COVID-19: Current Status in Gastrointestinal, Hepatic, and Pancreatic Diseases—A Concise Review
Abstract
:1. Introduction
2. The Gastrointestinal Tract in the Pathogenesis of COVID-19
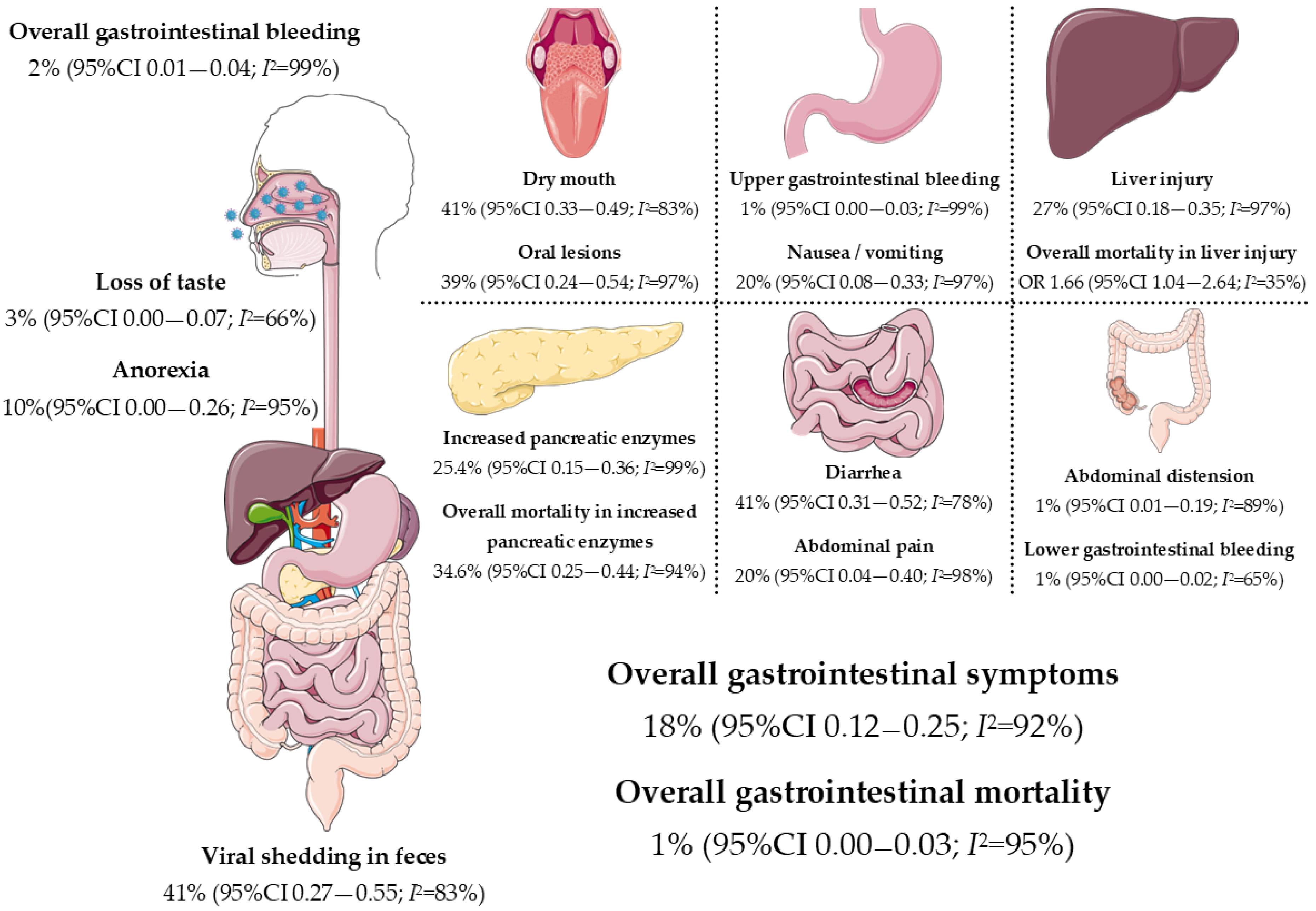
3. COVID-19 and Gastrointestinal Symptoms
4. COVID-19 and the Liver
4.1. Proposed Mechanisms of Liver Injury
4.2. Implications in Fatty Liver Disease
4.3. Implications in Liver Cirrhosis
4.4. Implications in Liver Transplantation
4.5. COVID-19 and Inflammatory Bowel Disease
4.6. COVID-19 and the Pancreas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 17 October 2020).
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 Infection: Origin, Transmission, and Characteristics of Human Coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Amoroso, L.; Simonato, L.E.; Ramos, R.R. Phylogeny and Pathogenesis of SARS-CoV-2: A Systematic Study. J. Mod. Med. Chem. 2020, 8, 49–55. [Google Scholar]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- D’Amico, F.; Baumgart, D.C.; Danese, S.; Peyrin-Biroulet, L. Diarrhea during COVID-19 Infection: Pathogenesis, Epidemiology, Prevention and Management. Clin. Gastroenterol. Hepatol. 2020, 18, 1663–1672. [Google Scholar] [CrossRef]
- Hindson, J. COVID-19: Faecal–Oral Transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 259. [Google Scholar] [CrossRef]
- Tian, Y.; Rong, L.; Nian, W.; He, Y. Review Article: Gastrointestinal Features in COVID-19 and the Possibility of Faecal Transmission. Aliment. Pharmacol. Ther. 2020, 51, 843–851. [Google Scholar] [CrossRef]
- Yeo, C.; Kaushal, S.; Yeo, D. Enteric Involvement of Coronaviruses: Is Faecal–Oral Transmission of SARS-CoV-2 Possible? Lancet Gastroenterol. Hepatol. 2020, 5, 335–337. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2 Fei. Gastroenterology 2020, 158, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Farsi, Y.; Tahvildari, A.; Arbabi, M.; Vazife, F.; Sechi, L.A.; Shahidi Bonjar, A.H.; Jamshidi, P.; Nasiri, M.J.; Mirsaeidi, M. Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 804644. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hsu, M.; Lee, M.; Chou, C. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses 2022, 14, 1188–1205. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Li, H.; Xu, J.; Yang, M.; Ma, C.; Li, J.; Zhao, S.; Wang, H.; Yang, Y.; Yu, W.; et al. The Gastrointestinal Tract Is an Alternative Route for SARS-CoV-2 Infection in a Nonhuman Primate Model. Gastroenterology 2021, 160, 1647–1661. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [PubMed]
