeger, Theo Daehne, Chukwuma Anyaike, Raman Velayudhan, and Marcus Panning. 2023. "Zika, Flavivirus and Malaria Antibody Cocirculation in Nigeria" Tropical Medicine and Infectious Disease 8, no. 3: 171. https://doi.org/10.3390/tropicalmed8030171
APA StyleMac, P. A., Kroeger, A., Daehne, T., Anyaike, C., Velayudhan, R., & Panning, M. (2023). Zika, Flavivirus and Malaria Antibody Cocirculation in Nigeria. Tropical Medicine and Infectious Disease, 8(3), 171. https://doi.org/10.3390/tropicalmed8030171






