Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections
Abstract
1. Introduction
2. Epidemiology
3. Molecular Mechanisms of Antibiotic Resistance
4. Rescue Therapy
5. Antimicrobial Susceptibility Testing
6. Novel Treatment Options
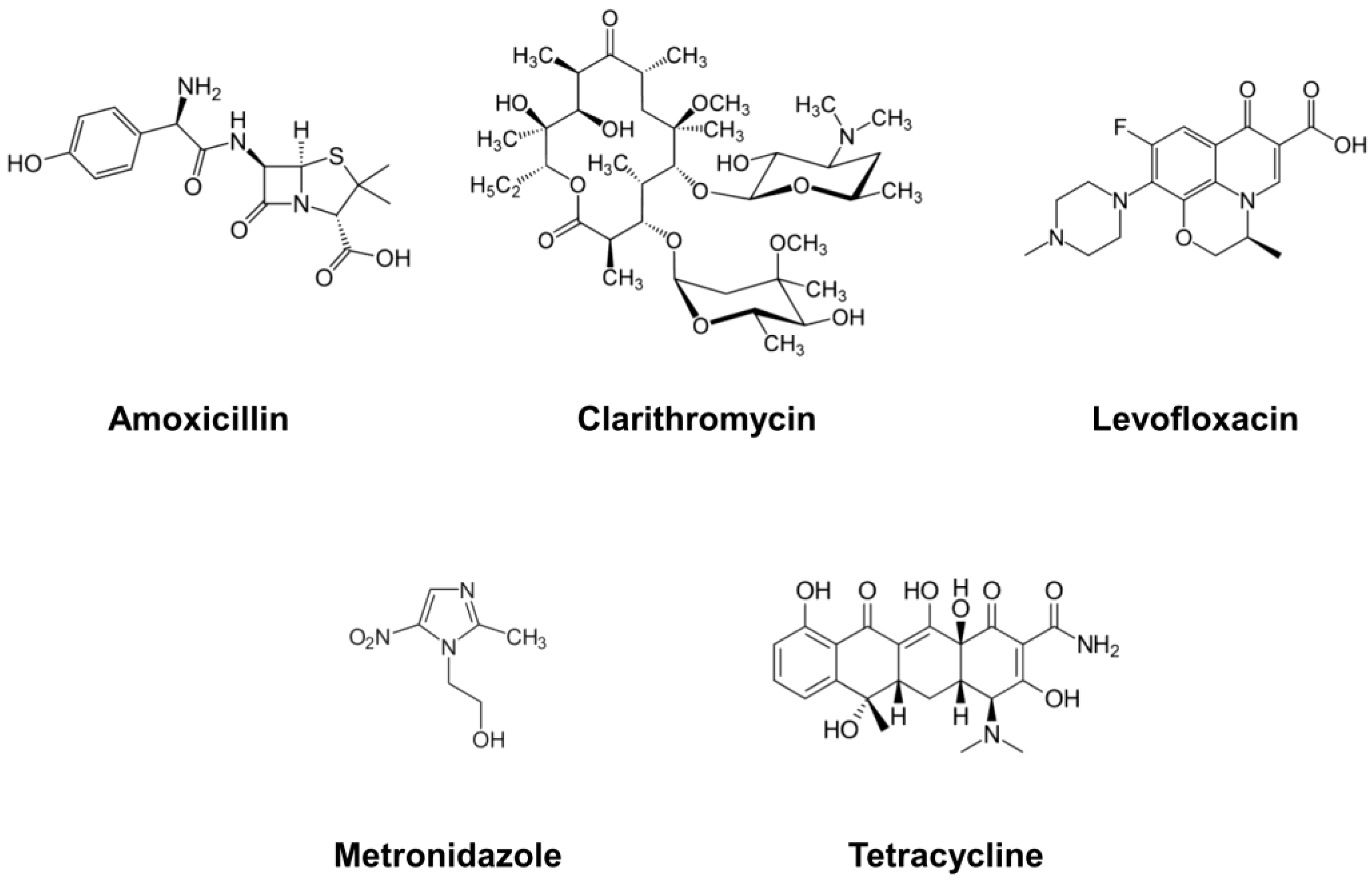
| Substance | Type | Mechanism of Action | Usage in Treatment | References |
|---|---|---|---|---|
| Amoxicillin | Antibiotic | Inhibition of cell wall biosynthesis | Conventional | [26,54,77] |
| Clarithromycin | Antibiotic | Inhibition of bacterial protein synthesis | Conventional | [28,35,60] |
| Levofloxacin | Antibiotic | Inhibition of bacterial DNA synthesis | Conventional | [39,78,79] |
| Metronidazole | Antibiotic | Inhibition of protein synthesis via DNA structure and strand breakage | Conventional | [35,52,72] |
| Tetracycline | Antibiotic | Inhibition of protein synthesis via the inhibition of mRNA-ribosome complex formation | Conventional | [75,80,81] |
| Rifabutin | Antibiotic | Inhibition of bacterial RNA polymerization | Alternative, Combination | [47,48,49] |
| Doxycycline | Antibiotic | Inhibition of protein synthesis via the inhibition of mRNA-ribosome complex formation | Alternative, Combination | [50,77,78] |
| Nitazoxanide | Antibiotic | Interfering with anaerobic energy metabolism | Alternative | [51,52,53] |
| Furazolidone | Antibiotic | Inhibition of protein synthesis via DNA cross-linkage | Clarithromycin-, Metronidazole-resistant | [54,73,74] |
| Armeniaspirol A | Antibiotic | Disruption of the bacterial cell membrane, inhibition of biofilm formation | Novel Alternative | [68,69,82] |
| Dihydrotanshinone I | Phytochemical | Elimination of preformed biofilm | Novel Alternative | [70,83] |
| Chrysin | Phytochemical | Interfering with cell wall formation, vesicle formation, and cell lysis | Novel Alternative | [71,84,85] |
| Galangin | Phytochemical | Interfering with cell wall formation, vesicle formation, and cell lysis | Novel Alternative | [71,84,85] |
| Curcumin | Phytochemical | Inhibition of vacuolation via binding to the virulence factor | Novel Alternative | [86,87,88] |
| Pexiganan | Peptide | Binding to the bacterial membrane, forming a toroidal pore | Novel Alternative | [89,90,91] |
| Tilapia Piscidins | Peptide | Induction of membrane micelle formation | Novel Alternative | [90,92] |
| Epinecidin-1 | Peptide | Generation of membrane curvature, vascularization, and pore formation | Novel Alternative | [90,92,93] |
| Cathelicidins | Peptide | Shrinking of the flagella and pore formation on bacterial membrane | Novel Alternative | [90,94] |
| Defensins | Peptide | Permeabilization of bacterial cell membrane | Novel Alternative | [90,95,96] |
| Bicarinalin | Peptide | Permeabilization of bacterial cell membrane | Novel Alternative | [90,97] |
| Odorranain-HP | Peptide | Unclear | Novel Alternative | [90,98] |
| PGLa-AM1 | Peptide | Binding to the bacterial cell membrane | Novel Alternative | [90,99] |
| Bacteriocins | Peptide | Binding to the bacterial cell membrane, pore formation | Novel Alternative | [90,100,101] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Roesler, B.M.; Rabelo-Gonçalves, E.M.; Zeitune, J.M. Virulence factors of Helicobacter pylori: A review. Clin. Med. Insights Gastroenterol. 2014, 7, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Salih, B.A. Helicobacter pylori infection in developing countries: The burden for how long? Saudi J. Gastroenterol. 2009, 15, 201–207. [Google Scholar] [CrossRef]
- Jafar, S.; Jalil, A.; Soheila, N.; Sirous, S. Prevalence of helicobacter pylori infection in children, a population-based cross-sectional study in west iran. Iran J. Pediatr. 2013, 23, 13–18. [Google Scholar]
