The Current Distribution of Oncomelania hupensis Snails in the People’s Republic of China Based on a Nationwide Survey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Area
2.2. Mapping Snail Habitats
2.3. Field Survey on Snails
2.4. Data Management and Analysis
3. Results
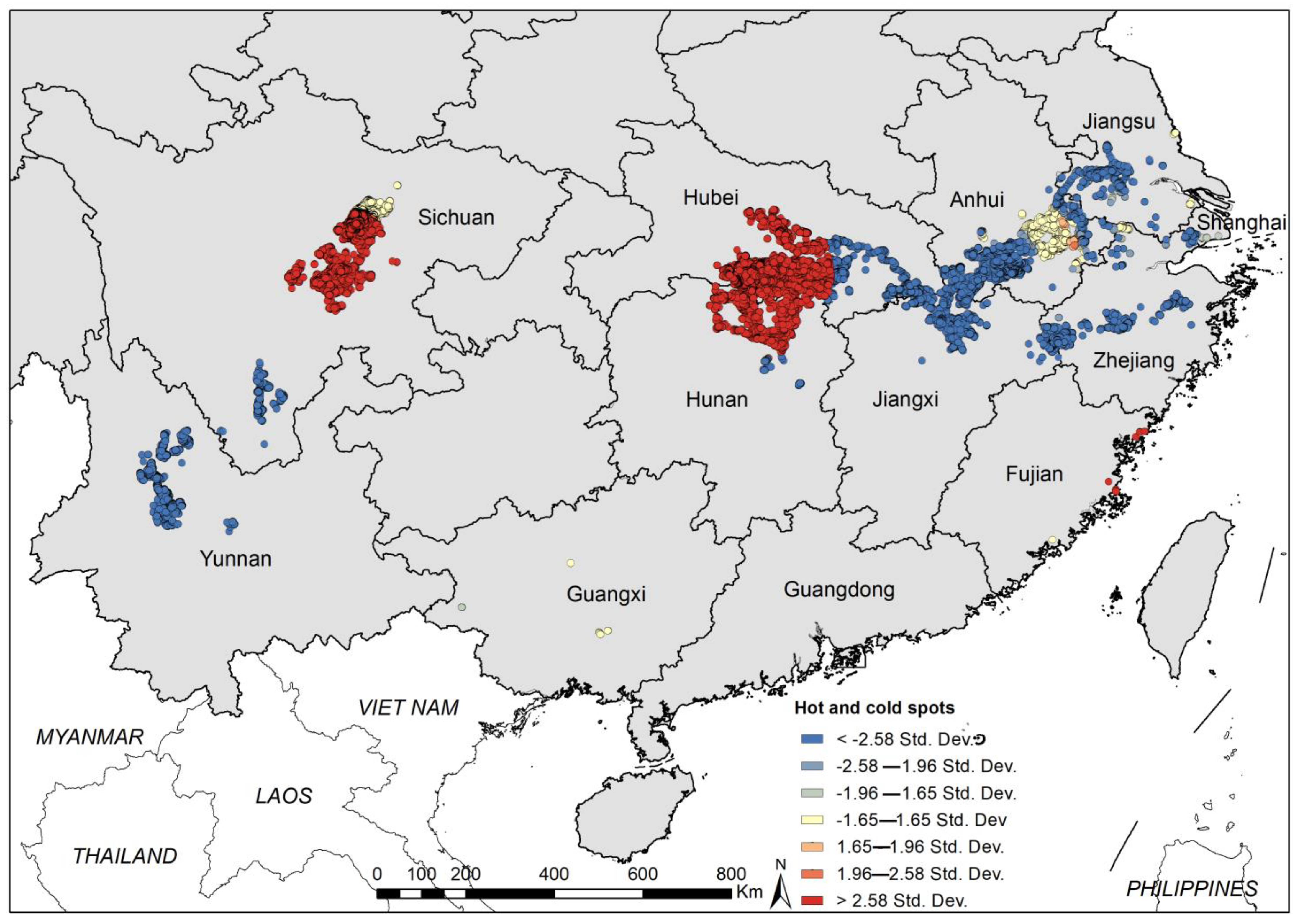
3.1. Geographical Distribution of Existing Snail Habitats
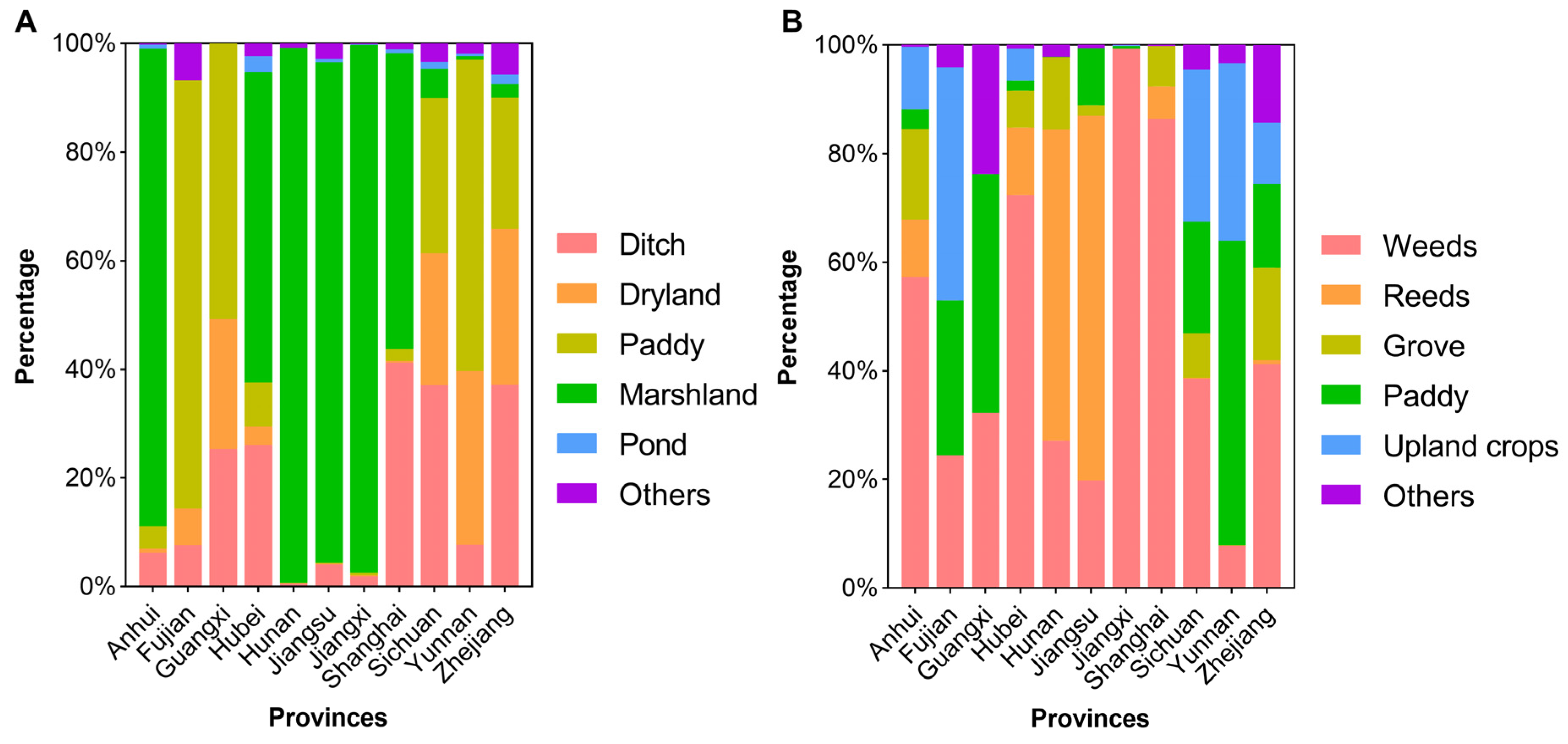
3.2. Environmental Characteristics of Existing Snail Habitats
3.3. Incidence and Density of O. hupensis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Primers. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.C.; Bezerra, F.S.M.; Colley, D.G.; Fleming, F.M.; Homeida, M.; Kabatereine, N.; Kabole, F.M.; King, C.H.; Mafe, M.A.; Midzi, N.; et al. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet. Infect. Dis. 2022, 22, e327–e335. [Google Scholar] [CrossRef]
- Webster, J.P.; Molyneux, D.H.; Hotez, P.J.; Fenwick, A. The contribution of mass drug administration to global health: Past, present and future. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130434. [Google Scholar] [CrossRef]
- Ogongo, P.; Nyakundi, R.K.; Chege, G.K.; Ochola, L. The Road to Elimination: Current State of Schistosomiasis Research and Progress Towards the End Game. Front. Immunol. 2022, 13, 846108. [Google Scholar] [CrossRef]
- Song, L.G.; Wu, X.Y.; Sacko, M.; Wu, Z.D. History of schistosomiasis epidemiology, current status, and challenges in China: On the road to schistosomiasis elimination. Parasitol. Res. 2016, 115, 4071–4081. [Google Scholar] [CrossRef]
- Xu, J.; Steinman, P.; Maybe, D.; Zhou, X.N.; Lv, S.; Li, S.Z.; Peeling, R. Evolution of the National Schistosomiasis Control Programmes in The People’s Republic of China. Adv. Parasitol. 2016, 92, 1–38. [Google Scholar] [CrossRef]
- Zhou, X.N.; Li, S.Z.; Hong, Q.B.; Yang, K.; Lv, S.; Xu, J. Remain true to our original aspiration for farewell to the God of Plague, compose the new chapter for the national schistosomiasis control programme scientifically—Commemoration of 60th anniversary of publishing Chairman Mao Zedong’s two poems “Farewell to the God of Plague”. Chin. J. Schisto. Control 2018, 30, 1–4. (In Chinese) [Google Scholar]
