Abstract
Trichomonas tenax is a flagellated protozoan parasite found in the oral cavities of humans and animals and has been associated with periodontal disease, the most prevalent inflammatory disease affecting them all. Studies have shown that T. tenax can cause damage to mammalian cells and secretes virulent proteins, such as cysteine. It is presently considered zoonotic. Despite the few studies that have been done, the pathogenicity of this oral protozoan is still not fully understood. A database search was performed in July 2022 using PubMed and Google Scholar to retrieve data eligible for this study. PRISMA-ScR guidelines were followed to conduct this scoping review. A total of 321 articles were found with 87 included in this review after applying the exclusion criteria. Due to its increasing prevalence worldwide in both humans and dogs, detecting and elucidating the pathogenicity of this parasite is paramount for effective global control and prevention of periodontal disease. However, there is a paucity in the literature on this neglected zoonotic trichomonad, which is in large contrast to the closely related human pathogen T. vaginalis. Here, we comprehensively review the history, morphology and reproduction, host, prevalence, diagnosis, pathogenicity, control, and prevention of T. tenax. Hopefully, this article will call attention to both medical and veterinary professionals as well as epidemiologists on this most neglected and zoonotic protozoan. More epidemiological and clinical studies need to be conducted on T. tenax to gain a better understanding of its pathogenicity, to increase the chances of developing effective drugs to aid in the control of this oral parasite, and reduce the spread of periodontal disease worldwide.
1. Introduction
Trichomonas tenax is an anaerobic flagellated protozoan of an ancient eukaryotic lineage without mitochondria that lives in low-oxygen environments [1,2]. In contrast to its close relative, Trichomonas vaginalis found in the human urogenital tract, T. tenax is mostly found to inhabit the oral cavities of humans and animals, which may lead to periodontal disease [3]. The protozoan has also been found in other parts of the body, such as the lungs and bronchi, submaxillary glands, tonsils, and lymph nodes [4,5]. Despite its importance, relative to both human and animal health, very little attention has been paid to T. tenax. Therefore, this scoping review aims not only to identify future research gaps but also to call attention to both medical and veterinary professionals on the epidemiology, detection, and risk associated with contracting this greatly neglected oral protozoan.
2. Materials and Methods
2.1. Data Search Strategy
This scoping review was executed to assess the following research questions: (1) Is T. tenax more prevalent in humans and dogs with periodontitis compared to those that are healthy? (2) Is there an association between T. tenax and periodontal disease? (3) What techniques are presently available to detect T. tenax? (4) Does T. tenax exosomes contains virulent proteins and RNA, such as its close relative T. vaginalis? (5) Are there any treatments or measures available for the control and prevention of T. tenax? This scoping review was conducted by adapting the guidelines of the preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) [6]. A literature search was conducted on 29 July 2022 with an open date using PubMed and Google Scholar databases for published articles. The databases were searched for the keywords Trichomonas tenax and Trichomonas elongata (Table 1). The results were exported in .csv format and then opened and organized in Microsoft Excel.

Table 1.
Results obtained from search strategies used in databases.
2.2. Study Inclusion and Exclusion Criteria
The inclusion criteria included all peer-reviewed published articles in English that included any information on the history, morphology, reproduction, host preference, the prevalence in humans and dogs, diagnosis, pathogenicity, and control and prevention of T. tenax. All articles were required to have an abstract and/or full text available to be included in this study. The search was not restricted to the publication date; therefore, all articles up to 29 July 2022 were included once inclusion criteria were met. Studies were excluded if no abstracts were available, if the article was not in English, if the article was not relevant based on the title and abstract, and if it was a duplicate (Figure 1).
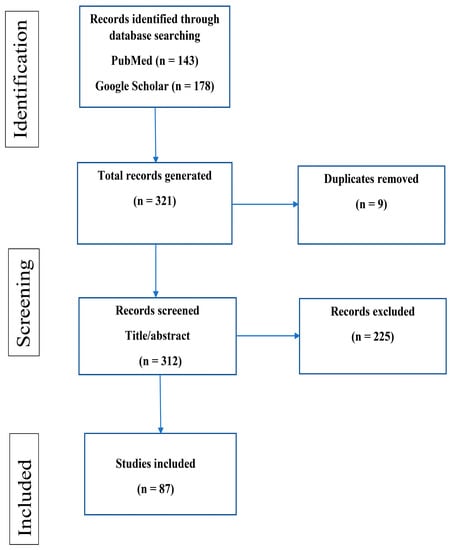
Figure 1.
Flow diagram of scoping review selection process.
2.3. Data Extraction
For data extraction, two reviewers, (MM) and (CY), independently read the title and abstract of each article retrieved from the search to determine if the article was eligible. Full-text screening was conducted by (MM), (CY), and (JK), and the data were extracted independently. Any differences or disagreements on eligibility were discussed and resolved by the team. The data extracted included the author, publication date, title, history, morphology, reproduction, host preference, the prevalence in humans and dogs, diagnosis, pathogenicity, and control and prevention.
3. Results
A total of 321 articles were retrieved after searching 2 databases (PubMed = 143; Google scholar = 178). Three hundred and twelve articles were screened after duplicates were removed. After inclusion and exclusion criteria were applied, 87 articles were eligible for this scoping review (Figure 1).
3.1. History
The name Trichomonas tenax originated from the Greek words “trichos” (meaning tiny hair), “monas” (meaning simple creature), and the Latin word “tenere”, which means to keep or to stick to [7]. The trichomonad protozoan was originally discovered by Muller in 1773, in aqueous solutions of tartar derived from the oral cavities of human beings [8]. He named it Cercaria tenax [9]. Up until the 1940s, different names were used, such as Trichomonas elongata, originating from Steinberg in 1862 [10], and Tetratrichomonas buccalis [11]. Although T. tenax was first observed by Muller in 1773, it was in 1917 that the first accepted name was given to the oral flagellate, which at that time was called Tetratrichomonas buccalis [11]. This was the first recorded report on the oral trichomonad in the 1900s. According to Honigberg and Lee (1959), it was Dobell (1939) and Wenrich (1944) who assumed that Trichomonas was the only flagellate in the buccal cavity and that its name should be tenax based on priority (hence, the name T. tenax) [9].
3.2. Morphology and Reproduction
Trophozoites of T. tenax are either ellipsoidal or ovoid (pear-shaped) in shape and 5–16 × 2–15 µm in size. Each has five flagella—four free anterior flagella and one that extends posteriorly. An undulating membrane extends two-thirds of the body length and its accompanying costa typically lies next to the posterior flagellum [12,13,14]. The four anterior flagella arise from a three-lobed blepharoplast, which gives rise to a chromatic basal rod and an axostyle. The nucleus is situated near the anterior end of the body and is generally ellipsoidal or ovoidal with an average size of 2.5 × 1.7 µm. It contains a single nucleolus, surrounded by clear halos [15,16]. The anterior end of the capitulum runs continuously with the pelta and there is no cell mouth present [9]. In addition to trophozoites, T. tenax has other forms. These forms include a round form that is usually larger than the trophozoite, an amoeboid cell form found swimming freely in axenic medium, and a pseudocyst form, which is found when the trichomonads are under stressful conditions [17]. These forms can range in size from approximately 5 to 16 µm in length and 5–6 µm wide [7,14].
In the past, these different forms of the trichomonad were considered different life-cycle stages. However, more recently, it has been shown that these different forms are due to different environmental conditions of the trichomonad’s habitat [17,18].