- Quach, D.T.; Vilaichone, R.K.; Vu, K.V.; Yamaoka, Y.; Sugano, K.; Mahachai, V. Helicobacter pylori infection and related gastrointestinal diseases in southeast Asian countries: An expert opinion survey. Asian Pac. J. Cancer Prev. 2018, 19, 3565–3569. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Y.; Lu, N.H. Recent progress in Helicobacter pylori treatment. Chin. Med. J. 2020, 133, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Long, X.; Chen, Q.; Yu, L.; Liang, X.; Liu, W.; Lu, H. Bismuth improves efficacy of proton-pump inhibitor clarithromycin, metronidazole triple Helicobacter pylori therapy despite a high prevalence of antimicrobial resistance. Helicobacter 2018, 23, e12485. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Kim, S.Y.; Chung, J.W. Best Helicobacter pylori eradication strategy in the era of antibiotic resistance. Antibiotics 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.T.; Liou, J.M.; El-Omar, E.M.; Wu, J.Y.; Leow, A.H.R.; Goh, K.L.; Das, R.; Lu, H.; Lin, J.T.; Tu, Y.K.; et al. Primary antibiotic resistance in Helicobacter pylori in the Asia-Pacific region: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 707–715. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Current and emerging methods of antibiotic susceptibility testing. Diagnostics 2019, 9, 49. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 11.0; European Committee on Antimicrobial Susceptibility Testing: Basel, Switzerland, 2021. [Google Scholar]
- De Francesco, V.; Giorgio, F.; Hassan, C.; Manes, G.; Vannella, L.; Panella, C.; Ierardi, E.; Zullo, A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointestin. Liver. Dis. 2010, 19, 409–414. [Google Scholar] [PubMed]
- Ghotaslou, R.; Leylabadlo, H.E.; Asl, Y.M. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J. Methodol. 2015, 5, 164–174. [Google Scholar] [CrossRef]
- Vilaichone, R.K.; Quach, D.T.; Yamaoka, Y.; Sugano, K.; Mahachai, V. Prevalence and pattern of antibiotic resistant strains of Helicobacter pylori infection in ASEAN. Asian Pac. J. Cancer Prev. 2018, 19, 1411–1413. [Google Scholar] [CrossRef]
- Shoosanglertwijit, R.; Kamrat, N.; Werawatganon, D.; Chatsuwan, T.; Chaithongrat, S.; Rerknimitr, R. Real-world data of Helicobacter pylori prevalence, eradication regimens, and antibiotic resistance in Thailand, 2013–2018. JGH Open 2020, 4, 49–53. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, M.; Lu, B.; Dai, J. Helicobacter pylori and antibiotic resistance, a continuing and intractable problem. Helicobacter 2016, 21, 349–363. [Google Scholar] [CrossRef]
- Hirschl, A.M.; Rotter, M.L. Amoxicillin for the treatment of Helicobacter pylori infection. J. Gastroenterol. 1996, 31 (Suppl. S9), 44–47. [Google Scholar]
- Nishizawa, T.; Suzuki, H.; Tsugawa, H.; Muraoka, H.; Matsuzaki, J.; Hirata, K.; Ikeda, F.; Takahashi, M.; Hibi, T. Enhancement of amoxicillin resistance after unsuccessful Helicobacter pylori eradication. Antimicrob. Agents Chemother. 2013, 57, 1106. [Google Scholar] [CrossRef]
- Chen, M.-J.; Wu, M.-S.; Chen, C.-C.; Chen, C.-C.; Fang, Y.-J.; Bair, M.-J.; Chang, C.-Y.; Lee, J.-Y.; Hsu, W.-F.; Luo, J.-C.; et al. Impact of amoxicillin resistance on the efficacy of amoxicillin-containing regimens for Helicobacter pylori eradication: Analysis of five randomized trials. J. Antimicrob. Chemother. 2017, 72, 3481–3489. [Google Scholar] [CrossRef]
- Cui, R.; Song, Z.; Suo, B.; Tian, X.; Xue, Y.; Meng, L.; Niu, Z.; Jin, Z.; Zhang, H.; Zhou, L. Correlation analysis among genotype resistance, phenotype resistance and eradication effect of Helicobacter pylori. Infect. Drug Resist. 2021, 14, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Albasha, A.M.; Elnosh, M.M.; Osman, E.H.; Zeinalabdin, D.M.; Fadl, A.A.M.; Ali, M.A.; Altayb, H.N. Helicobacter pylori 23S rRNA gene A2142G, A2143G, T2182C, and C2195T mutations associated with clarithromycin resistance detected in Sudanese patients. BMC Microbiol 2021, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.H.; Ha, T.M.T.; Le, P.T.Q.; Nguyen, V.N.; Phan, T.N.; Paglietti, B. Helicobacter pylori 23S rRNA gene mutations associated with clarithromycin resistance in chronic gastritis in Vietnam. J. Infect. Dev. Ctries 2018, 12, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Shetty, V.; Lamichhane, B.; Tay, C.Y.; Pai, G.C.; Lingadakai, R.; Balaraju, G.; Shetty, S.; Ballal, M.; Chua, E.G. High primary resistance to metronidazole and levofloxacin, and a moderate resistance to clarithromycin in Helicobacter pylori isolated from Karnataka patients. Gut. Pathog. 