- Chen, J.; Xu, J.; Bergquist, R.; Li, S.Z.; Zhou, X.N. “Farewell to the God of Plague”: The Importance of Political Commitment Towards the Elimination of Schistosomiasis. Trop. Med. Infect. Dis. 2018, 3, 108. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Kurscheid, J.; Williams, G.M.; Clements, A.C.A.; Li, Y.; Zhou, X.N.; Utzinger, J.; McManus, D.P.; Gray, D.J. Asian Schistosomiasis: Current Status and Prospects for Control Leading to Elimination. Trop. Med. Infect. Dis. 2019, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.N.; Bergquist, R.; Leonardo, L.; Yang, G.J.; Yang, K.; Sudomo, M.; Olveda, R. Schistosomiasis japonica control and research needs. Adv. Parasitol. 2010, 72, 145–178. [Google Scholar] [CrossRef] [PubMed]
- Zayed, K.M.; Guo, Y.H.; Lv, S.; Zhang, Y.; Zhou, X.N. Molluscicidal and antioxidant activities of silver nanoparticles on the multi-species of snail intermediate hosts of schistosomiasis. PLoS. Negl. Trop. Dis. 2022, 16, e0010667. [Google Scholar] [CrossRef]
- Muhsin, M.A.; Wang, X.; Kabole, F.M.; Zilabumba, J.; Yang, K. The Indispensability of Snail Control for Accelerating Schistosomiasis Elimination: Evidence from Zanzibar. Trop. Med. Infect. Dis. 2022, 7, 347. [Google Scholar] [CrossRef]
- Sun, L.P.; Wang, W.; Zuo, Y.P.; Hong, Q.B.; Du, G.L.; Ma, Y.C.; Wang, J.; Yang, G.J.; Zhu, D.J.; Liang, Y.S. A multidisciplinary, integrated approach for the elimination of schistosomiasis: A longitudinal study in a historically hyper-endemic region in the lower reaches of the Yangtze River, China from 2005 to 2014. Infect. Dis. Poverty. 2017, 6, 56. [Google Scholar] [CrossRef]
- Wang, L.D.; Chen, H.G.; Guo, J.G.; Zeng, X.J.; Hong, X.L.; Xiong, J.J.; Wu, X.H.; Wang, X.H.; Wang, L.Y.; Xia, G.; et al. A strategy to control transmission of Schistosoma japonicum in China. N. Engl. J. Med. 2009, 360, 121–128. [Google Scholar] [CrossRef]