Trichomonas tenax can be readily differentiated from other species of the Trichomonadidae family, except for two, T. vaginalis and T. gallinae. Honigberg and Lee (1959) stated that from a morphological standpoint, T. vaginalis is on average larger than the other two trichomonads and the Paracostal granules are larger and more numerous compared to the other two trichomonads. Paracostal granules are mostly absent or small in T. tenax whereas in T. gallinae the granules are found in the region of the axostyle [9]. Furthermore, these trichomonads exhibit host and site predilection, with the T. vaginalis site of predilection being in the urogenital tract of humans, T. tenax in the oral cavities of humans and animals, and T. gallinae in the upper digestive tracts of avian hosts [19].
Trichomonas tenax reproduces by asexual reproduction, followed by a process of mitosis involving six morphologically distinct chromosomes [20]. Honigberg and Lee (1959) reported that during division, the parental flagella are divided equally among the daughter mastigonts with full flagellar completion taking place right before cytokinesis. Additionally, each daughter cell receives one of the parabasal, one daughter cell retains the old undulating membrane and costa while the other develops new organelles. By the end of the division, the old axostyle and pelta are destroyed and a new supporting organelle develops in both daughter cells [9].
3.3. Host
Trichomonas tenax has been cited and recorded in different animal hosts over the years. It was first reported in the oral cavities of humans by Muller in 1773 [8]. This was confirmed by other studies that also found T. tenax in the oral cavities of humans [21,22,23]. It has also been observed in samples taken from the vaginas of monkeys [24]. Several studies reported that T. tenax can cause urogenital invasions in humans in addition to its close relative T. vaginalis [25,26,27]. Dybicz et al. (2018) detected T. tenax in the oral samples of small companion animals, such as dogs and cats, as well as large animals, such as horses [28]. Other studies have reported on the occurrence of T. tenax in the oral cavities of dogs and cats [3,29]. Recently, T. tenax was detected in the cloaca of birds [30,31,32,33,34,35]. With T. tenax being found in so many different hosts, it is important to determine whether T. tenax is a human parasite, which is the predominant current view, or if it is a parasite of animal origin with zoonotic potential. This question on host preference and specificity requires further investigation.
3.4. Culture
Culturing T. tenax is important for studying the biochemistry, physiology, and metabolism of parasites, and to determine the nutrient requirements, morphological structure, pathophysiology, life cycle, and host–parasite relationship [13]. Before 1917, T. tenax was of little or no interest to clinicians and biochemists since it could not be maintained in an axenic culture unlike its close relatives, T. vaginalis and T. gallinae [9]. Although there were reports of T. tenax being cultured as early as 1915, these reports were not credible [36]. In 1917, Ohira and Noguchi continued culture work on T. tenax, but this work was also brief with no final conclusions as to whether the culture was successful or not [37]. Culture studies on T. tenax have increased over the years as they have (simultaneously) attracted the attention of researchers. Other culture work was followed by Hinshaw and Hogue in 1926, giving a better understanding of environmental conditions and nutrient requirements necessary to culture the oral trichomonad [38,39]. Honigberg et al. (1959) tried without success to culture T. tenax axenically [9]. Success was finally reached in 1962 when L. S. Diamond was able to induce axenic growth in a nutrient broth supplemented with serum and a cell-free extract of the chick embryo, which is now referred to as Diamond’s medium [40]. Currently, Diamond’s medium is still used by researchers worldwide to culture T. tenax. Although Diamond and Bargit (1962) were successful in culturing T. tenax, other studies continued as the oral trichomonad gained interest. Work by Wantland et al. (1963) and Asai et al. (1986) was somewhat unsuccessful but still contributed to the overall understanding of the oral flagellate [41,42]. Wantland et al. (1963) used egg yolk, a non-synthetic serum, to culture T. tenax and Entamoeba gingivalis, which resulted in the near-axenic and mono-axenic culture of T. tenax and E. gingivalis. Asai et al. (1986) used RPMI 1640 and Eagle’s minimum essential medium (MEM) to culture T. tenax but cultures failed to support the growth of T. tenax without bacterial growth. Additionally, researchers have shown that T. tenax isolated from humans can be successfully cultured if incubated at a temperature between 31 and 37 °C with a pH between 7.0 and 7.5 for 72 h [41,42,43]. All of these contributions to the culture of T. tenax were mainly from human strains with only one being successful, i.e., culturing T. tenax from dogs [3].
3.5. Molecular Diagnosis
Currently, microscopy and molecular methods are used in the diagnosis of T. tenax infection in humans and animals. Trichomonads of ellipsoidal or ovoid (pear-shape) shapes and 5–16 × 2–15 µm in size, as revealed by the microscopy along with the predilection site of the mouth, either directly or after being cultured, can be used as criteria for diagnosing T. tenax infection [19] (Table 2). However, molecular methods such as PCR are preferred for confirmation since they are considered more sensitive and specific than the conventional techniques of culture and microscopy [13,44]. PCR was first used to detect T. tenax in human oral samples in 1997 [1], where primers were designed for the 18S rRNA gene of T. tenax, which was aligned with T. vaginalis and T. foetus (Table 3). Kikuta et al. (1997) concluded that the method was specific to T. tenax and had a limit of detection of 100fg DNA or as low as 5 cells [1]. In comparison with the gold standard microscopy in the detection of T. tenax (in ten healthy individuals and nine patients of periodontitis or gingivitis), the PCR only detected T. tenax in five patients, whereas microscopy was negative for all patients and healthy individuals [1]. The data clearly show that PCR surpasses microscopy in sensitivity and yet maintains specificity. However, it was not until a case study by Szczepaniak (2016) that PCR was used to detect T. tenax in canine oral samples where primers for the ITS1-5.8S rRNA-ITS2 regions were used [45].

Table 2.
Morphological characteristics of trichomonad protozoa found in human infections a.
Over the last two decades, the loop-mediated isothermal amplification (LAMP), a nucleic acid amplification test, was developed to amplify the DNA of different pathogens for detection and diagnostic purposes [46]. This technique has been proven to be more sensitive than traditional techniques of culture and microscopy and PCR that are presently used [47,48,49]. It is rapid, does not require expensive equipment, and is ideal for future diagnoses, especially in developing countries [50,51]. To date, only one LAMP assay has been developed to detect T. tenax in human and/or canine oral samples, which can be directly used for clinical samples without prior DNA extraction. The test has a limit of detection of 10fg DNA or 1 cell [52]. A total of 8 out of 44 clinical canine samples were microscopically positive for T. tenax after culturing. They were also LAMP-positive when two cells were used in each reaction without prior DNA extraction [52]. Therefore, this LAMP assay has the great advantage of being used in point of care in both developed and developing countries. No other new molecular techniques have been reported in the literature to specifically detect T. tenax in humans and/or animals.
3.6. Prevalence
3.6.1. Humans
The only reliable method available that could have identified or detected T. tenax in the 1900s and most of the 20th century was microscopy [38]. Hinshaw (1926) found that 90% of the prisoners with advanced pyorrhea (periodontitis) were infected with T. tenax. Several prevalence studies in humans have followed since that time (Table 4) [21,22,23,53,54,55,56]. In Iraq, the prevalence of T. tenax was 8.4% in 143 mouth disease patients and 4.1% in 271 controls [23]. Norberg (2014) reported the prevalence of both E. gingivalis and T. tenax in patients with oral infections in Brazil with the prevalence of T. tenax being 51% (51 out of 100 patients). The study by Norberg (2014) also showed that the flagellate infection decreased with age in the control group and increased with age in those who were ill. Additionally, E. gingivalis and T. tenax infections increased in individuals with tooth loss, indicating a positive correlation between tooth loss and both infections [21].