2019, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- López-Gasca, M.; Peña, J.; García-Amado, M.A.; Michelangeli, F.; Contreras, M. Point mutations at gyrA and gyrB genes of levofloxacin-resistant Helicobacter pylori isolates in the esophageal mucosa from a Venezuelan population. Am. J. Trop. Med. Hyg. 2018, 98, 1051–1055. [Google Scholar] [CrossRef]
- Rimbara, E.; Noguchi, N.; Kawai, T.; Sasatsu, M. Fluoroquinolone resistance in Helicobacter pylori: Role of mutations at position 87 and 91 of GyrA on the level of resistance and identification of a resistance conferring mutation in GyrB. Helicobacter 2012, 17, 36–42. [Google Scholar] [CrossRef]
- Chandan, V.; Logan, S.M.; Harrison, B.A.; Vinogradov, E.; Aubry, A.; Stupak, J.; Li, J.; Altman, E. Characterization of a waaF mutant of Helicobacter pylori strain 26,695 provides evidence that an extended lipopolysaccharide structure has a limited role in the invasion of gastric cancer cells. Biochem. Cell Biol. 2007, 85, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, X.; Wen, Y.; Chen, H.; She, F. A newly discovered drug resistance gene rfaF in Helicobacter pylori. Infect. Drug Resist. 2019, 12, 3507–3514. [Google Scholar] [CrossRef]
- Hashemi, S.J.; Sheikh, A.F.; Goodarzi, H.; Yadyad, M.J.; Seyedian, S.S.; Aslani, S.; Assarzadegan, M.A. Genetic basis for metronidazole and clarithromycin resistance in Helicobacter pylori strains isolated from patients with gastroduodenal disorders. Infect. Drug Resist. 2019, 12, 535–543. [Google Scholar] [CrossRef]
- Jenks, P.J.; Edwards, D.I. Metronidazole resistance in Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 19, 1–7. [Google Scholar] [CrossRef]
- Martínez-Júlvez, M.; Rojas, A.L.; Olekhnovich, I.; Espinosa Angarica, V.; Hoffman, P.S.; Sancho, J. Structure of RdxA—An oxygen-insensitive nitroreductase essential for metronidazole activation in Helicobacter pylori. Febs. J. 2012, 279, 4306–4317. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joo, Y.M.; Lee, H.S.; Chung, I.S.; Yoo, Y.J.; Merrell, D.S.; Cha, J.H. Genetic analysis of Helicobacter pylori clinical isolates suggests resistance to metronidazole can occur without the loss of functional rdxA. J. Antibiot. 2009, 62, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Carothers, J.J.; Bruce, M.G.; Hennessy, T.W.; Bensler, M.; Morris, J.M.; Reasonover, A.L.; Hurlburt, D.A.; Parkinson, A.J.; Coleman, J.M.; McMahon, B.J. The relationship between previous fluoroquinolone use and levofloxacin resistance in Helicobacter pylori infection. Clin. Infect. Dis. 2007, 44, e5–e8. [Google Scholar] [CrossRef]
- Shetty, V.; Lingadakai, R.; Pai, G.C.; Ballal, M. Profile of Helicobacter pylori cagA &vacA genotypes and its association with the spectrum of gastroduodenal disease. Indian. J. Med. Microbiol. 2021, 39, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Taneike, I.; Nami, A.; O’Connor, A.; Fitzgerald, N.; Murphy, P.; Qasim, A.; O’Connor, H.; O’Morain, C. Analysis of drug resistance and virulence-factor genotype of Irish Helicobacter pylori strains: Is there any relationship between resistance to metronidazole and cagA status? Aliment Pharmacol. Ther. 2009, 30, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Linn, A.K.; Javadi, M.B.; Al-Gubare, S.; Ali, N.; Katzenmeier, G. Vacuolating cytotoxin A (VacA)—A multi-talented pore-forming toxin from Helicobacter pylori. Toxicon 2016, 118, 27–35. [Google Scholar] [CrossRef]