- Lei, Z.L.; Zhou, X.N. Eradication of schistosomiasis: A new target and a new task for the National Schistosomiasis Control Porgramme in the People’s Republic of China. Chin. J. Schisto. Control. 2015, 27, 1–4. (In Chinese) [Google Scholar]
- Bergquist, R.; Zhou, X.N.; Rollinson, D.; Reinhard-Rupp, J.; Klohe, K. Elimination of schistosomiasis: The tools required. Infect. Dis. Poverty. 2017, 6, 158. [Google Scholar] [CrossRef]
- Xu, J.; Lv, S.; Cao, C.L.; Li, S.Z.; Zhou, X.N. Progress and challenges of schistosomiasis elimination in China. Chin. J. Schisto. Control 2018, 30, 605–609. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, L.J.; Xu, Z.M.; Yang, F.; Dang, H.; Li, Y.L.; Lu, S.; Cao, C.L.; Xu, J.; Li, S.Z.; Zhou, X.N. Endemic status of schistosomiasis in People’s Republic of China in 2020. Chin. J. Schisto. Control. 2021, 33, 225–233. [Google Scholar] [CrossRef]
- Li, Z.J.; Ge, J.; Dai, J.R.; Wen, L.Y.; Lin, D.D.; Madsen, H.; Zhou, X.N.; Lv, S. Biology and Control of Snail Intermediate Host of Schistosoma japonicum in The People’s Republic of China. Adv. Parasitol. 2016, 92, 197–236. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Li, L.; Zhang, L.J.; Li, Y.L.; Li, S.Z.; Xu, J. From the One Health Perspective: Schistosomiasis Japonica and Flooding. Pathogens 2021, 10, 1538. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Raso, G.; Zhao, Z.Y.; He, Y.K.; Ellis, M.K.; McManus, D.P. Large water management projects and schistosomiasis control, Dongting Lake region, China. Emerg. Infect. Dis. 2007, 13, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Li, Y.L.; Zhang, L.J.; Lu, S.; Xu, J. Thinking on schistosomiasis control under the strategy of China’s Yangtze River Economic Belt. Chin. J. Schisto. Control 2019, 31, 459–462. (In Chinese) [Google Scholar] [CrossRef]
- Kirby, R.S.; Delmelle, E.; Eberth, J.M. Advances in spatial epidemiology and geographic information systems. Ann. Epidemiol. 2017, 27, 1–9. [Google Scholar] [CrossRef]
- He, J.; Li, W.; Bergquist, R.; Zhang, J.F.; Shi, L.; Zhao, S.; Wu, F.; Yang, K. The spatio-temporal distribution of Oncomelania hupensis along Yangtze river in Jiangsu Province, China after implementation of a new, integrated schistosomiasis control strategy. Geospat. Health 2016, 11, 480. [Google Scholar] [CrossRef]
