Systematic Surveillance of Rickettsial Diseases in 27 Hospitals from 26 Provinces throughout Vietnam
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Specimen Collection and Processing
2.2.1. DNA Extraction
2.2.2. Quantitative Real-Time PCR Assays
2.2.3. Multilocus Sequence Typing (MLST) for Rickettsia Species Identification
2.3. Data Entry and Analysis
2.4. Ethical Considerations
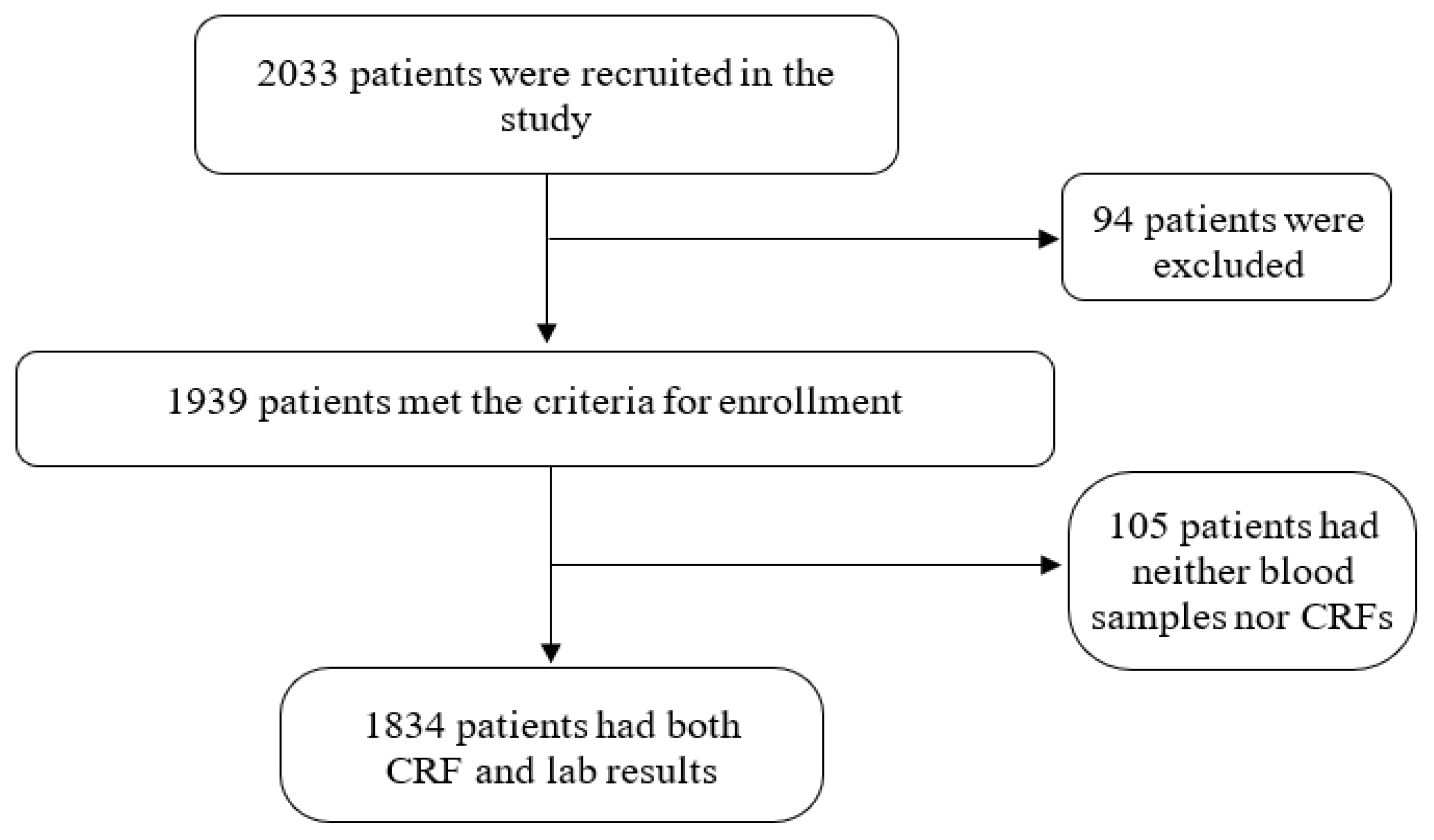
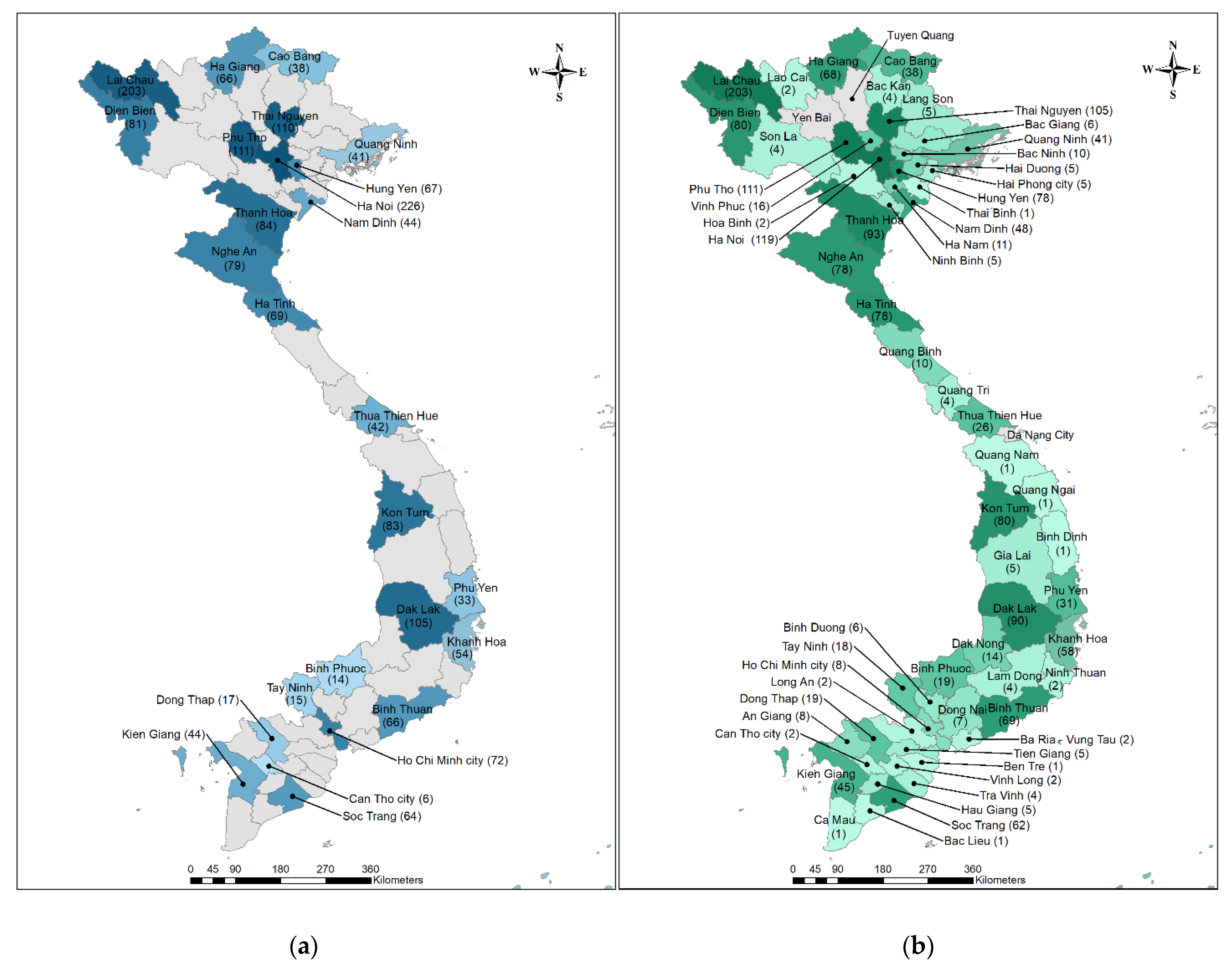
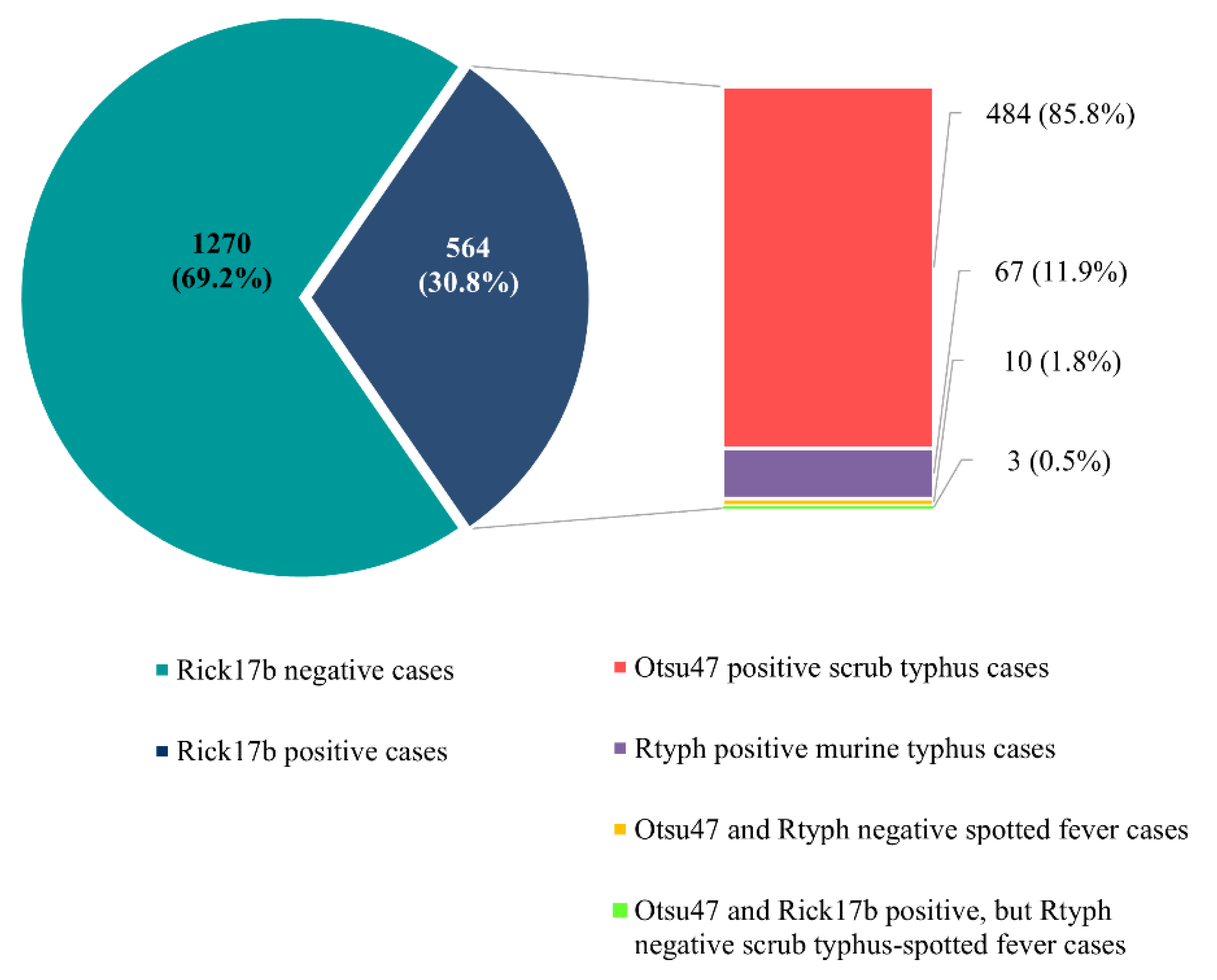
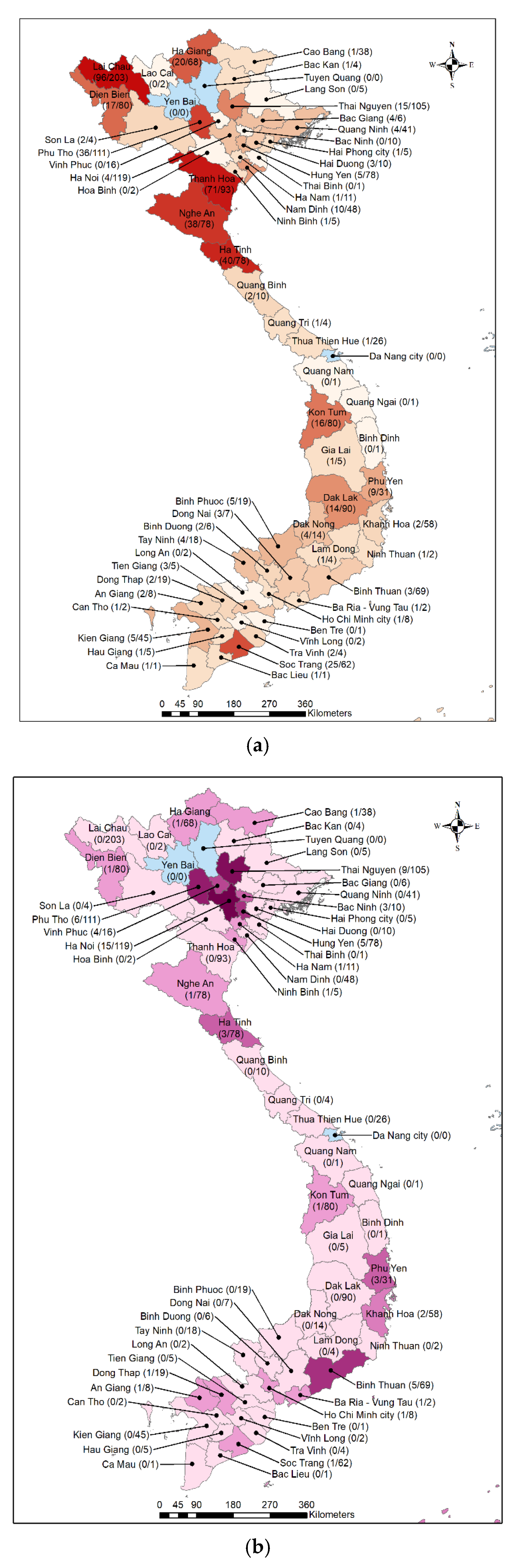
3. Results
Patient Population and Enrollment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ericsson, C.D.; Jensenius, M.; Fournier, P.-E.; Raoult, D. Rickettsioses and the International Traveler. Clin. Infect. Dis. 2004, 39, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: London, UK, 2019; pp. 2244–2248. [Google Scholar]
- Walker, D.H.; Fishbein, D.B. Epidemiology of rickettsial diseases. Eur. J. Epidemiol. 1991, 7, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Fuerst, P.A.; Ching, W.; Richards, A.L. Scrub Typhus: The Geographic Distribution of Phenotypic and Genotypic Variants of Orientia tsutsugamushi. Clin. Infect. Dis. 2009, 48 (Suppl. S3), S203–S230. [Google Scholar] [CrossRef] [PubMed]
- Satjanadumrong, J.; Robinson, M.T.; Hughes, T.; Blacksell, S.D. Distribution and Ecological Drivers of Spotted Fever Group Rickettsia in Asia. EcoHealth 2019, 16, 611–626. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on Tick-Borne Rickettsioses around the World: A Geographic Approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef]