- Boyanova, L.; Markovska, R.; Yordanov, D.; Gergova, G.; Mitov, I. Clarithromycin resistance mutations in Helicobacter pylori in association with virulence factors and antibiotic susceptibility of the strains. Microb. Drug Resist. 2016, 22, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Karabiber, H.; Selimoglu, M.A.; Otlu, B.; Yildirim, O.; Ozer, A. Virulence factors and antibiotic resistance in children with Helicobacter pylori gastritis. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 608–612. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Q.; Yuan, Y.; Gong, Y. The antibiotic resistance of Helicobacter pylori to five antibiotics and influencing factors in an area of China with a high risk of gastric cancer. BMC Microbiol. 2019, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Guevara, B.; Cogdill, A.G. Helicobacter pylori: A review of current diagnostic and management strategies. Dig. Dis. Sci. 2020, 65, 1917–1931. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Canaan, Y.; Maher, J.; Wiener, G.; Hulten, K.G.; Kalfus, I.N. Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication: A double-blind, randomized, controlled trial. Ann. Intern. Med. 2020, 172, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, T.; Suzuki, H.; Matsuzaki, J.; Muraoka, H.; Tsugawa, H.; Hirata, K.; Hibi, T. Helicobacter pylori resistance to rifabutin in the last 7 years. Antimicrob. Agents Chemother. 2011, 55, 5374–5375. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Fagoonee, S.; Astegiano, M.; Durazzo, M.; Morgando, A.; Sprujevnik, T.; Giordanino, C.; Baronio, M.; De Angelis, C.; Saracco, G.M.; et al. Rifabutin-based rescue therapy for Helicobacter pylori eradication: A long-term prospective study in a large cohort of difficult-to-treat patients. J. Clin. Med. 2019, 8, 199. [Google Scholar] [CrossRef]
- Basu, P.P.; Rayapudi, K.; Pacana, T.; Shah, N.J.; Krishnaswamy, N.; Flynn, M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am. J. Gastroenterol. 2011, 106, 1970–1975. [Google Scholar] [CrossRef]
- Mégraud, F.; Occhialini, A.; Rossignol, J.F. Nitazoxanide, a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob. Agents Chemother. 1998, 42, 2836–2840. [Google Scholar] [CrossRef]
- Sisson, G.; Goodwin, A.; Raudonikiene, A.; Hughes, N.J.; Mukhopadhyay, A.K.; Berg, D.E.; Hoffman, P.S. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 2002, 46, 2116–2123. [Google Scholar] [CrossRef]
- Guttner, Y.; Windsor, H.M.; Viiala, C.H.; Dusci, L.; Marshall, B.J. Nitazoxanide in treatment of Helicobacter pylori: A clinical and in vitro study. Antimicrob. Agents Chemother. 2003, 47, 3780–3783. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.W.; Hu, W.L.; Cai, Y.; Zheng, W.F.; Du, Q.; Kim, J.J.; Kao, J.Y.; Dai, N.; Si, J.M. Outcomes of furazolidone- and amoxicillin-based quadruple therapy for Helicobacter pylori infection and predictors of failed eradication. World J. Gastroenterol. 2018, 24, 4596–4605. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Dore, M.P. Update on the use of vonoprazan: A competitive acid blocker. Gastroenterology 2018, 154, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, H.; Ando, K.; Sato, K.; Ito, T.; Goto, M.; Sato, T.; Fujinaga, A.; Kawamoto, T.; Utsumi, T.; Yanagawa, N.; et al. Efficacy of vonoprazan-based triple therapy for Helicobacter pylori eradication: A multicenter study and a review of the literature. Dig. Dis. Sci. 2017, 62, 3069–3076. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, N.; Nam, R.H.; Lee, S.M.; Kwon, Y.H.; Sohn, S.D.; Kim, J.M.; Lee, D.H.; Jung, H.C. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter 2019, 24, e12561. [Google Scholar] [CrossRef]
- Klesiewicz, K.; Nowak, P.; Karczewska, E.; Skiba, I.; Wojtas-Bonior, I.; Sito, E.; Budak, A. PCR-RFLP detection of point mutations A2143G and A2142G in 23S rRNA gene conferring resistance to clarithromycin in Helicobacter pylori strains. Acta Biochim. Pol. 2014, 61, 311–315. [Google Scholar] [CrossRef]