- Irham, L.M.; Chou, W.H.; Calkins, M.J.; Adikusuma, W.; Hsieh, S.L.; Chang, W.C. Genetic Variants That Influence SARS-CoV-2 Receptor TMPRSS2 Expression among Population Cohorts from Multiple Continents. Biochem. Biophys. Res. Commun. 2020, 529, 263–269. [Google Scholar] [CrossRef]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative Genetic Analysis of the Novel Coronavirus (2019-NCoV/SARS-CoV-2) Receptor ACE2 in Different Populations. Cell Discov. 2020, 6, 11. [Google Scholar] [CrossRef]
- Patel, K.P.; Patel, P.A.; Vunnam, R.R.; Hewlett, A.T.; Jain, R.; Jing, R.; Vunnam, S.R. Gastrointestinal, Hepatobiliary, and Pancreatic Manifestations of COVID-19. J. Clin. Virol. 2020, 128, 104386. [Google Scholar] [CrossRef]
- Schmulson, M.; Dávalos, M.F.; Berumen, J. Beware: Gastrointestinal Symptoms Can Be a Manifestation of COVID-19. Rev. Gastroenterol. Mex. 2020, 85, 282–287. [Google Scholar] [CrossRef]
- Deidda, S.; Tora, L.; Firinu, D.; Del Giacco, S.; Campagna, M.; Meloni, F.; Orrù, G.; Chessa, L.; Carta, M.G.; Melis, A.; et al. Gastrointestinal Coronavirus Disease 2019: Epidemiology, Clinical Features, Pathogenesis, Prevention, and Management. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 41–50. [Google Scholar] [CrossRef]
- Mao, R.; Qiu, Y.; He, J.S.; Tan, J.Y.; Li, X.H.; Liang, J.; Shen, J.; Zhu, L.R.; Chen, Y.; Iacucci, M.; et al. Manifestations and Prognosis of Gastrointestinal and Liver Involvement in Patients with COVID-19: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Xiang, B.J.; Jiang, M.; Sun, M.J.; Dai, C. The Prevalence of Gastrointestinal Symptoms, Abnormal Liver Function, Digestive System Disease and Liver Disease in COVID-19 Infection. J. Clin. Gastroenterol 2021, 55, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Samanta, J.; Suri, V.; Bhalla, A.; Puri, G.D.; Sehgal, R.; Kochhar, R. Presence of Diarrhea Associated with Better Outcomes in Patients with COVID-19—A Prospective Evaluation. Indian J. Med. Microbiol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal Symptoms and Fecal Shedding of SARS-CoV-2 RNA Suggest Prolonged Gastrointestinal Infection. Med. (N. Y.) 2022, 3, 371–387.e9. [Google Scholar] [CrossRef]
- Sneller, M.C.; Liang, C.J.; Marques, A.R.; Chung, J.Y.; Shanbhag, S.M.; Fontana, J.R.; Raza, H.; Okeke, O.; Dewar, R.L.; Higgins, B.P.; et al. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann. Intern. Med. 2022, 175, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, P.; Rafiee, F.; Kavandi, H.; Goudarzi, S.; Heidari, F.; Gholamrezanezhad, A. Ischemic Gastrointestinal Complications of COVID-19: A Systematic Review on Imaging Presentation. Clin. Imaging 2021, 73, 86–95. [Google Scholar] [CrossRef]
- Qi, X.; Northridge, M.E.; Hu, M.; Wu, B. Oral Health Conditions and COVID-19: A Systematic Review and Meta-Analysis of the Current Evidence. Aging Health Res. 2022, 2, 100064. [Google Scholar] [CrossRef]