- Lee, N.; Tsang, O.T.Y.; Lui, C.Y.G.; Lai, S.T.; Wong, B.; Cockram, C.S.; Chan, Y.Y.; Ng, T.K.; Lam, R.; Ip, M.; et al. Risk Factors Associated with Life-threatening Rickettsial Infections. Am. J. Trop. Med. Hyg. 2008, 78, 973–978. [Google Scholar] [CrossRef]
- Taylor, A.J.; Paris, D.; Newton, P. A Systematic Review of Mortality from Untreated Scrub Typhus (Orientia tsutsugamushi). PLoS Negl. Trop. Dis. 2015, 9, e0003971. [Google Scholar] [CrossRef]
- Trung, N.V.; Hoi, L.T.; Dien, V.M.; Huong, D.T.; Hoa, T.M.; Lien, V.N.; Van Luan, P.; Lewycka, S.; Choisy, M.; Bryant, J.E.; et al. Clinical Manifestations and Molecular Diagnosis of Scrub Typhus and Murine Typhus, Vietnam, 2015–2017. Emerg. Infect. Dis. 2019, 25, 633–641. [Google Scholar] [CrossRef]
- Katoh, S.; Cuong, N.C.; Hamaguchi, S.; Thuy, P.T.; Cuong, D.D.; Anh, L.K.; Anh, N.T.H.; Anh, D.D.; Sando, E.; Suzuki, M.; et al. Challenges in diagnosing scrub typhus among hospitalized patients with undifferentiated fever at a national tertiary hospital in northern Vietnam. PLoS Negl. Trop. Dis. 2019, 13, e0007928. [Google Scholar] [CrossRef]
- Le-Viet, N.; Le, V.-N.; Chung, H.; Phan, D.-T.; Phan, Q.-D.; Cao, T.-V.; Abat, C.; Raoult, D.; Parola, P. Prospective case-control analysis of the aetiologies of acute undifferentiated fever in Vietnam. Emerg. Microbes Infect. 2019, 8, 339–352. [Google Scholar] [CrossRef]
- Noc, F.; Gautron, P. Deux cas de fièvre indéterminée rappelant le pseudo-typhus de Delhi observés à Saigon. Bull. Soc. Med. Chir. D’ Indoch. 1915, 6, 108. [Google Scholar]