- Sun, L.; Talarico, S.; Yao, L.; He, L.; Self, S.; You, Y.; Zhang, H.; Zhang, Y.; Guo, Y.; Liu, G.; et al. Droplet digital PCR-based detection of clarithromycin resistance in Helicobacter pylori isolates reveals frequent heteroresistance. J. Clin. Microbiol. 2018, 56, e00019-18. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shen, W.X.; Chen, C.F.; Sheng, H.H.; Cheng, H.; Li, J.; Hu, F.; Lu, D.R.; Gao, H.J. Detection of the clarithromycin resistance of Helicobacter pylori in gastric mucosa by the amplification refractory mutation system combined with quantitative real-time PCR. Cancer Med. 2019, 8, 1633–1640. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Urruzuno, P.; Barrio, J.; Martinez, M.J.; Agudo, S.; Somodevilla, A.; Llorca, L.; Alarcón, T. Detection of Helicobacter pylori and the genotypes of resistance to clarithromycin and the heterogeneous genotype to this antibiotic in biopsies obtained from symptomatic children. Diagn. Microbiol. Infect. Dis. 2017, 87, 150–153. [Google Scholar] [CrossRef]
- Pastukh, N.; Binyamin, D.; On, A.; Paritsky, M.; Peretz, A. GenoType® HelicoDR test in comparison with histology and culture for Helicobacter pylori detection and identification of resistance mutations to clarithromycin and fluoroquinolones. Helicobacter 2017, 22, e12447. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Jeon, S.W.; Nam, S.Y.; Lee, H.S.; Park, J.H. Efficacy of tailored therapy for Helicobacter pylori eradication based on clarithromycin resistance and survey of previous antibiotic exposure: A single-center prospective pilot study. Helicobacter 2019, 24, e12585. [Google Scholar] [CrossRef]
- Liou, J.M.; Chen, C.C.; Chang, C.Y.; Chen, M.J.; Fang, Y.J.; Lee, J.Y.; Chen, C.C.; Hsu, S.J.; Hsu, Y.C.; Tseng, C.H.; et al. Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: A multicentre clinical trial. J. Antimicrob. Chemother. 2013, 68, 450–456. [Google Scholar] [CrossRef]
- Romano, M.; Marmo, R.; Cuomo, A.; De Simone, T.; Mucherino, C.; Iovene, M.R.; Montella, F.; Tufano, M.A.; Del Vecchio Blanco, C.; Nardone, G. Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin. Gastroenterol. Hepatol. 2003, 1, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Beckman, E.; Saracino, I.; Fiorini, G.; Clark, C.; Slepnev, V.; Patel, D.; Gomez, C.; Ponaka, R.; Elagin, V.; Vaira, D. A novel stool pcr test for Helicobacter pylori may predict clarithromycin resistance and eradication of infection at a high rate. J. Clin. Microbiol. 2017, 55, 2400–2405. [Google Scholar] [CrossRef]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, C.; Liu, Y.; Huang, Y.; Bai, Y.; Hang, X.; Zeng, L.; Zhu, D.; Bi, H. Armeniaspirol A: A novel anti-Helicobacter pylori agent. Microb. Biotechnol. 2021, 15, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xie, F.; Hoffmann, J.; Wang, Q.; Bauer, A.; Brönstrup, M.; Mahmud, T.; Müller, R. Armeniaspirol antibiotic biosynthesis: Chlorination and oxidative dechlorination steps affording Spiro[4.4]non-8-ene. Chembiochem 2019, 20, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Huang, Y.; Hang, X.; Tong, Q.; Zeng, L.; Jia, J.; Zhang, G.; Bi, H. Dihydrotanshinone I is effective against drug-resistant Helicobacter pylori in vitro and in vivo. Antimicrob. Agents Chemother. 2021, 65, e01921-20. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Salillas, S.; Velázquez-Campoy, A.; Espinosa Angarica, V.; Fillat, M.F.; Sancho, J.; Lanas, Á. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Sci. Rep. 2019, 9, 11294. [Google Scholar] [CrossRef]
- Olekhnovich, I.N.; Vitko, S.; Valliere, M.; Hoffman, P.S. Response to metronidazole and oxidative stress is mediated through homeostatic regulator HsrA (HP1043) in Helicobacter pylori. J. Bacteriol. 2014, 196, 729–739. [Google Scholar] [CrossRef]
- Kwon, D.H.; Lee, M.; Kim, J.J.; Kim, J.G.; El-Zaatari, F.A.; Osato, M.S.; Graham, D.Y. Furazolidone- and nitrofurantoin-resistant Helicobacter pylori: Prevalence and role of genes involved in metronidazole resistance. Antimicrob. Agents Chemother. 2001, 45, 306–308. [Google Scholar] [CrossRef] [PubMed]
- National Center for Advancing Translational Sciences. Inxight Drugs; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013.