- Yadav, D.K.; Singh, A.; Zhang, Q.; Bai, X.; Zhang, W.; Yadav, R.K.; Singh, A.; Zhiwei, L.; Adhikari, V.P.; Liang, T. Involvement of Liver in COVID-19: Systematic Review and Meta-Analysis. Gut 2021, 70, 807–809. [Google Scholar] [CrossRef]
- Yang, F.; Xu, Y.; Dong, Y.; Huang, Y.; Fu, Y.; Li, T.; Sun, C.; Pandanaboyana, S.; Windsor, J.A.; Fu, D. Prevalence and Prognosis of Increased Pancreatic Enzymes in Patients with COVID-19: A Systematic Review and Meta-Analysis. Pancreatology 2022, 22, 539–546. [Google Scholar] [CrossRef]
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the Liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef]
- Cai, Q.; Huang, D.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Li, Z.; Zhou, G.; Gou, J.; Qu, J.; et al. COVID-19: Abnormal Liver Function Tests. J. Hepatol. 2020, 73, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jaiswal, P.; Kerakhan, Y.; Saravanan, L.; Murtaza, Z.; Zergham, A.; Honganur, N.-S.; Akbar, A.; Deol, A.; Francis, B.; et al. Liver Disease and Outcomes among COVID-19 Hospitalized Patients—A Systematic Review and Meta-Analysis. Ann. Hepatol. 2021, 21, 100273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, L.; Wang, F.S. Liver Injury in COVID-19: Management and Challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Lizardo-Thiebaud, M.J.; Cervantes-Alvarez, E.; Limon-de la Rosa, N.; Tejeda-Dominguez, F.; Palacios-Jimenez, M.; Méndez-Guerrero, O.; Delaye-Martinez, M.; Rodriguez-Alvarez, F.; Romero-Morales, B.; Liu, W.-H.; et al. Direct or Collateral Liver Damage in SARS-CoV-2-Infected Patients. Semin. Liver Dis. 2020, 40, 321–330. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Liu, B.; Wu, R.; Zou, N.; Xu, Y.Z.; Yang, Y.Y.; Zhang, F.; Zhou, H.M.; Wan, K.Q.; et al. Pioglitazone Upregulates Hepatic Angiotensin Converting Enzyme 2 Expression in Rats with Steatohepatitis. Ann. Hepatol. 2013, 12, 892–900. [Google Scholar] [CrossRef]
- Méndez-Sánchez, N.; Valencia-Rodríguez, A.; Qi, X.; Yoshida, E.M.; Romero-Gómez, M.; George, J.; Eslam, M.; Abenavoli, L.; Xie, W.; Teschke, R.; et al. What Has the COVID-19 Pandemic Taught Us so Far? Addressing the Problem from a Hepatologist’s Perspective. J. Clin. Transl. Hepatol. 2020, 8, 109–112. [Google Scholar] [CrossRef]
- Tian, S.; Xiong, Y.; Liu, H.; Niu, L.; Guo, J.; Liao, M.; Xiao, S.Y. Pathological Study of the 2019 Novel Coronavirus Disease (COVID-19) through Postmortem Core Biopsies. Mod. Pathol. 2020, 33, 1007–1014. [Google Scholar] [CrossRef]
- Barton, L.M.; Duval, E.J.; Stroberg, E.; Ghosh, S.; Mukhopadhyay, S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020, 153, 725–733. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Waseem, N.; Chen, P.H. Hypoxic Hepatitis: A Review and Clinical Update. J. Clin. Transl. Hepatol. 2016, 4, 263–268. [Google Scholar]