The difficulty in culturing and identifying the parasites made way for the development of molecular techniques, such as PCR and LAMP. Athari et al. (2007) reported on the prevalence of oral trichomoniasis in 160 patients with gingivitis and periodontitis using the PCR-amplifying 18S rRNA gene and microscopy. A total of 33 patients (20.6%) were PCR-positive whilst 28 (15.5%) were diagnosed microscopically. The study further found an association between the prevalence of T. tenax and the severity of periodontitis [57]. Mehr et al. (2015) investigated the prevalence of T. tenax in the periodontal lesions of patients who also had Down’s syndrome in Iran. Moreover, 52 patients were presented with periodontal disease and 52 with healthy gingiva; the prevalence was 14 (26.9%) and 5 (9.6%), respectively [58]. Dybicz et al. (2018) used PCR as a method of detection for T. tenax in patients with health issues, including diabetes, renal transplant, and rheumatoid arthritis. Healthy individuals were used as controls and the primers targeted the ITS1-5.8S rRNA-ITS2 region rather than the 18S rRNA, as mentioned earlier. The prevalence of T. tenax in the oral cavity of the control group was 10.2% (33 of 226), 14.1% (13 of 92) in diabetics, 12% (6 of 50) in renal transplant patients, and 14% (7 of 50) in rheumatoid arthritis patients. A higher prevalence of T. tenax was revealed in adults from all groups involved [4]. Two additional studies have been published, which used PCR to determine the prevalence of T. tenax in patients with periodontal disease. In one study, primers designed from the RNA polymerase II rpbI gene for T. tenax strain NIH4 [59] were used and the other study used primers designed from the β-tubulin gene of T. vaginalis [60]. Benabdelkader et al. (2019) included 50 patients in the study—20 with gingivitis and 30 with periodontitis. The overall prevalence of T. tenax was 56% (28/50). Interestingly, it was found to be more prevalent in patients with periodontitis than in those with gingivitis, i.e., 70% (21/30) and 35% (7/20), respectively [59]. In a later study using PCR by Bracamonte-Wolf et al. (2019), out of 106 periodontitis patients and 85 healthy controls, the prevalence was 34% (36/106) in periodontitis patients and 28% (30/85) in the control group [60]. Collectively, these data showed a strong correlation between T. tenax and periodontal disease in humans. Additionally, these data represent the evolution of techniques developed over the years to detect and assess T. tenax in humans.
The variability in the prevalence of T. tenax in humans is possibly due to the difference in the standards of oral hygiene in the different populations and due to the increased sensitivity of the detection methods. In populations with low oral hygiene and poor socioeconomic backgrounds, the prevalence of T. tenax is high compared to populations with average or good oral hygiene and socioeconomic backgrounds [14]. In a cross-sectional survey carried out in Iran, Azadbakht K et al. found that the odds ratio (OR) of individuals brushing their teeth was 0.43 (95% CI: 0.21–0.88) in comparison with those who did not brush. Further, the OR of people who resided in urban areas was 0.22 (95% CI: 0.1–0.47) compared with those in rural places [61].
3.6.2. Domestic Dogs
Trichomonas tenax was first reported in canines in 1927 with 22 out of 23 canines testing positive [62]. It was not until there was a PCR method designed to detect T. tenax that studies on canines started to increase. This was probably due to the difficulty in culturing and detecting the flagellate by microscopy in a canine host. Alternatively, it may be due to a lack of interest in this protozoan in veterinary medicine (until recently). To date, only three recorded studies have been reported on the prevalence of T. tenax in canines using PCR (Table 5) [3,28,29]. The first study was reported by Patel et al. (2017) in the United Kingdom. A total of 92 samples were collected from canine dental plaque and screened for the presence of T. tenax and Entamoeba spp.; the prevalence of T. tenax was 56.2% (52/92) and Entamoebae spp. was 4.34% (4/92). Furthermore, the next-generation sequencing of healthy, gingivitis, early-stage periodontitis, and severe periodontitis samples showed the prevalence of T. tenax at 3.51%, 2.84%, 6.07%, and 35.0%, respectively. These findings were the first conclusive evidence of the presence of T. tenax in canine oral plaque [29]. Kellerová and Tachezy (2017) also investigated the occurrence of oral trichomonads in 111 domestic dogs and 122 cats using cell culture, PCR, and sequencing of the ITS1-5.8S rRNA-ITS2 regions. The prevalence percentages of T. tenax in dogs and cats were 8.1% and 4.1%, whilst for the different Trichomonas spp., they were 30.6% and 6.6%, respectively. The study also identified T. brixi as a new species. It concluded that dogs 3 years or older, as well as crossbred dogs, showed an increased prevalence of T. tenax [3]. Dybicz et al. (2018) used PCR to detect T. tenax in domesticated animals, such as horses, dogs, and cats. In the study, 142 dogs, 57 cats, and 102 horses were examined for the presence of T. tenax. The prevalence of T. tenax in canines was 4.92% (7 of 142). Additionally, 9 of 11 DNA sequences of trichomonad isolates showed 100% identity with T. tenax sequence obtained from the GenBank. The study concluded that oral trichomoniasis spreading between humans and domestic animals should be taken into consideration since the owners of three positive dogs also tested positive for T. tenax [4]. Studies on the prevalence of T. tenax in canines remain limited and more studies are needed worldwide.
3.7. Pathogenesis and Virulence Factors
Two systematic reviews on T. tenax have concluded that there is an association between T. tenax and periodontal disease [63,64]. The increase in the prevalence and association of T. tenax with periodontal disease from previous studies leads to questions concerning the pathogenicity of the oral flagellate. Extensive studies have been conducted on the pathogenicity of its close relative of the urogenital tract, T. vaginalis, and bacteria associated with periodontal disease [17,65]. However, the pathogenicity of T. tenax with regard to periodontal disease is far less documented [14]. To date, only seven studies have focused on the pathogenicity of T. tenax, and the proteins secreted by the oral flagellate that exhibit virulent characteristics. The first reported study on the pathogenicity of T. tenax was by Ribaux et al. (1979), who investigated the proteolytic activity of T. tenax in whole cells. Unfortunately, only the abstract could be obtained for this scoping review [66]. Ribaux et al. (1983) reported another study on the immunohistochemical location of fibronectin-like proteins on the cell surface of T. tenax. Two strains of T. tenax were used to establish an immunofluorescence staining procedure. The cells gave a positive fluorescence stain with anti-fibronectin anti-serum and the controls remained negative. They concluded that T. tenax produces fibronectin-like proteins that could be responsible for tissue adhesion [67]. These early works led other researchers to further investigate the proteolytic activity of T. tenax.
Bózner and Demeš (1991) carried out a study on the proteolytic activity in crude extracts and culture filtrates from T. tenax in SDS-polyacrylamide gels containing copolymerized gelatin. A total of seven distinct proteolytic bands were found, of which, three had molecular weights ranging from 35 to 56 kDa. These bands were SH-dependent, and their inhibitory sensitivities were characteristic of cysteine proteinases. The other four bands had molecular weights ranging from 76 to 270 kDa; these were SH-independent and were inhibited by a chelating agent, EDTA, suggesting they belong to the metalloproteinase family [65]. The authors continued studying the degradation of collagens I, III, IV, and V by extracellular proteinases of T. tenax. They concluded that the degradation of all four collagen types was temperature-dependent, with collagen IV being digested most effectively. They further stated that E-64 and the activation by reducing the agent dithiothreitol indicate that cysteine proteinases from T. tenax are responsible for the cleavage of collagen [68].