- Wang, J.; Cao, Y.; He, W.; Li, X. Efficacy and safety of bismuth quadruple regimens containing tetracycline or furazolidone for initial eradication of Helicobacter pylori. Medicine 2021, 100, e28323. [Google Scholar] [CrossRef] [PubMed]
- Salillas, S.; Alías, M.; Michel, V.; Mahía, A.; Lucía, A.; Rodrigues, L.; Bueno, J.; Galano-Frutos, J.J.; De Reuse, H.; Velázquez-Campoy, A.; et al. Design, synthesis, and efficacy testing of nitroethylene- and 7-nitrobenzoxadiazol-based flavodoxin inhibitors against Helicobacter pylori drug-resistant clinical strains and in Helicobacter pylori-infected mice. J. Med. Chem. 2019, 62, 6102–6115. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Xu, C.; Liu, X.; Wu, H.; Xie, X.; Liu, P.; Li, H.; Zhang, G.; Xu, M.; Li, C.; et al. A comparison of doxycycline and amoxicillin containing quadruple eradication therapy for treating helicobacter pylori-infected duodenal ulcers: A multicenter, opened, randomized controlled trial in china. Pathogens 2022, 11, 1549. [Google Scholar] [CrossRef] [PubMed]
- Alhalabi, M.; Alassi, M.W.; Alaa Eddin, K.; Cheha, K. Efficacy of two-week therapy with doxycycline-based quadruple regimen versus levofloxacin concomitant regimen for helicobacter pylori infection: A prospective single-center randomized controlled trial. BMC Infect. Dis. 2021, 21, 642. [Google Scholar] [CrossRef] [PubMed]
- Ogata, S.K.; Godoy, A.P.; da Silva Patricio, F.R.; Kawakami, E. High Helicobacter pylori resistance to metronidazole and clarithromycin in Brazilian children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 645–648. [Google Scholar] [CrossRef]
- Hsu, P.I.; Tsay, F.W.; Kao, J.Y.; Peng, N.J.; Chen, Y.H.; Tang, S.Y.; Kuo, C.H.; Kao, S.S.; Wang, H.M.; Wu, I.T.; et al. Tetracycline-levofloxacin versus amoxicillin-levofloxacin quadruple therapies in the second-line treatment of Helicobacter pylori infection. Helicobacter 2021, 26, e12840. [Google Scholar] [CrossRef]
- Siavoshi, F.; Saniee, P.; Latifi-Navid, S.; Massarrat, S.; Sheykholeslami, A. Increase in resistance rates of H. pylori isolates to metronidazole and tetracycline--comparison of three 3-year studies. Arch. Iran Med. 2010, 13, 177–187. [Google Scholar]
- Miri, A.H.; Kamankesh, M.; Llopis-Lorente, A.; Liu, C.; Wacker, M.G.; Haririan, I.; Asadzadeh Aghdaei, H.; Hamblin, M.R.; Yadegar, A.; Rad-Malekshahi, M.; et al. The potential use of antibiotics against Helicobacter pylori infection: Biopharmaceutical implications. Front. Pharmacol. 2022, 13, 917184. [Google Scholar] [CrossRef]
- Yu, Y.F.; Zhou, M.L.; Wang, Y.P.; Zhu, Y. Mechanism of dihydrotanshinone I in the treatment of helicobacter pylori infection based on network pharmacology and molecular docking technology. Med. Data Min. 2022, 5, 9. [Google Scholar] [CrossRef]
- Romero, M.; Freire, J.; Pastene, E.; García, A.; Aranda, M.; González, C. Propolis polyphenolic compounds affect the viability and structure of Helicobacter pylori in vitro. Rev. Bras. Farmacogn. 2019, 29, 325–332. [Google Scholar] [CrossRef]
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Bozhadze, A.; Jokhadze, M.; Korona-Głowniak, I. Correlation between chemical profile of Georgian propolis extracts and their activity against Helicobacter pylori. Molecules 2023, 28, 1374. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, M.; Mishra, M.; Pandey, A.; Gupta, J.; Pandey, J.; Gupta, S.; Malik, M.Z.; Somvanshi, P.; Chaturvedi, R. Oxidative products of curcumin rather than curcumin bind to Helicobacter Pylori virulence factor VacA and are required to inhibit Its vacuolation activity. Molecules 2022, 27, 6727. [Google Scholar] [CrossRef]
- Ray, A.K.; Luis, P.B.; Mishra, S.K.; Barry, D.P.; Asim, M.; Pandey, A.; Chaturvedi, M.; Gupta, J.; Gupta, S.; Mahant, S.; et al. Curcumin oxidation Is required for inhibition of Helicobacter pylori growth, translocation and phosphorylation of Cag A. Front. Cell Infect. Microbiol. 2021, 11, 765842. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; De, R.; Mukhopadhyay, A.K. Curcumin as a potential therapeutic candidate for Helicobacter pylori associated diseases. World. J. Gastroenterol. 2016, 22, 2736–2748. [Google Scholar] [CrossRef]