- Hamid, S.; Alvares Da Silva, M.R.; Burak, K.W.; Chen, T.; Drenth, J.P.H.; Esmat, G.; Gaspar, R.; Labrecque, D.; Lee, A.; Macedo, G.; et al. WGO Guidance for the Care of Patients with COVID-19 and Liver Disease. J. Clin. Gastroenterol. 2021, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lagana, S.M.; Kudose, S.; Iuga, A.C.; Lee, M.J.; Fazlollahi, L.; Remotti, H.E.; Del Portillo, A.; De Michele, S.; de Gonzalez, A.K.; Saqi, A.; et al. Hepatic Pathology in Patients Dying of COVID-19: A Series of 40 Cases Including Clinical, Histologic, and Virologic Data. Mod. Pathol. 2020, 33, 2147–2155. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-Chronic Liver Failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; O’Leary, J.G. Acute-On-Chronic Liver Failure. Clin. Liver Dis. 2014, 18, 561–574. [Google Scholar] [CrossRef]
- Noor, M.T.; Manoria, P. Immune Dysfunction in Cirrhosis. J. Clin. Transl. Hepatol. 2017, 5, 50–58. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, M.; Sulthana, S.F.; Kulkarni, A.; Rao, P.N.; Reddy, D.N. SARS-CoV-2 Related Acute on Chronic Liver Failure (S-ACLF). J. Clin. Exp. Hepatol. 2021, 11, 404–406. [Google Scholar] [CrossRef]
- Suk, K.T.; Kim, D.J. Drug-Induced Liver Injury: Present and Future. Clin. Mol. Hepatol. 2012, 18, 249–257. [Google Scholar] [CrossRef]
- Alqahtani, S.A.; Schattenberg, J.M. Liver Injury in COVID-19: The Current Evidence. United Eur. Gastroenterol. J. 2020, 8, 509–519. [Google Scholar] [CrossRef]
- Boeckmans, J.; Rodrigues, R.M.; Demuyser, T.; Piérard, D.; Vanhaecke, T.; Rogiers, V. COVID-19 and Drug-Induced Liver Injury: A Problem of Plenty or a Petty Point? Arch. Toxicol. 2020, 94, 1367–1369. [Google Scholar] [CrossRef]
- Charan, J.; Bhardwaj, P.; Dutta, S.; Kaur, R.; Bist, S.K.; Detha, M.D.; Kanchan, T.; Yadav, D.; Mitra, P.; Sharma, P. Use of Complementary and Alternative Medicine (CAM) and Home Remedies by COVID-19 Patients: A Telephonic Survey. Indian J. Clin. Biochem. 2020, 36, 108–111. [Google Scholar] [CrossRef]
- Olry, A.; Meunier, L.; Délire, B.; Larrey, D.; Horsmans, Y.; Le Louët, H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020, 43, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Qin, E.; Xu, J.; Zhang, D.; Cheng, G.; Wang, Y.; Lau, G. Non-Alcoholic Fatty Liver Diseases in Patients with COVID-19: A Retrospective Study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.I.; Gao, F.; Wang, X.B.; Sun, Q.F.; Pan, K.H.; Wang, T.Y.; Ma, H.L.; Liu, W.Y.; George, J.; Zheng, M.H. Letter to the Editor: Obesity as a Risk Factor for Greater Severity of COVID-19 in Patients with Metabolic Associated Fatty Liver Disease. Metabolism 2020, 108, 154244. [Google Scholar] [CrossRef]