- Beytout, D. Rickettsioses Diagnostiquées Par Microagglutination De Janvier 1962 a Juin 1963 a Saigon. Bull. Société Pathol. Exot. 1964, 57, 257–263. [Google Scholar]
- Berman, S.J.; Kundin, W.D. Scrub Typhus in South Vietnam. Ann. Intern. Med. 1973, 79, 26–30. [Google Scholar] [CrossRef]
- Miller, M.B.; Bratton, J.L.; Hunt, J.; Blankenship, R.; Lohr, D.C.; Reynolds, R.D. Murine Typhus in Vietnam. Mil. Med. 1974, 139, 184–186. [Google Scholar] [CrossRef]
- Hamaguchi, S.; Cuong, N.C.; Tra, D.T.; Doan, Y.H.; Shimizu, K.; Tuan, N.Q.; Yoshida, L.-M.; Mai, L.Q.; Duc-Anh, D.; Ando, S.; et al. Clinical and Epidemiological Characteristics of Scrub Typhus and Murine Typhus among Hospitalized Patients with Acute Undifferentiated Fever in Northern Vietnam. Am. J. Trop. Med. Hyg. 2015, 92, 972–978. [Google Scholar] [CrossRef]
- Le Viet, N.; Laroche, M.; Thi Pham, H.L.; Viet, N.L.; Mediannikov, O.; Raoult, D.; Parola, P. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. PLoS Negl. Trop Dis. 2017, 11, e0005397. [Google Scholar] [CrossRef]
- Trung, N.V.; Hoi, L.T.; Toan, T.K.; Hoa, T.M.; Fox, A.; Van Kinh, N.; Van Doorn, H.R.; Wertheim, H.F.L.; Bryant, J.E.; Nadjm, B.; et al. Seroprevalence of Scrub Typhus, Typhus, and Spotted Fever Among Rural and Urban Populations of Northern Vietnam. Am. J. Trop. Med. Hyg. 2017, 96, 1084–1087. [Google Scholar] [CrossRef]