- Iwahori, A.; Hirota, Y.; Sampe, R.; Miyano, S.; Takahashi, N.; Sasatsu, M.; Kondo, I.; Numao, N. On the antibacterial activity of normal and reversed magainin 2 analogs against Helicobacter pylori. Biol. Pharm. Bull. 1997, 20, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Neshani, A.; Zare, H.; Akbari Eidgahi, M.R.; Hooshyar Chichaklu, A.; Movaqar, A.; Ghazvini, K. Review of antimicrobial peptides with anti-Helicobacter pylori activity. Helicobacter 2019, 24, e12555. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Thennarasu, S.; Lee, D.K.; Tan, A.; Maloy, L. Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys. J. 2006, 91, 206–216. [Google Scholar] [CrossRef]
- Narayana, J.L.; Huang, H.N.; Wu, C.J.; Chen, J.Y. Efficacy of the antimicrobial peptide TP4 against Helicobacter pylori infection: In vitro membrane perturbation via micellization and in vivo suppression of host immune responses in a mouse model. Oncotarget 2015, 6, 12936–12954. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, W.J.; Wu, J.L.; Her, G.M.; Hui, C.F. Epinecidin-1 peptide induces apoptosis which enhances antitumor effects in human leukemia U937 cells. Peptides 2009, 30, 2365–2373. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, W.K.; Gallo, R.L.; Fang, E.F.; Hu, W.; Ling, T.K.; Shen, J.; Chan, R.L.; Lu, L.; Luo, X.M.; et al. Critical role of antimicrobial peptide cathelicidin for controlling Helicobacter pylori survival and infection. J. Immunol. 2016, 196, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A. Mechanism of mammalian cell lysis mediated by peptide defensins. Evidence for an initial alteration of the plasma membrane. J. Clin. Investig. 1991, 88, 93–100. [Google Scholar] [CrossRef]
- Sahl, H.G.; Pag, U.; Bonness, S.; Wagner, S.; Antcheva, N.; Tossi, A. Mammalian defensins: Structures and mechanism of antibiotic activity. J. Leukoc. Biol. 2005, 77, 466–475. [Google Scholar] [CrossRef]
- Téné, N.; Bonnafé, E.; Berger, F.; Rifflet, A.; Guilhaudis, L.; Ségalas-Milazzo, I.; Pipy, B.; Coste, A.; Leprince, J.; Treilhou, M. Biochemical and biophysical combined study of bicarinalin, an ant venom antimicrobial peptide. Peptides 2016, 79, 103–113. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, J.; Xu, X.; Lai, R.; Zou, Q. An antimicrobial peptide with antimicrobial activity against Helicobacter pylori. Peptides 2007, 28, 1527–1531. [Google Scholar] [CrossRef]
- Owolabi, B.O.; Musale, V.; Ojo, O.O.; Moffett, R.C.; McGahon, M.K.; Curtis, T.M.; Conlon, J.M.; Flatt, P.R.; Abdel-Wahab, Y.H.A. Actions of PGLa-AM1 and its [A14K] and [A20K] analogues and their therapeutic potential as anti-diabetic agents. Biochimie 2017, 138, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.T.; Breukink, E.; Tischenko, E.; Lutters, M.A.; de Kruijff, B.; Kaptein, R.; Bonvin, A.M.; van Nuland, N.A. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 2004, 11, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Tewari, M.; Shukla, H.S.; Roy, B.K. In silico profiling of the potentiality of curcumin and conventional drugs for CagA oncoprotein inactivation. Arch. Pharm. 2015, 348, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. Curcumin may (not) defy science. ACS Med. Chem. Lett. 2017, 8, 467–470. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, W.; Hu, G.; Sun, H.; Kong, Q. Bioactivities of EF24, a novel curcumin analog: A review. Front. Oncol. 2018, 8, 614. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, A.M.; Ma, Z.Y.; Li, X.B.; Xiong, Y.Y.; Dou, J.F.; Wang, J.F. The synthetic antimicrobial peptide pexiganan and its nanoparticles (PNPs) exhibit the anti-helicobacter pylori activity in vitro and in vivo. Molecules 2015, 20, 3972–3985. [Google Scholar] [CrossRef]
- Homan, M.; Orel, R. Are probiotics useful in Helicobacter pylori eradication? World J. Gastroenterol. 2015, 21, 10644–10653. [Google Scholar] [CrossRef]