Another study that focused on the pathogenicity of T. tenax, carried out by Nagao et al. (2000), investigated the ability of T. tenax to lyse the red blood cells of sheep, horses, and humans. To achieve this, five fractions derived from intact cells, culture supernatant, cultural filtrate, cell debris, and lipid enriched fractions were used to assess the hemolytic activities under various conditions; only the culture supernatant was negative for hemolytic activity, the other four samples were positive for hemolytic activity. The authors concluded that the hemolytic activities were due to two types of hemolysins, one which is protein-like and the other lipid-like. The protein-like hemolysin was heat-labile and inhibited by various cysteine–proteinase inhibitors [69]. A decade later, El Sibaei et al. (2012) investigated the proteinase activities of seven isolates of T. tenax obtained from clinical patients in Egypt. The study also concluded that proteinase bands were observed, and these bands were intensified with a cysteine proteinase activator and disappeared completely in the presence of the cysteine proteinase inhibitor, further suggesting that the proteinases found were also cysteine proteinases [70]. These cysteine proteinases are the same virulent proteins that have been detected in T. vaginalis [71]. Recently, Ribeiro et al. (2015) performed a study where T. tenax fulfilled the requisites of a parasite, damaging different mammalian cells and behaving in a similar manner to T. vaginalis [17]. In short, limited work has been done on the pathogenicity of T. tenax, although there is growing evidence that it contributes to periodontal disease. Therefore, more studies are needed to better understand the pathophysiological processes of T. tenax infections in humans and other mammalian hosts with reference to periodontal disease.
Regarding the proteomics of parasites, exosome release has been gaining attention. Research has shown that many parasites excrete proteins enclosed in exosomes, which are considered potential virulent factors [72]. Exosomal studies performed on T. vaginalis have found that it secretes exosomes (such as those found in mammals) containing RNA and parasite-specific proteins [73]. Twu et al. (2013) illustrated that T. vaginalis exosomes deliver their contents to the host cell, modulating the cell’s immune response when fused. Additionally, the study was the first to show the potential role of exosomes in parasite-to-parasite communication [73]. Another study examined the major surface proteins (MSP) in the exosomes of the Leishmania spp. These proteins have been shown to digest extracellular matrix proteins. The study classified the MSP proteins released in L. infantum exosomes from promastigotes in avirulent procyclic (logarithmic), virulent stationary, and metacyclic stages, respectively, and found high levels of MSP in exosomes released from the stationary and metacyclic promastigotes than in the logarithmic promastigotes [74]. Work on exosomes is the new path that scientists are taking to gain a better idea of the pathogenicity of parasites. Therefore, it is important to investigate the pathogenicity of T. tenax by isolating exosomes, finding virulent factors by proteomic analysis, and elucidating exosome interactions with epithelial cells. This would be a significant contribution and addition to the pathogenicity of T. tenax.
3.8. Control and Prevention
Limited studies have been reported on the control and prevention of T. tenax despite its high prevalence among the human and canine populations. This could be because the pathogenesis of the oral protozoa remains unclear. However, studies have been documented on the treatment and prevention of its close relative T. vaginalis [73,75,76,77]. Trichomonas tenax can be transmitted between individuals by droplets from the mouth, kissing, or the use of contaminated dishes and drinking water [23]. Few studies have investigated treatment and prevention measures to control T. tenax infections. One study investigated the effects of non-surgical periodontal therapy (i.e., deep cleaning with scaling and root planning) on T. tenax and E. gingivalis in patients with chronic periodontitis. Rashidi Maybodi et al. (2016) reported that non-surgical treatment can reduce T. tenax and E. gingivalis in the oral environments of patients with chronic periodontitis [77]. Another study investigated the in vitro activities of selected mouth rinses on the reference strains of T. tenax and E. gingivalis. In this study, two standard strains of T. tenax (ATCC 30207) and E. gingivalis (ATCC 30927) were used, and metronidazole was used along with fourteen mouth rinses. The activities of the preparations were evaluated based on the ratio of dead to living cells after incubation at (37 °C) for 1, 10, and 30 min. The death of protozoa was categorized by the lack of movement and changes in the shape and characteristics of cell disintegration. The study concluded that all mouth rinses tested were effective on both protozoa [78]. There are no vaccines or drugs reported in the literature to effectively treat T. tenax infections in the oral cavity. However, metronidazole and tinidazole are the drugs approved by the U.S. FDA and EMA to treat vaginal trichomoniasis caused by T. vaginalis [72,73,75,79]. Since both protozoa are flagellates and closely related, there may be a possibility that metronidazole and tinidazole are effective at treating oral trichomoniasis caused by T. tenax as well [80]. Moreover, studies focusing on the treatment and prevention of T. tenax are needed since it is prevalent in both humans and animals that exhibit signs of periodontal disease [81]. It is worth noting that T. tenax has been found in the urogenital tracts of humans [25,26] and, hence, possibly contributes to human trichomoniasis (although how significant a contribution is to be determined).
4. Discussion
Periodontal disease is a public health concern for humans and dogs worldwide. For years, the disease has been associated with the oral flagellate T. tenax, although the cause and effect have not been confirmed, and much is unknown about the pathology of the parasite. Trichomonas tenax was first seen in the oral cavities of humans, and site and host predilections were assumed. However, it is now found in the lungs [82,83,84,85], lymph nodes [86], vaginal samples [27], and subhepatic abscesses [87]. When it comes to the host, T. tenax has been found in humans, dogs, birds, cats, horses, and monkeys. A parasite that has been found in so many different sites and hosts, with zoonotic potential, should not be neglected, especially since the transmission between these hosts is not yet understood. Studies have shown its parasitic and pathogenic capabilities when in contact with mammalian cells. Researchers are now questioning this protozoan zoonotic potential because of the wide range of hosts it has discovered [17,88]. Its prevalence is increasing in humans and dogs, with the latter needing more research. The prevalence of T. tenax ranges between 1 and 90% in humans and 8–96% in dogs (Table 4 and Table 5). This gradual increase in prevalence worldwide should not be taken lightly. The prevalence in humans is in accordance with results reported by Szczepaniak et al. 2016; however, the prevalence in dogs was higher than what was reported by Norberg et al. 2014, due to limited studies. Moreover, based on studies published so far, it is clear that there is a direct association between periodontal disease and T. tenax infection [89,90,91]. Presently, several methods can detect T. tenax, including culture, microscopy, PCR, and LAMP. Thus far, LAMP is the most sensitive and specific method used to detect T. tenax, with a limit of detection of one cell, followed by PCR, and then microscopy, which is considered a gold standard [1,52]. Virulent proteins have been extracted from T. tenax similar to the ones found in its close relative, T. vaginalis [65,66,67,68,92]. However, the pathogenicity is still largely unknown. The area of exosome studies has shown great potential in finding the pathogenicity of various eukaryotic cells. With this new area of proteomics and the recent publication of the T. tenax draft genome [93], we can possibly move closer to mapping the pathway of T. tenax and developing a possible drug therapy that can control and prevent the transmission of this oral flagellate.