- Henry, K.M.; Jiang, J.; Rozmajzl, P.J.; Azad, A.F.; Macaluso, K.R.; Richards, A.L. Development of quantitative real-time PCR assays to detect Rickettsia typhi and Rickettsia felis, the causative agents of murine typhus and flea-borne spotted fever. Mol. Cell. Probes 2007, 21, 17–23. [Google Scholar] [CrossRef]
- Jiang, J.; Temenak, J.J.; Richards, A.L. Real-time PCR duplex assay for Rickettsia prowazekii and Borrelia recurrentis. Ann. N. Y. Acad. Sci. 2003, 990, 302–310. [Google Scholar] [CrossRef]
- Luce-Fedrow, A.; Mullins, K.; Kostik, A.P.; John, H.K.S.; Jiang, J.; Richards, A.L. Strategies for detecting rickettsiae and diagnosing rickettsial diseases. Futur. Microbiol. 2015, 10, 537–564. [Google Scholar] [CrossRef]
- Jiang, J.; Dasch, G.; Ching, W.-M.; Richards, A.L.; Chan, T.-C.; Temenak, J.J. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am. J. Trop. Med. Hyg. 2004, 70, 351–356. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Dumler, J.S.; Greub, G.; Zhang, J.; Wu, Y.; Raoult, D. Gene Sequence-Based Criteria for Identification of New Rickettsia Isolates and Description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 2003, 41, 5456–5465. [Google Scholar] [CrossRef]
- Jiang, J.; Sangkasuwan, V.; Lerdthusnee, K.; Sukwit, S.; Chuenchitra, T.; Rozmajzl, P.J.; Eamsila, C.; Jones, J.W.; Richards, A.L. Molecular evidence of human infection with TT-118, Rickettsia honei, from Thailand. Emerg. Infect. Dis. 2005, 11, 1475–1477. [Google Scholar] [CrossRef]
- Hieu, N.T.; NNgu, D.; Thang, N.V.; Khiem, M.V.; Mau, N.D.; Thuy, T.T.T. Developing the climatic sub-zones from the climate zones of Vietnam. Vietnam. J. Clim. Chang. Sci. 2017, 2. [Google Scholar]