- Hu, F.; Ge, J.; Lv, S.B.; Li, Y.F.; Li, Z.J.; Yuan, M.; Chen, Z.; Liu, Y.M.; Li, Y.S.; Ross, A.G.; et al. Distribution pattern of the snail intermediate host of schistosomiasis japonica in the Poyang Lake region of China. Infect. Dis. Poverty 2019, 8, 23. [Google Scholar] [CrossRef]
- Niu, Y.; Li, R.; Qiu, J.; Xu, X.; Huang, D.; Shao, Q.; Cui, Y. Identifying and Predicting the Geographical Distribution Patterns of Oncomelania hupensis. Int. J. Environ. Res. Public. Health 2019, 16, 2206. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xu, Z.M.; Yang, F.; He, J.Y.; Dang, H.; Li, Y.L.; Cao, C.L.; Xu, J.; Li, S.Z.; Zhou, X.N. Progress of schistosomiasis control in People’s Republic of China in 2021. Chin. J. Schisto. Control 2022, 34, 329–336. (In Chinese) [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Wang, P. Long-term effectiveness of the integrated schistosomiasis control strategy with emphasis on infectious source control in China: A 10-year evaluation from 2005 to 2014. Parasitol. Res. 2017, 116, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.G.; Zhao, Y.E.; Lee Willingham, A.; Wang, T.P. Towards the Elimination of Schistosomiasis japonica through Control of the Disease in Domestic Animals in The People’s Republic of China: A Tale of over 60 Years. Adv. Parasitol. 2016, 92, 269–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Wang, T.P.; Wang, Q.Z.; Yin, X.M.; Zhou, L.; Wang, F.F.; Wang, Y.; Fang, G.R. Infection status of sources of schistosomiasis japonica in marshaland and hill regions. J. Pathogen. Biol. 2013, 8, 445–447. [Google Scholar]
- Lv, S.B.; Chen, N.G.; Liu, Y.M.; Zhou, L.Y.; Wang, Y.S.; Hu, F.; Li, Y.F.; Yuan, M.; Lin, D.D. Survey of Schistosoma japonicum infections in wild animals in hilly transmission-controlled areas of Jiangxi Province. Chin. J. Schisto. Control 2019, 31, 463–467. (In Chinese) [Google Scholar]