5. Conclusions
Periodontal disease is a major public health concern worldwide with prevalence ranging from 1 to 90%; it was ranked the 11th most prevalent disease condition in the world in 2016 [94]. Presently, microscopy, PCR, and LAMP are the available techniques used to detect T. tenax, with the latter being the most sensitive. In this scoping review, gaps in the knowledge and areas of research on T. tenax are indicated. The evidence, however, suggests that not only does T. tenax play a role in periodontal disease but there is an association between both oral flagellate and periodontal disease patients. It is imperative that the mechanism in which T. tenax adheres (and causes damage) to gums be functionally elucidated. This knowledge could increase the ability to develop other effective drug therapies to control this parasite and potentially decrease the prevalence of periodontal disease.

Table 3.
Molecular diagnosis of Trichomonas tenax.
Table 3.
Molecular diagnosis of Trichomonas tenax.
| Method | Target Gene | Primers | Expected Amplicon Size (bp) | Limit of Detection | References |
|---|---|---|---|---|---|
| PCR | 18S rRNA | 5′AGTTCCATCGATGCCATTC3′ 5′GCATCTAAGGACTTAGACG3′ | 862 | 100 fg or 5 cells | [1] |
| PCR | ITS1-5.8S rRNA-ITS2 | 5′GAGAAGTCGTAACAAGGTACG3′ 5′ATGCTTCAGTTCAGCGGGTCT3′ | 368 | N/A | [4] |
| PCR | rpb1 gene | 5′GCTGTCATCTCTTGTGGGGCTG3′ 5′AAACTCATGGGAGCTGCTGGTTC3′ | 3048 | N/A | [59] |
| PCR | Beta-tubulin gene | 5′ATACTCTATCGTCCCATCTC3′ 5′GCCATCATGTTCTTGTTATCG3′ | 405 | N/A | [60] |
| LAMP | ITS1-5.8S rRNA-ITS2 | 5′GTCATGATGTATGCAACTCCGG-TCCTCACACGATGAAGAACG3′ 5′GGTTAATCTTTGAATGCAAATTGCG-TGTACTGTTACACGCATGCTTCT3′ 5′ACATTATGCCACGTTCTTCATCG3′ 5′TGCGCTAAACTTGGCTTCGG3′ 5′AGCAATGGATGTCTTGGC3′ 5′GCAGACAACGTAAGTTTGT3′ | N/A | 10 fg or 1 cell | [52] |
N/A—not available; LAMP—loop-mediated isothermal amplification.

Table 4.
Prevalence of Trichomonas tenax in humans with or without periodontitis.
Table 4.
Prevalence of Trichomonas tenax in humans with or without periodontitis.
| Periodontitis Patients | Healthy Individuals | |||||
|---|---|---|---|---|---|---|
| Country or Region | No. Tested (Positive) | Prevalence (%) | No. Tested (Positive) | Prevalence (%) | Method * (M, PCR) | References |
| America | ||||||
| Brazil | 100 (51) | 51 | N/A | N/A | M | [21] |
| Chile | 30 (21) | 70 | N/A | N/A | PCR | [60] |
| USA | 350 (315) | 90 | N/A | N/A | M | [38] |
| Asia | ||||||
| Indonesia | 373 (19) | 5.1 | N/A | N/A | M | [95] |
| Iran | 50 (3) | 6 | 50 (0) | 0% | M | [56] |
| Iran Iran | 160 (34) | 21 | 160 (3) | 2 | PCR | [57] |
| 52 (14) | 19 | 52 (5) | 3 | PCR | [58] | |
| Iraq Iraq | 143 (12) | 8 | 271 (11) | 4 | M | [23] |
| 383 (31) | 8 | N/A | N/A | M | [55] | |
| Japan | 9 (5) | 56 | N/A | N/A | PCR | [1] |
| Turkey Turkey | 220 (2) | 1 | N/A | N/A | M | [22] |
| 107 (3) | 3 | N/A | N/A | M | [54] | |
| Thailand | 90(23) | 25.6 | 94(3) | 3.2 | PCR | [96] |
| Europe | ||||||
| Croatia | 51 (18) | 36 | N/A | N/A | M | [53] |
| France | 106 (37) | 35 | 85 (16) | 19 | PCR | [59] |
| Poland | 192 (26) | 14 | 226 (33) | 15 | PCR | [4] |
* M—microscopy; N/A—not available.

Table 5.
Prevalence of Trichomonas tenax in a domestic dog with or without periodontitis.
Table 5.
Prevalence of Trichomonas tenax in a domestic dog with or without periodontitis.
| Periodontitis Patients | Healthy Individuals | |||||
|---|---|---|---|---|---|---|
| Country or Region | No. Tested (positive) | Prevalence (%) | No. Tested (positive) | Prevalence (%) | Method * (M, PCR) | References |
| Czechia | 111 (9) | 8 | N/A | N/A | PCR | [3] |
| Poland | 142 (7) | 5 | N/A | N/A | PCR | [28] |
| UK | 92 (52) | 56 | 20 (4) | 20 | PCR | [29] |
| USA | 23 (22) | 96 | N/A | N/A | M | [62] |
* M—microscopy; N/A—not available.
Author Contributions
Conceptualization, C.Y.; methodology, M.A.M., N.Y. and S.M..; investigation, M.A.M., J.K. and C.Y.; writing—original draft preparation, M.A.M.; writing—review and editing, N.Y., J.K., S.M. and C.Y.; supervision, C.Y.; project administration, C.Y.; funding acquisition, C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by intramural research grants from the Ross University School of Veterinary Medicine (RUSVM), grants 41002-2021 and 41004-2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Grace Carr Benjamin of the Ross University School of Veterinary Medicine for accessing the full articles. The publication cost was provided by the Associate Dean for Research and Postgraduate Studies of Ross University School of Veterinary Medicine.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kikuta, N.; Yamamoto, A.; Fukura, K.; Goto, N. Specific and sensitive detection of Trichomonas tenax by the polymerase chain reaction. Lett. Appl. Microbiol. 1997, 24, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kucknoor, A.S.; Mundodi, V.; Alderete, J.F. Genetic identity and differential gene expression between Trichomonas vaginalis and Trichomonas tenax. BMC Microbiol. 2009, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Kellerová, P.; Tachezy, J. Zoonotic Trichomonas tenax and a new trichomonad species, Trichomonas brixi n. sp., from the oral cavities of dogs and cats. Int. J. Parasitol. 2017, 47, 247–255. [Google Scholar] [CrossRef]
- Dybicz, M.; Perkowski, K.; Sędzikowska, A.; Baltaza, W.; Chomicz, L. Studies on prevalence of infection with Trichomonas tenax identified by molecular techniques—In respect to oral health of patients with various systemic disease requiring immunosuppressive therapy. Ann. Parasitol. 2018, 64, 193–197. [Google Scholar] [PubMed]
- Duboucher, C.; Caby, S.; Chabé, M.; Gantois, N.; Delgado-Viscogliosi, P.; Pierce, R.; Capron, M.; Dei-Cas, E.; Viscogliosi, E. Human pulmonary trichomonoses. Press Med. 2007, 36, 835–839. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Mehlhorn, H. Human Parasites: Diagnosis, Treatment, Prevention; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Hamadto, H.H.A.; El Hayawan, I.A.H.; Abdallah, K.F.; Abd El-Maboud, A.I.; Mohammed, O.I.; Omar, G.H.E. Relation between Trichomonas tenax and pulmonary diseases. Egypt J. Med. Sci. 2014, 35, 633–652. [Google Scholar]