- Hernaez, R.; Lazo, M.; Bonekamp, S.; Kamel, I.; Brancati, F.L.; Guallar, E.; Clark, J.M. Diagnostic Accuracy and Reliability of Ultrasonography for the Detection of Fatty Liver: A Meta-Analysis. Hepatology 2011, 54, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Hayat, U.; Ashfaq, M.Z.; Johnson, L.; Ford, R.; Wuthnow, C.; Kadado, K.; El Jurdi, K.; Okut, H.; Kilgore, W.R.; Assi, M.; et al. The Association of Metabolic-Associated Fatty Liver Disease with Clinical Outcomes of COVID-19: A Systematic Review and Meta-Analysis. Kansas J. Med. 2022, 15, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Zamarripa-Dorsey, F.; Panduro, A.; Purón-González, E.; Coronado-Alejandro, E.U.; Cortez-Hernández, C.A.; de la Tijera, F.H.; Pérez-Hernández, J.L.; Cerda-Reyes, E.; Rodríguez-Hernández, H.; et al. Current Trends of Liver Cirrhosis in Mexico: Similitudes and Differences with Other World Regions. World J. Clin. Cases 2018, 6, 922–930. [Google Scholar] [CrossRef]
- Ibáñez-Samaniego, L.; Bighelli, F.; Usón, C.; Caravaca, C.; Carrillo, C.F.; Romero, M.; Barreales, M.; Perelló, C.; Madejón, A.; Marcos, A.C.; et al. Elevation of Liver Fibrosis Index FIB-4 Is Associated With Poor Clinical Outcomes in Patients With COVID-19. J. Infect. Dis. 2020, 222, 726–733. [Google Scholar] [CrossRef]
- Marjot, T.; Moon, A.M.; Cook, J.A.; Abd-Elsalam, S.; Aloman, C.; Armstrong, M.J.; Brenner, E.J.; Cargill, T. Outcomes Following SARS-CoV-2 Infection in Patients with Chronic Liver Disease: An International Registry Study. J. Hepatol. 2021, 74, 567–577. [Google Scholar] [CrossRef]
- Lleo, A.; Invernizzi, P.; Lohse, A.W.; Aghemo, A.; Carbone, M. Management of Patients with Autoimmune Liver Disease during COVID-19 Pandemic. J. Hepatol. 2020, 73, 453–455. [Google Scholar] [CrossRef]
- Kumar, D.; Manuel, O.; Natori, Y.; Egawa, H.; Grossi, P.; Han, S.H.; Fernández-Ruiz, M.; Humar, A. COVID-19: A Global Transplant Perspective on Successfully Navigating a Pandemic. Am. J. Transplant. 2020, 20, 1773–1779. [Google Scholar] [CrossRef]
- Colmenero, J.; Rodríguez-Perálvarez, M.; Salcedo, M.; Arias-Milla, A.; Muñoz-Serrano, A.; Graus, J.; Nuño, J.; Gastaca, M.; Bustamante-Schneider, J.; Cachero, A.; et al. Epidemiological Pattern, Incidence, and Outcomes of COVID-19 in Liver Transplant Patients. J. Hepatol. 2021, 74, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Mouch, C.A.; Alexopoulos, S.P.; LaRue, R.W.; Kim, H.P. Successful Liver Transplantation in Patients with Active 2 Infection. Am. J. Transpl. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Burba, K. Liver Transplantation Linked to Lower Antibody, T-Cell Response to COVID-19 Vaccine. Healio Gastroenterology. Available online: https://www.healio.com/news/gastroenterology/20220628/liver-transplantation-linked-to-lower-antibody-tcell-response-to-covid19-vaccine (accessed on 20 June 2022).