- Seong, S.-Y.; Choi, M.-S.; Kim, I.-S. Orientia tsutsugamushi infection:overview and immune responses. Microbes Infect. 2001, 3, 11–21. [Google Scholar] [CrossRef]
- Jiang, J.; Richards, A.L. Scrub Typhus: No Longer Restricted to the Tsutsugamushi Triangle. Trop. Med. Infect. Dis. 2018, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Wangrangsimakul, T.; Althaus, T.; Mukaka, M.; Kantipong, P.; Wuthiekanun, V.; Chierakul, W.; Blacksell, S.D.; Day, N.P.; Laongnualpanich, A.; Paris, D.H. Causes of acute undifferentiated fever and the utility of biomarkers in Chiangrai, northern Thailand. PLoS Negl. Trop. Dis. 2018, 12, e0006477. [Google Scholar] [CrossRef] [PubMed]
- Mayxay, M.; Castonguay-Vanier, J.; Chansamouth, V.; Dubot-Pérès, A.; Paris, D.H.; Phetsouvanh, R.; Tangkhabuanbutra, J.; Douangdala, P.; Inthalath, S.; Souvannasing, P.; et al. Causes of non-malarial fever in Laos: A prospective study. Lancet Glob. Health 2013, 1, e46–e54. [Google Scholar] [CrossRef]
- Lokida, D.; Hadi, U.; Lau, C.-Y.; Kosasih, H.; Liang, C.J.; Rusli, M.; Sudarmono, P.; Lukman, N.; Laras, K.; Asdie, R.H.; et al. Underdiagnoses of Rickettsia in patients hospitalized with acute fever in Indonesia: Observational study results. BMC Infect. Dis. 2020, 20, 364. [Google Scholar] [CrossRef]
- Basra, G.; Berman, M.A.; Blanton, L.S. Murine Typhus: An Important Consideration for the Nonspecific Febrile Illness. Case Rep. Med. 2012, 2012, 134601. [Google Scholar] [CrossRef][Green Version]