- Honigberg, B.M.; Lee, J.J. Structure and division of Trichomonas tenax (O. F. Müller). Am. J. Epidemiol. 1959, 69, 177–201. [Google Scholar] [CrossRef]
- Dobell, C. The common flagellate of the human mouth, Trichomonas tenax (O.F.M.): Its discovery and its nomenclature. Parasitology 1939, 31, 138–146. [Google Scholar] [CrossRef]
- Goodey, T.; Wellings, A.W. Observations on Entamoeba gingivalis from the human mouth, with a note on the trichomonad flagellate Tetratrichomonas buccalis n. sp. Parasitology 1917, 9, 537–559. [Google Scholar] [CrossRef]
- Wenrich, D.H. Comparative morphology of the trichomonad flagellates of man. Am. J. Trop. Med. Hyg. 1944, 24, 39–51. [Google Scholar] [CrossRef]
- Mehlhorn, H. Trichomonas tenax. In Encyclopedia of Parasitology; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Marty, M.; Lemaitre, M.; Kémoun, P.; Morrier, J.J.; Monsarrat, P. Trichomonas tenax and periodontal diseases: A concise review. Parasitology 2017, 144, 1417–1425. [Google Scholar] [CrossRef]
- Kofoid, C.A.; Hinshaw, H.C.; Johnstone, H.G. Animal parasites of the mouth and their relation to dental disease**from the protozoological section of the California Stomatological Research Group and the Department of Zoology of the University of California, under the direction of Prof. Charles A. Kof. J. Am. Dent. Assoc. 1929, 16, 1436–1455. [Google Scholar] [CrossRef]
- Ribaux, C.L.; Joffre, A.; Magloire, H. Trichomonas tenax: Ultrastructure of giant forms. J. Biol. Buccale 1988, 16, 19–23. [Google Scholar] [PubMed]
- Ribeiro, L.C.; Santos, C.; Benchimol, M. Is Trichomonas tenax a parasite or a commensal? Protist 2015, 166, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef]
- Yao, C.; Ketzis, J.K. Aberrant and accidental trichomonad flagellate infections: Rare or underdiagnosed? Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 64–72. [Google Scholar] [CrossRef]
- Atwood Kofoid, C. The protozoa of the human mouth. J. Parasitol. 1929, 15, 151–174. [Google Scholar] [CrossRef]
- Norberg, C.M.B.M. Entamoeba Gingivalis (Gros, 1849) and Trichomonas Tenax (Muller, 1773) oral infections in patients from Baixada Fluminense, Province of Rio de Janeiro, Brazil. Sci. J. Public Health 2014, 2, 288–292. [Google Scholar] [CrossRef]
- Özçelik, S.; Gedik, T.; Gedik, R.; Malatyali, E. Investigation of the relationship between oral and dental health and presence of Entamoeba gingivalis and Trichomonas tenax. Turk. Parazitol. Derg. 2010, 34, 155–159. [Google Scholar] [CrossRef]
- Mahdi, N.K.; al-Saeed, A.T. Trichomonas tenax in Basrah, Iraq. J. Pak. Med. Assoc. 1993, 43, 261–262. [Google Scholar]
- Hegner, R.; Ratcliffe, H. Trichomonads from the vagina of the monkey, from the mouth of the cat and man, and from the intestine of the monkey, Opossum and Prairie-dog. J. Parasitol. 1927, 14, 27. [Google Scholar] [CrossRef]
- Fedorych, P.V.; Mavrov, G.I.; Osinska, T.V.; Shcherbakova, Y.V. Protozoan genital invasions caused by the representatives of trichomonas and giardia. Wiad. Lek. 2020, 73, 380–383. [Google Scholar] [CrossRef]
- Brosh-Nissimov, T.; Hindiyeh, M.; Azar, R.; Smollan, G.; Belausov, N.; Mandelboim, M.; Rahav, G.; Keller, N.; Gefen-Halevi, S. A false-positive Trichomonas vaginalis result due to Trichomonas tenax presence in clinical specimens may reveal a possible T. tenax urogenital infection. Clin. Microbiol. Infect. 2019, 25, 123–124. [Google Scholar] [CrossRef]
- Crucitti, T.; Jespers, V.; Mulenga, C.; Khondowe, S.; Vandepitte, J.; Buvé, A. Trichomonas vaginalis is highly prevalent in adolescent girls, pregnant women, and commercial sex workers in Ndola, Zambia. Sex. Transm. Dis. 2010, 37, 223–227. [Google Scholar] [CrossRef]
- Dybicz, M.; Perkowski, K.; Baltaza, W.; Padzik, M.; Sędzikowska, A.; Chomicz, L. Molecular identification of trichomonas tenax in the oral environment of domesticated animals in poland—Potential effects of host diversity for human health. Ann. Agric. Environ. Med. 2018, 25, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Colyer, A.; Harris, S.; Holcombe, L.; Andrew, P. The prevalence of canine oral protozoa and their association with periodontal disease. J. Eukaryot. Microbiol. 2017, 64, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrero, M.C.; Garijo-Toledo, M.M.; Liebhart, D.; Ganas, P.; Martínez-Díaz, R.A.; Ponce-Gordo, F.; Carrero-Ruiz, A.; Hess, M.; Gómez-Muñoz, M.T. Novel avian oropharyngeal trichomonads isolated from European turtle doves (Streptopelia turtur) and racing pigeons (Columba livia): Genetic and morphometric characterisation of clonal cultures. Infect. Genet. Evol. 2017, 55, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Landman, W.J.M.; Gantois, N.; Sawant, M.; Majoor, F.A.; van Eck, J.H.H.; Viscogliosi, E. Prevalence of trichomonads in the cloaca of wild wetland birds in the Netherlands. Avian Pathol. 2021, 50, 465–476. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, J.; Wang, F.; Li, H.; Zhao, X. Prevalence of Trichomonas spp. in domestic pigeons in Shandong Province, China, and genotyping by restriction fragment length polymorphism. Vet. J. 2016, 211, 88–93. [Google Scholar] [CrossRef]
- Lennon, R.J.; Dunn, J.C.; Stockdale, J.E.; Goodman, S.J.; Morris, A.J.; Hamer, K.C. Trichomonad parasite infection in four species of Columbidae in the UK. Parasitology 2013, 140, 1368–1376. [Google Scholar] [CrossRef]
- Grabensteiner, E.; Bilic, I.; Kolbe, T.; Hess, M. Molecular analysis of clonal trichomonad isolates indicate the existence of heterogenic species present in different birds and within the same host. Vet. Parasitol. 2010, 172, 53–64. [Google Scholar] [CrossRef]
- Gerhold, R.W.; Yabsley, M.J.; Smith, A.J.; Ostergaard, E.; Mannan, W.; Cann, J.D.; Fischer, J.R. Molecular characterization of the Trichomonas gallinae morphologic complex in the United States. J. Parasitol. 2008, 94, 1335–1341. [Google Scholar] [CrossRef]
- Lynch, K. Trichomoniasis of the vagina and the mouth. Cultivation of the causative organism and experimental infection. (A Preliminary Communication). Am. J. Trop. Dis. Prev. Med. 1915, 2, 627–634. [Google Scholar]
- Ohira, T.; Noguchi, H. The cultivation of trichomonas of the human mouth (Tetratrichomonas hominis). J. Exp. Med. 1917, 25, 341–347. [Google Scholar] [CrossRef]