- Monteleone, G.; Ardizzone, S. Are Patients with Inflammatory Bowel Disease at Increased Risk for Covid-19 Infection? J. Crohns. Colitis 2020, 14, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, Y.; Zhou, Y.; Liu, S.; Zheng, B.J.; Jiang, S. The Spike Protein of SARS-CoV-A Target for Vaccine and Therapeutic Development. Nat. Rev. Microbiol. 2009, 7, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khan, A.; Chowdhry, M.; Bilal, M.; Kochhar, G.S.; Clarke, K. Risk of Severe Coronavirus Disease 2019 in Patients with Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020, 159, 1575–1578.e4. [Google Scholar] [CrossRef] [PubMed]
- Brenner, E.J.; Pigneur, B.; Focht, G.; Zhang, X.; Ungaro, R.C.; Colombel, J.-F.; Turner, D.; Kappelman, M.D.; Ruemmele, F.M. Benign Evolution of SARS-CoV-2 Infections in Children with Inflammatory Bowel Disease: Results from Two International Databases. Clin. Gastroenterol. Hepatol. 2020, 19, 394–396.e5. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jena, A.; Kumar-M, P.; Sharma, V.; Sebastian, S. Risk and Outcomes of Coronavirus Disease (COVID-19) in Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. United Eur. Gastroenterol. J. 2021, 9, 159–176. [Google Scholar] [CrossRef]
- Amiot, A.; Rahier, J.-F.; Baert, F.; Nahon, S.; Hart, A.; Viazis, N.; Biancone, L.; Domenech, E.; Reenears, C.; Peyrin-Biroulet, L.; et al. The Impact of COVID-19 on Patients with IBD in a Prospective European Cohort Study. J. Crohn’s Colitis, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Al-Ani, A.H.; Prentice, R.E.; Rentsch, C.A.; Johnson, D.; Ardalan, Z.; Heerasing, N.; Garg, M.; Campbell, S.; Sasadeusz, J.; Macrae, F.A.; et al. Review Article: Prevention, Diagnosis and Management of COVID-19 in the IBD Patient. Aliment. Pharmacol. Ther. 2020, 52, 54–72. [Google Scholar] [CrossRef]
- Samanta, J.; Gupta, R.; Singh, M.P.; Patnaik, I.; Kumar, A.; Kochhar, R. Coronavirus Disease 2019 and the Pancreas. Pancreatology 2020, 20, 1567–1575. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128–2130.e2. [Google Scholar] [CrossRef]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ Distribution of Severe Acute Respiratory Syndrome (SARS) Associated Coronavirus (SARS-CoV) in SARS Patients: Implications for Pathogenesis and Virus Transmission Pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef]
- McNabb-Baltar, J.; Jin, D.X.; Grover, A.S.; Redd, W.D.; Zhou, J.C.; Hathorn, K.E.; McCarty, T.R.; Bazarbashi, A.N.; Shen, L.; Chan, W.W. Lipase Elevation in Patients With COVID-19. Am. J. Gastroenterol. 2020, 115, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- Barlass, U.; Wiliams, B.; Dhana, K.; Adnan, D.; Khan, S.R.; Mahdavinia, M.; Bishehsari, F. Marked Elevation of Lipase in COVID-19 Disease: A Cohort Study. Clin. Transl. Gastroenterol. 2020, 11, e00215. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Benias, P.C.; Liu, Y.; Sejpal, D.V.; Satapathy, S.K.; Trindade, A.J. Prevalence, Risk Factors, and Outcomes of Hospitalized Patients with COVID-19 Presenting as Acute Pancreatitis. Gastroenterology 2020, 159, 2226–2228.e2. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.L.; Patel, A.; Sobotka, L.A.; Lim, W.; Kirkpatrick, R.B.; Han, S.; Hart, P.A.; Krishna, S.G.; Papachristou, G.I. Hospital Trends of Acute Pancreatitis During the Coronavirus Disease 2019 Pandemic. Pancreas, 2022; online ahead of print. [Google Scholar] [CrossRef]