- Le Van, N.; Van, C.P.; Dang, M.N.; Van, T.D.; Do, Q.L.T.; Hoang, H.V.; Viet, T.T.; Do, B.N. Clinical Features, Laboratory Characteristics and Prognostic Factors of Severity in Patients with Rickettsiaceae at Two Military Hospitals, Northern Vietnam. Infect. Drug Resist. 2020, 13, 2129–2138. [Google Scholar] [CrossRef]
- Azuma, M.; Nishioka, Y.; Ogawa, M.; Takasaki, T.; Sone, S.; Uchiyama, T. Murine Typhus from Vietnam, Imported into Japan. Emerg. Infect. Dis. 2006, 12, 1466–1468. [Google Scholar] [CrossRef]
- Lim, C.; Paris, D.; Blacksell, S.; Laongnualpanich, A.; Kantipong, P.; Chierakul, W.; Wuthiekanun, V.; Day, N.P.J.; Cooper, B.; Limmathurotsakul, D. How to Determine the Accuracy of an Alternative Diagnostic Test when It Is Actually Better than the Reference Tests: A Re-Evaluation of Diagnostic Tests for Scrub Typhus Using Bayesian LCMs. PLoS ONE 2015, 10, e0114930. [Google Scholar] [CrossRef]
- Rajapakse, S.; Weeratunga, P.; Sivayoganathan, S.; Fernando, S.D. Clinical manifestations of scrub typhus. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 43–54. [Google Scholar] [CrossRef]
- van Eekeren, L.; de Vries, S.G.; Wagenaar, J.F.; Spijker, R.; Grobusch, M.P.; Goorhuis, A. Under-diagnosis of rickettsial disease in clinical practice: A systematic review. Travel Med. Infect. Dis. 2018, 26, 7–15. [Google Scholar] [CrossRef]
- Blanton, L.S.; Lea, A.S.; Kelly, B.C.; Walker, D.H. An Unusual Cutaneous Manifestation in a Patient with Murine Typhus. Am. J. Trop. Med. Hyg. 2015, 93, 1164–1167. [Google Scholar] [CrossRef]