- Song, J.; Du, C.H.; Dong, Y.; Shen, M.F.; Zhang, Z.Y.; Bie, S.S. Review on changes in major infectious sources and control effects of schistosomiasis japonica in hilly areas of Yunnan and Sichuan during different prevention and treatment stages. J. Med. Pest. Control 2022, 38, 370–373. [Google Scholar]
- Zhou, X.N. Report on National Survey of Oncomelania Hupensis in China; Shanghai Science and Technology Press: Shanghai, China, 2022. [Google Scholar]
- Zhou, X.N. A high-quality driver to accelerate the progress towards schistosomiasis elimination by science and technology-led innovation in Jiangsu Province. Chin. J. Schisto. Control 2020, 31, 573–575. (In Chinese) [Google Scholar] [CrossRef]
- Wen, L.Y.; Zhu, D.M.; Yan, X.L.; Chen, J.H.; Zhang, J.F.; Tao, H.Q. Report on surveillance of schistosomiasis in Zhejiang province from1996 to 2005. Chin. J. Schisto. Control 2007, 23, 605–607. (In Chinese) [Google Scholar]
- Wang, X.M. Review on the Progress of Schistosomiasis Elimination; Shanghai Science and Technology Press: Shanghai, China, 1988. [Google Scholar]
- Zhang, S.Q.; Sun, C.S.; Wang, M.; Lin, D.D.; Zhou, X.N.; Wang, T.P. Epidemiological Features and Effectiveness of Schistosomiasis Control Programme in Lake and Marshland Region in The People’s Republic of China. Adv. Parasitol. 2016, 92, 39–71. [Google Scholar] [CrossRef]
- Chen, S.; Lu, D.; Duan, L.; Ma, B.; Lv, C.; Li, Y.L.; Lu, S.N.; Li, L.H.; Xu, L.; Wu, Z.S.; et al. Cross-watershed distribution pattern challenging the elimination of Oncomelania hupensis, the intermediate host of Schistosoma japonicum, in Sichuan province, China. Parasit. Vectors. 2022, 15, 363. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.B.; Li, R.Z.; Wan, J.J.; Yang, Y.; Qiu, D.C.; Zhong, B. Epidemiological Features and Effectiveness of Schistosomiasis Control Programme in Mountainous and Hilly Region of The People’s Republic of China. Adv. Parasitol. 2016, 92, 73–95. [Google Scholar] [CrossRef]
- Zou, H.Y.; Yu, Q.F.; Qiu, C.; Webster, J.P.; Lu, D.B. Meta-analyses of Schistosoma japonicum infections in wild rodents across China over time indicates a potential challenge to the 2030 elimination targets. PLoS. Negl. Trop. Dis. 2020, 14, e0008652. [Google Scholar] [CrossRef] [PubMed]