- Hinshaw, H.C. Correlation of protozoan infections of human mouth with extent of certain lesions in pyorrhea alveolaris. Proc. Soc. Exp. Biol. Med. 1926, 24, 71–73. [Google Scholar] [CrossRef]
- Hogue, M.J. Studies on Trichomonas buccalis. Am. J. Trop. Med. Hyg. 1926, s1-6, 75–89. [Google Scholar] [CrossRef]
- Diamond, L.S.; Bartgis, I.L. Axenic cultivation of Trichomonas tenax, the oral flagellate of man I. Establishment of cultures. J. Protozool. 1962, 9, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Wantland, W.W.; Wantland, E.M.; Winquist, D.L. Collection, identification, and cultivation of oral protozoa. J. Dent. Res. 1963, 42, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Hayashi, A.; Nakamura, Y.; Kato, M.; Sato, M.; Nitta, H.; Namikawa, I. Growth of Trichomonas tenax in tissue culture medium containing complement and antiserum to the accompanying bacteria. Jpn. J. Oral Biol. 1986, 28, 731–736. [Google Scholar] [CrossRef]
- Pardi, G.; Perrone, M.; Mazzali de Ilja, R. Incidencia de Trichomonas tenax en pacientes con periodontitis marginal crónica. Acta Odontológica Venez. 2002, 40, 152–159. [Google Scholar]
- Lin, C.; Ying, F.; Lai, Y.; Li, X.; Xue, X.; Zhou, T.; Hu, D. Use of nested PCR for the detection of trichomonads in bronchoalveolar lavage fluid. BMC Infect. Dis. 2019, 19, 512. [Google Scholar] [CrossRef]
- Szczepaniak, K.; Łojszczyk-Szczepaniak, A.; Tomczuk, K.; Skrzypek, T.; Lisiak, B.; Abd-Al-Hammza Abbass, Z. Canine Trichomonas tenax mandibular gland infestation. Acta Vet. Scand. 2016, 58, 15. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Lenkowski, M.; Nijakowski, K.; Kaczmarek, M.; Surdacka, A. The loop-mediated isothermal amplification technique in periodontal diagnostics: A systematic review. J. Clin. Med. 2021, 10, 1189. [Google Scholar] [CrossRef]
- Kato, H.; Yoshida, A.; Ansai, T.; Watari, H.; Notomi, T.; Takehara, T. Loop-mediated isothermal amplification method for the rapid detection of Enterococcus faecalis in infected root canals. Oral Microbiol. Immunol. 2007, 22, 131–135. [Google Scholar] [CrossRef] [PubMed]
- de Lira Nunes, M.; Mendes-Marques, C.L.; de Almeida, A.M.P.; Leal, N.C. The development of a Loop-mediated isothermal amplification (LAMP) procedure for plague diagnostic. Am. J. Anal. Chem. 2014, 05, 1069–1077. [Google Scholar] [CrossRef]
- Reyes, J.C.B.; Solon, J.A.A.; Rivera, W.L. Development of a loop-mediated isothermal amplification assay for detection of Trichomonas vaginalis. Diagn. Microbiol. Infect. Dis. 2014, 79, 337–341. [Google Scholar] [CrossRef]
- Li, W.; Lee, S.Y.; Back, C.G.; Ten, L.N.; Jung, H.Y. Loop-mediated isothermal amplification for the detection of Xanthomonas arboricola pv. Pruni in peaches. Plant Pathol. J. 2019, 35, 635–643. [Google Scholar] [CrossRef]
- Matthew, M.A.; Christie, J.; Yang, N.; Yao, C. A Loop-mediated isothermal amplification (LAMP) assay specific to Trichomonas tenax is suitable for use at point-of-care. Microorganisms 2022, 10, 594. [Google Scholar] [CrossRef]
- Potočki-Tukša, K.; Granić, J.; Šegović, S.; Buntak-Kobler, D. Trichomonas Tenax in human oral cavity. Acta Stomatol. Croat. 1993, 27, 255–261. [Google Scholar]
- Yazar, S.; Çetinkaya, Ü.; Hamamcı, B.; Alkan, A.; Şişman, Y.; Esen, Ç.; Kolay, M. Investigation of Entamoeba gingivalis and Trichomonas tenax in periodontitis or gingivitis patients in Kayseri. Turk. Parazitolojii Derg. 2016, 40, 17. [Google Scholar] [CrossRef]
- Ali Mohammed, S.A.; Mohsen Alwaaly, A.B. Prevalence Trichomonas tenax in Karbala Governorate. J. Phys. Conf. Ser. 2019, 1294, 062030. [Google Scholar] [CrossRef]
- Ghabanchi, J.; Zibaei, M.; Afkar, M.D.; Sarbazie, A.H. Prevalence of oral Entamoeba gingivalis and Trichomonas tenax in patients with periodontal disease and healthy population in Shiraz, southern Iran. Indian J. Dent. Res. 2010, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Athari, A.; Soghandi, L.; Haghighi, A.; Kazemi, B. Prevalence of oral trichomoniasis in patients with periodontitis and gingivitis using PCR and direct smear. Iran J. Public Health 2007, 36, 33–37. [Google Scholar]
- Mehr, A.K.; Zarandi, A.; Anush, K. Prevalence of oral Trichomonas tenax in periodontal lesions of down syndrome in Tabriz, Iran. J. Clin. Diagn. Res. 2015, 9, ZC88. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkader, S.; Andreani, J.; Gillet, A.; Terrer, E.; Pignoly, M.; Chaudet, H.; Aboudharam, G.; La Scola, B. Specific clones of Trichomonas tenax are associated with periodontitis. PLoS ONE 2019, 14, e0213338. [Google Scholar] [CrossRef]
- Bracamonte-Wolf, C.; Orrego, P.R.; Muñoz, C.; Herrera, D.; Bravo, J.; Gonzalez, J.; Varela, H.; Catalán, A.; Araya, J.E. Observational cross-sectional study of Trichomonas tenax in patients with periodontal disease attending a Chilean university dental clinic. BMC Oral Health 2019, 19, 207. [Google Scholar] [CrossRef]
- Azadbakht, K.; Baharvand, P.; Artemes, P.; Niazi, M.; Mahmoudvand, H. Prevalence and risk factors of oral cavity parasites in pregnant women in Western Iran. Parasite Epidemiol. Control 2022, 19, e00275. [Google Scholar] [CrossRef]
- Hegner, R.; Ratcliffe, H. Trichomonads from the mouth of the dog. J. Parasitol. 1927, 14, 51. [Google Scholar] [CrossRef]
- Eslahi, A.V.; Olfatifar, M.; Abdoli, A.; Houshmand, E.; Johkool, M.G.; Zarabadipour, M.; Abadi, P.A.; Ghorbani, A.; Mirzadeh, M.; Badri, M. The neglected role of Trichomonas tenax in oral diseases: A systematic review and meta-analysis. Acta Parasitol. 2021, 66, 715–732. [Google Scholar] [CrossRef]
- Bisson, C.; Dridi, S.M.; Machouart, M. Assessment of the role of Trichomonas tenax in the etiopathogenesis of human periodontitis: A systematic review. PLoS ONE 2019, 14, e0226266. [Google Scholar] [CrossRef] [PubMed]
- Bózner, P.; Demeš, P. Cell-associated and extracellular proteolytic activity of an oral flagellate, Trichomonas tenax. Arch. Oral Biol. 1991, 36, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Ribaux, C.L. Study of an oral protozoan Trichomonas tenax using scanning and transmission electron microscopy. J. Biol. Buccale 1979, 7, 157–168. [Google Scholar] [PubMed]