- Ji, J.; Yang, H. Using probiotics as supplementation for Helicobacter pylori antibiotic therapy. Int. J. Mol. Sci. 2020, 21, 1136. [Google Scholar] [CrossRef]
- Francavilla, R.; Polimeno, L.; Demichina, A.; Maurogiovanni, G.; Principi, B.; Scaccianoce, G.; Ierardi, E.; Russo, F.; Riezzo, G.; Di Leo, A.; et al. Lactobacillus reuteri strain combination in Helicobacter pylori infection: A randomized, double-blind, placebo-controlled study. J. Clin. Gastroenterol. 2014, 48, 407–413. [Google Scholar] [CrossRef]
- Urrutia-Baca, V.H.; Escamilla-García, E.; de la Garza-Ramos, M.A.; Tamez-Guerra, P.; Gomez-Flores, R.; Urbina-Ríos, C.S. In vitro antimicrobial activity and downregulation of virulence gene expression on Helicobacter pylori by reuterin. Probiotics Antimicrob. Proteins 2018, 10, 168–175. [Google Scholar] [CrossRef]
- Vale, F.F.; Oleastro, M. Overview of the phytomedicine approaches against Helicobacter pylori. World J. Gastroenterol. 2014, 20, 5594–5609. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Tan, Z. An overview of traditional Chinese medicine therapy for Helicobacter pylori-related gastritis. Helicobacter 2021, 26, e12799. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori infections: What are we doing now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, L.; Zhu, S.; Wei, M. Effectiveness and safety of Chinese medicine combined with omeprazole in the treatment of gastric ulcer: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25744. [Google Scholar] [CrossRef]
- Ye, H.; Shi, Z.M.; Chen, Y.; Yu, J.; Zhang, X.Z. Innovative perspectives of integrated Chinese medicine on H. pylori. Chin. J. Integr. Med. 2018, 24, 873–880. [Google Scholar] [CrossRef]
- Ma, F.; Chen, Y.; Li, J.; Qing, H.P.; Wang, J.D.; Zhang, Y.L.; Long, B.G.; Bai, Y. Screening test for anti-Helicobacter pylori activity of traditional Chinese herbal medicines. World. J. Gastroenterol. 2010, 16, 5629–5634. [Google Scholar] [CrossRef]
- Argueta, E.A.; Ho, J.J.C.; Elfanagely, Y.; D’Agata, E.; Moss, S.F. Clinical implication of drug resistance for H. pylori management. Antibiotics 2022, 11, 1684. [Google Scholar] [CrossRef]
- Mégraud, F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut 2004, 53, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Fatima, N.; Alvi, A. Epidemiology and pattern of antibiotic resistance in Helicobacter pylori: Scenario from Saudi Arabia. Saudi J. Gastroenterol. 2014, 20, 212–218. [Google Scholar] [CrossRef]
- Nestegard, O.; Moayeri, B.; Halvorsen, F.A.; Tønnesen, T.; Sørbye, S.W.; Paulssen, E.; Johnsen, K.M.; Goll, R.; Florholmen, J.R.; Melby, K.K. Helicobacter pylori resistance to antibiotics before and after treatment: Incidence of eradication failure. PLoS ONE 2022, 17, e0265322. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisuphanunt, M.; Wilairatana, P.; Kooltheat, N.; Duangchan, T.; Katzenmeier, G.; Rose, J.B. Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections. Trop. Med. Infect. Dis. 2023, 8, 163. https://doi.org/10.3390/tropicalmed8030163
Srisuphanunt M, Wilairatana P, Kooltheat N, Duangchan T, Katzenmeier G, Rose JB. Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections. Tropical Medicine and Infectious Disease. 2023; 8(3):163. https://doi.org/10.3390/tropicalmed8030163
Chicago/Turabian StyleSrisuphanunt, Mayuna, Polrat Wilairatana, Nateelak Kooltheat, Thitinat Duangchan, Gerd Katzenmeier, and Joan B. Rose. 2023. "Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections" Tropical Medicine and Infectious Disease 8, no. 3: 163. https://doi.org/10.3390/tropicalmed8030163
APA StyleSrisuphanunt, M., Wilairatana, P., Kooltheat, N., Duangchan, T., Katzenmeier, G., & Rose, J. B. (2023). Molecular Mechanisms of Antibiotic Resistance and Novel Treatment Strategies for Helicobacter pylori Infections. Tropical Medicine and Infectious Disease, 8(3), 163. https://doi.org/10.3390/tropicalmed8030163