- VAN Dorssen, C.F.; Gordon, C.A.; Li, Y.; Williams, G.M.; Wang, Y.; Luo, Z.; Gobert, G.N.; You, H.; McManus, D.P.; Gray, D.J. Rodents, goats and dogs—Their potential roles in the transmission of schistosomiasis in China. Parasitology 2017, 144, 1633–1642. [Google Scholar] [CrossRef]
- Zhang, L.J.; Li, S.Z.; Wen, L.Y.; Lin, D.D.; Abe, E.M.; Zhu, R.; Du, Y.; Lv, S.; Xu, J.; Webster, B.L.; et al. The Establishment and Function of Schistosomiasis Surveillance System Towards Elimination in The People’s Republic of China. Adv. Parasitol. 2016, 92, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lv, S. Watershed ecology-based thinking of Oncomelania snail control. Chin. J. Schisto. Control 2022, 34, 542. [Google Scholar] [CrossRef]
- Wang, W.; Bergquist, R.; King, C.H.; Yang, K. Elimination of schistosomiasis in China: Current status and future prospects. PLoS. Negl. Trop. Dis. 2021, 15, e0009578. [Google Scholar] [CrossRef]
- Huang, S.; Mao, Q.; Zhong, Q.; Fan, X.; Li, W.; Rao, Y.; Pei, F.; Li, S.; Deng, Z. Reappearance of Risk of Schistosomiasis Transmission and the Response After 27 Years of Interrupted Transmission—Guangdong Province, China, 2019. China. CDC. Wkly. 2021, 3, 1093–1097. [Google Scholar] [CrossRef]
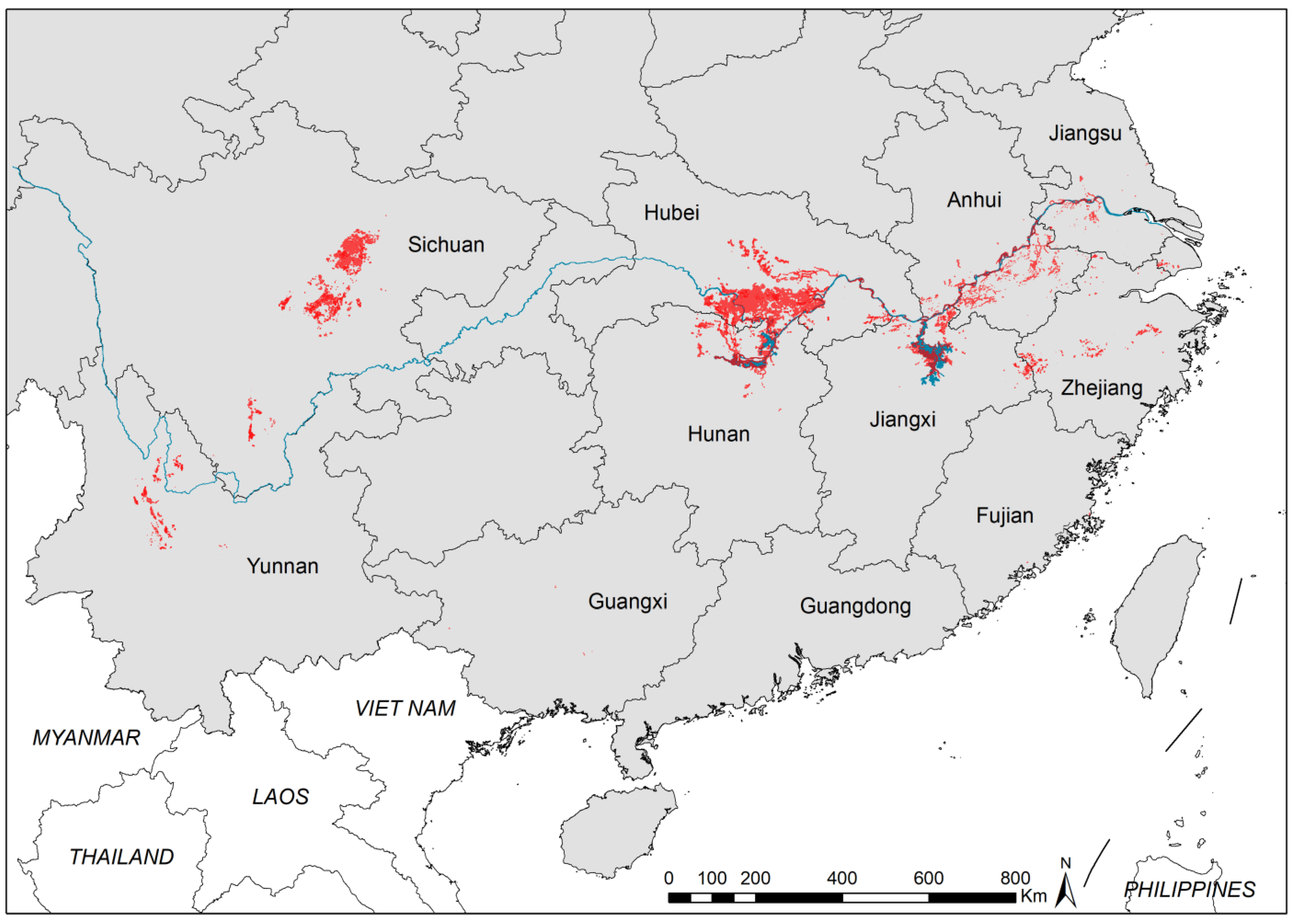
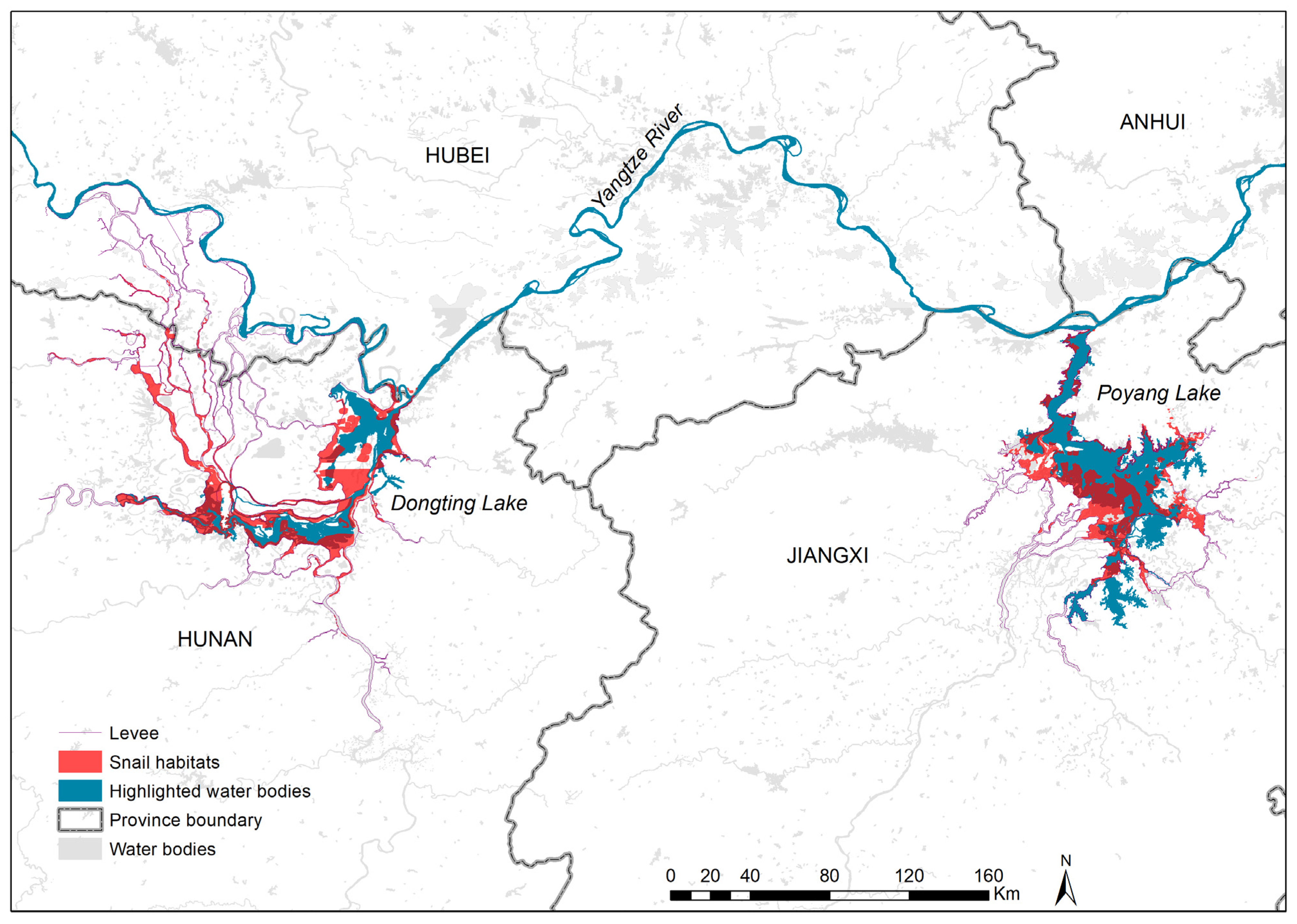

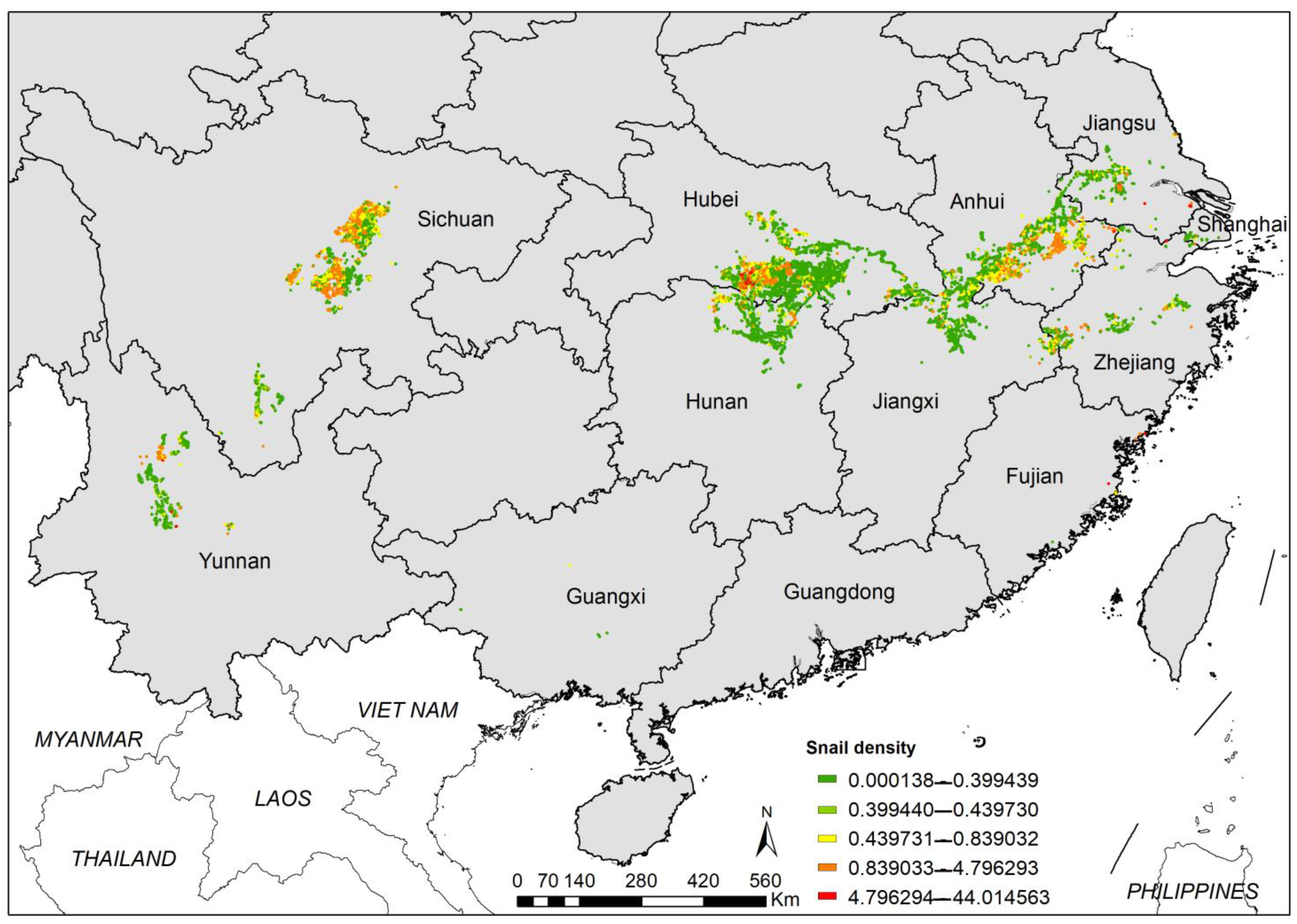
| Province | No. ESH | EASH (Million m2) | Percentage of EASH (%) | AIS (Million m2) | Percentage of AIS (%) | No. Surveyed ESH | No. Systematic Sampling Frames | Percentage of Frames with Living Snails (%) | Maximum Snail Density (/0.11 m2) | Median Snail Density (/0.11 m2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sichuan | 19,314 | 143.77 | 2.75 | 45.79 | 1.28 | 19,124 | 2,944,065 | 35.37 | 18.00 | 0.75 |
| Hubei | 16,718 | 1132.75 | 21.63 | 721.11 | 20.16 | 15,964 | 3,962,343 | 18.72 | 44.01 | 0.53 |
| Yunnan | 6440 | 44.24 | 0.84 | 20.31 | 0.57 | 6262 | 782,564 | 8.64 | 39.33 | 0.20 |