- Ribaux, C.L.; Magloire, H.; Joffre, A.; Morrier, J.J. Immunohistochemical localization of fibronectin-like protein on the cell surface of the oral flagelatte Trichomonas tenax. J. Biol. Buccale 1983, 11, 41–51. [Google Scholar]
- Bózner, P.; Demeš, P. Degradation of collagen types I, III, IV and V by extracellular proteinases of an oral flagellate Trichomonas tenax. Arch. Oral Biol. 1991, 36, 765–770. [Google Scholar] [CrossRef]
- Nagao, E.; Yamamoto, A.; Igarashi, T.; Goto, N.; Sasa, R. Two distinct hemolysins in Trichomonas tenax ATCC 30207. Oral Microbiol. Immunol. 2000, 15, 355–359. [Google Scholar] [CrossRef]
- El Sibaei, M.M.; Abdel-Fattah, N.S.; Ahmed, S.A.; Abou-Seri, H.M. Growth kinetics, antigen profiling, and proteinase activity of Egyptian Trichomonas tenax isolates derived from patients having oral infections. Exp. Parasitol. 2012, 130, 416–422. [Google Scholar] [CrossRef]
- Neale, K.A.; Alderete, J.F. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect. Immun. 1990, 58, 157–162. [Google Scholar] [CrossRef]
- Nawaz, M.; Malik, M.I.; Hameed, M.; Zhou, J. Research progress on the composition and function of parasite-derived exosomes. Acta Trop. 2019, 196, 30–36. [Google Scholar] [CrossRef]
- Twu, O.; de Miguel, N.; Lustig, G.; Stevens, G.C.; Vashisht, A.A.; Wohlschlegel, J.A.; Johnson, P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host:parasite interactions. PLoS Pathog. 2013, 9, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Kelly, P.H.; Singh, B.K.; Pope, R.M.; Kim, P.; Zhanbolat, B.; Wilson, M.E.; Yao, C. Extracellular release of virulence factor major surface protease via exosomes in Leishmania infantum promastigotes. Parasites Vectors 2018, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Burgess, D. Trichomoniasis. Clin. Microbiol. Rev. 2004, 17, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Cudmore, S.L.; Garber, G.E. Prevention or treatment: The benefits of Trichomonas vaginalis vaccine. J. Infect. Public Health 2010, 3, 47–53. [Google Scholar] [CrossRef]
- Rashidi Maybodi, F.; Haerian Ardakani, A.; Fattahi Bafghi, A.; Haerian Ardakani, A.; Zafarbakhsh, A. The effect of nonsurgical periodontal therapy on Trichomonas tenax and Entamoeba gingivalis in patients with chronic periodontitis. J. Dent. 2016, 17, 171–176. [Google Scholar]
- Moroz, J.; Kurnatowska, A.J.; Kurnatowski, P. The in vitro activity of selected mouthrinses on the reference strains of Trichomonas tenax and Entamoeba gingivalis. Ann. Parasitol. 2019, 65, 257–265. [Google Scholar] [CrossRef]
- Bouchemal, K.; Bories, C.; Loiseau, P.M. Strategies for prevention and treament of Trichomonas vaginalis infections. Am. Soc. Microbiol. 2017, 30, 811–825. [Google Scholar] [CrossRef]
- Shiota, T.; Arizono, N.; Morimoto, T.; Shimatsu, A.; Nakao, K. Trichomonas tenax empyema in an immunocompromised patient with advanced cancer. Parasite 1998, 5, 375–377. [Google Scholar] [CrossRef]
- Prieto-Prieto, J.; Calvo, A. Microbiological basis of oral infections and sensitivity to antibiotics. Med. Oral Patol. Oral Cir. Bucal 2004, 9 (Suppl. S15-8), 11–14. [Google Scholar]
- Wu, Y.; Ye, Y.; Yang, Y.; Yang, W.; Lin, J.; Cao, K. Pyopneumothorax from coinfection by Trichomonas tenax and Geotrichum capitatum in a child from China: A case report. BMC Infect. Dis. 2021, 21, 842. [Google Scholar] [CrossRef]
- Dimasuay, K.G.B.; Rivera, W.L. First report of Trichomonas tenax infections in the Philippines. Parasitol. Int. 2014, 63, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.S.E.; Rahman, G.A. Pulmonary trichomoniasis: Improved diagnosis by using polymerase chain reaction targeting Trichomonas tenax 18S rRNA gene in sputum specimens. J. Egypt. Soc. Parasitol. 2004, 34, 197–211. [Google Scholar]
- Hersh, S.M. Pulmonary trichomoniasis and Trichomonas tenax. J. Med. Microbiol. 1985, 20, 1–10. [Google Scholar] [CrossRef]
- Duboucher, C.; Farto-Bensasson, F.; Chéron, M.; Peltier, J.Y.; Beaufils, F.; Périé, G. Lymph node infection by Trichomonas tenax: Report of a case with co-infection by Mycobacterium tuberculosis. Hum. Pathol. 2000, 31, 1317–1321. [Google Scholar] [CrossRef]
- Jakobsen, E.B.; Friis-Møller, A.; Friis, J. Trichomonas species in a subhepatic abscess. Eur. J. Clin. Microbiol. 1987, 6, 296–297. [Google Scholar] [CrossRef]
- Maritz, J.M.; Land, K.M.; Carlton, J.M.; Hirt, R.P. What is the importance of zoonotic trichomonads for human health? Trends Parasitol. 2014, 30, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, D.F.; Sallam, M.; Garcia, G.; Santi-Rocca, J. Parasites in periodontal health and disease: A systematic review and meta-analysis. Adv. Exp. Med. Biol. 2022, 1373, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Arpag, O.F.; Kaya, O.M. Presence of Trichomonas tenax and Entamoeba gingivalis in peri-implantitis lesions. Quintessence Int. 2020, 51, 212–218. [Google Scholar] [CrossRef]
- Dubar, M.; Zaffino, M.L.; Remen, T.; Thilly, N.; Cunat, L.; Machouart, M.C.; Bisson, C. Protozoans in subgingival biofilm: Clinical and bacterial associated factors and impact of scaling and root planing treatment. J. Oral Microbiol. 2020, 12, 12. [Google Scholar] [CrossRef]
- Yamamoto, A.; Asaga, E.; Nagao, E.; Igarashi, T.; Goto, N. Characterization of the cathepsin B-like proteinases of Trichomonas tenax ATCC 30207. Oral Microbiol. Immunol. 2000, 15, 360–364. [Google Scholar] [CrossRef]
- Yang, N.; Christine, J.; Keen, H.L.; Matthew, M.A.; Yao, C. Draft genome sequence of Trichomonas tenax strain Hs-4:NIH. Microbiol. Resour. Announc. 2022, 11, e00157-22. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2020. [Google Scholar] [CrossRef]
- Palmieri, J.R.; Halverson, B.A.; Sudjadi, S.T.; Purnomo; Masbar, S. Parasites found in the mouths of inhabitants of three villages of South Kalimantan (Borneo), Indonesia. Trop. Geogr. Med. 1984, 36, 57–59. [Google Scholar] [PubMed]
- Yaseen, A.; Mahafzah, A.; Dababseh, D.; Taim, D.; Hamdan, A.A.; Al-Fraihat, E.; Hassona, Y.; Şahin, G.Ö.; Santi-Rocca, J.; Sallam, M. Oral colonization by Entamoeba gingivalis and Trichomonas tenax: A PCR-based study in health, gingivitis, and periodontitis. Front. Cell. Infect. Microbiol. 2021, 1204. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).