| Study | Hypothesis | Design | Results | Implications |
|---|---|---|---|---|
| Jiao [15] | The gastrointestinal tract could play a central role in the pathogenesis of COVID-19 | Infection of Rhesus monkeys with an intragastric or intranasal challenge with SARS-CoV-2 | Both intranasal and intragastric inoculation caused pneumonia and gastrointestinal dysfunction | Possible connections through inflammatory cytokines |
| Wang [16] | SARS-CoV-2 could be potentially transmitted other than through the respiratory tract | Biodistribution of SARS-CoV-2 among different tissues of inpatients | SARS-CoV-2 detected in respiratory tissue, feces, and blood but not in urine | Transmission of the virus through extra-respiratory routes (feces) could explain the rapid spread |
| Irham [17] | Individual expression of TMPRSS2 may influence SARS-CoV-2 susceptibility | Multiple large genome databases (GTEx portal, SNP nexus, Ensembl genome project) | Four variants (rs464397, rs469390, rs2070788, and rs383510) affect expression of TMPRSS2 in lung tissue | Higher frequency of upregulating variants in European and American populations |
| Cao [18] | ACE2 variants could reduce the binding of S protein in SARS-CoV-2 | Analysis of variants of ACE2 gene and allele frequencies in ChinaMAP and 1 KgP databases | Singleton truncating variant of ACE2 (Gln300X) and higher allele frequency in China of the SNP rs2285666 | Lack of natural resistant mutations for coronavirus S protein binding |
| Summary of Recommendations |
|---|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino-Matus, J.; Uribe, M.; Chavez-Tapia, N. COVID-19: Current Status in Gastrointestinal, Hepatic, and Pancreatic Diseases—A Concise Review. Trop. Med. Infect. Dis. 2022, 7, 187. https://doi.org/10.3390/tropicalmed7080187
Aquino-Matus J, Uribe M, Chavez-Tapia N. COVID-19: Current Status in Gastrointestinal, Hepatic, and Pancreatic Diseases—A Concise Review. Tropical Medicine and Infectious Disease. 2022; 7(8):187. https://doi.org/10.3390/tropicalmed7080187
Chicago/Turabian StyleAquino-Matus, Jorge, Misael Uribe, and Norberto Chavez-Tapia. 2022. "COVID-19: Current Status in Gastrointestinal, Hepatic, and Pancreatic Diseases—A Concise Review" Tropical Medicine and Infectious Disease 7, no. 8: 187. https://doi.org/10.3390/tropicalmed7080187
APA StyleAquino-Matus, J., Uribe, M., & Chavez-Tapia, N. (2022). COVID-19: Current Status in Gastrointestinal, Hepatic, and Pancreatic Diseases—A Concise Review. Tropical Medicine and Infectious Disease, 7(8), 187. https://doi.org/10.3390/tropicalmed7080187