| Anhui | 4830 | 545.34 | 10.41 | 265.03 | 7.41 | 4769 | 1,192,716 | 14.27 | 20.56 | 0.53 |
| Jiangxi | 2175 | 1274.58 | 24.34 | 832.35 | 23.27 | 2033 | 1,530,114 | 9.43 | 6.50 | 0.25 |
| Hunan | 1707 | 1940.76 | 37.06 | 1663.64 | 46.51 | 1683 | 1,737,538 | 9.59 | 3.46 | 0.18 |
| Zhejiang | 1315 | 16.82 | 0.32 | 0.97 | 0.03 | 1315 | 952,078 | 3.37 | 24.44 | 0.21 |
| Jiangsu | 641 | 137.56 | 2.63 | 27.92 | 0.78 | 641 | 358,080 | 12.47 | 38.18 | 0.17 |
| Shanghai | 90 | 0.19 | 0.00 | 0.03 | 0.00 | 90 | 26,175 | 13.27 | 6.17 | 0.33 |
| Fujian | 14 | 0.22 | 0.00 | 0.02 | 0.00 | 14 | 11,084 | 6.11 | 16.25 | 2.09 |
| Guangxi | 10 | 0.20 | 0.00 | 0.06 | 0.00 | 10 | 6904 | 18.11 | 0.81 | 0.05 |
| Guangdong | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0 | 0 | 0.00 | 0.00 | 0.00 |
| Total | 53,254 | 5236.45 | 100.00 | 3577.25 | 100.00 | 51,905 | 13,503,661 | 17.88 | 44.01 | 0.50 |
| Types | No. ESH | EASH (Million m2) | AIS (Million m2) | Percentage of AIS (%) | No. Surveyed ESH | No. Systematic Sampling Frames | Percentage of Frames with Living Snails (%) | Maximum Density of Snails (/0.11 m2) | Median Density of Snails (/0.11 m2) |
|---|---|---|---|---|---|---|---|---|---|
| Environment | |||||||||
| Ditch | 35,025 | 448.47 | 247.79 | 6.93 | 34,397 | 6,299,097 | 24.85 | 44.01 | 0.57 |
| Dryland | 3568 | 123.00 | 49.14 | 1.37 | 3384 | 920,401 | 17.57 | 38.18 | 0.50 |
| Paddy | 6760 | 218.54 | 99.32 | 2.78 | 6521 | 1,570,437 | 16.88 | 39.33 | 0.47 |
| Marshland | 5414 | 4306.53 | 3120.21 | 87.22 | 5265 | 3,993,404 | 8.39 | 6.53 | 0.20 |
| Pond | 1019 | 75.95 | 23.90 | 0.67 | 892 | 175,288 | 11.24 | 13.50 | 0.35 |
| Other | 1468 | 63.97 | 36.88 | 1.03 | 1446 | 545,034 | 12.31 | 15.42 | 0.40 |
| Total | 53,254 | 5236.45 | 3577.25 | 100.00 | 51,905 | 13,503,661 | 17.88 | 44.01 | 0.50 |
| Vegetation | |||||||||
| Weeds | 39,139 | 2999.70 | 1976.83 | 55.26 | 38,111 | 8,871,150 | 20.09 | 44.01 | 0.53 |
| Reeds | 903 | 1360.40 | 1088.49 | 30.43 | 897 | 1,009,701 | 8.74 | 12.88 | 0.18 |
| Grove | 1369 | 493.57 | 319.48 | 8.93 | 1363 | 718,425 | 11.00 | 15.42 | 0.28 |
| Paddy | 5841 | 147.58 | 51.32 | 1.43 | 5737 | 1,317,745 | 15.82 | 39.33 | 0.49 |
| Dryland crops | 3465 | 165.14 | 95.25 | 2.66 | 3272 | 897,400 | 18.87 | 21.00 | 0.51 |
| Other | 2537 | 70.05 | 45.88 | 1.28 | 2525 | 689,240 | 12.55 | 16.67 | 0.40 |
| Total | 53,254 | 5236.45 | 3577.25 | 100.00 | 51,905 | 13,503,661 | 17.88 | 44.01 | 0.50 |
| Endemic Types | No. Surveyed ESH | No. Systematic Sampling Frames | Percentage of Frames with Living Snails (%) | Maximum Density of Snails (/0.11 m2) | Median Density of Snails (/0.11 m2) |
|---|---|---|---|---|---|
| Marshland and lakes | 17,300 | 6,820,036 | 13.93 | 44.01 | 0.44 |
| Mountains and hills | 34,182 | 6,421,207 | 22.26 | 39.33 | 0.51 |
| Plain water networks | 423 | 262,418 | 13.17 | 38.18 | 0.21 |
| Total | 51,905 | 13,503,661 | 17.88 | 44.01 | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, C.; Li, Y.-L.; Deng, W.-P.; Bao, Z.-P.; Xu, J.; Lv, S.; Li, S.-Z.; Zhou, X.-N. The Current Distribution of Oncomelania hupensis Snails in the People’s Republic of China Based on a Nationwide Survey. Trop. Med. Infect. Dis. 2023, 8, 120. https://doi.org/10.3390/tropicalmed8020120
Lv C, Li Y-L, Deng W-P, Bao Z-P, Xu J, Lv S, Li S-Z, Zhou X-N. The Current Distribution of Oncomelania hupensis Snails in the People’s Republic of China Based on a Nationwide Survey. Tropical Medicine and Infectious Disease. 2023; 8(2):120. https://doi.org/10.3390/tropicalmed8020120
Chicago/Turabian StyleLv, Chao, Yin-Long Li, Wang-Ping Deng, Zi-Ping Bao, Jing Xu, Shan Lv, Shi-Zhu Li, and Xiao-Nong Zhou. 2023. "The Current Distribution of Oncomelania hupensis Snails in the People’s Republic of China Based on a Nationwide Survey" Tropical Medicine and Infectious Disease 8, no. 2: 120. https://doi.org/10.3390/tropicalmed8020120
APA StyleLv, C., Li, Y.-L., Deng, W.-P., Bao, Z.-P., Xu, J., Lv, S., Li, S.-Z., & Zhou, X.-N. (2023). The Current Distribution of Oncomelania hupensis Snails in the People’s Republic of China Based on a Nationwide Survey. Tropical Medicine and Infectious Disease, 8(2), 120. https://doi.org/10.3390/tropicalmed8020120