- Newton, P.N.; Keolouangkhot, V.; Lee, S.J.; Choumlivong, K.; Sisouphone, S.; Choumlivong, K.; Vongsouvath, M.; Mayxay, M.; Chansamouth, V.; Davong, V.; et al. A Prospective, Open-label, Randomized Trial of Doxycycline Versus Azithromycin for the Treatment of Uncomplicated Murine Typhus. Clin. Infect. Dis. 2018, 68, 738–747. [Google Scholar] [CrossRef]
- Bonell, A.; Lubell, Y.; Newton, P.N.; Crump, J.A.; Paris, D.H. Estimating the burden of scrub typhus: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005838. [Google Scholar] [CrossRef]
- Doppler, J.F.; Newton, P.N. A systematic review of the untreated mortality of murine typhus. PLoS Negl. Trop. Dis. 2020, 14, e0008641. [Google Scholar] [CrossRef]
- Helmick, C.G.; Bernard, K.W.; D’Angelo, L.J. Rocky Mountain Spotted Fever: Clinical, Laboratory, and Epidemiological Features of 262 Cases. J. Infect. Dis. 1984, 150, 480–488. [Google Scholar] [CrossRef]
- Parola, P. Rickettsia felis: From a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin. Microbiol. Infect. 2011, 17, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-López, D.I.; Ochoa-Mora, E.; Heitman, K.N.; Binder, A.M.; Álvarez-Hernández, G.; Armstrong, P.A. Epidemiology and Clinical Features of Rocky Mountain Spotted Fever from Enhanced Surveillance, Sonora, Mexico: 2015–2018. Am. J. Trop. Med. Hyg. 2021, 104, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.; Artsob, H. Spotted Fever Group Rickettsiae: A Brief Review and a Canadian Perspective. Zoonoses Public Health 2012, 59 (Suppl. S2), 65–79. [Google Scholar] [CrossRef] [PubMed]
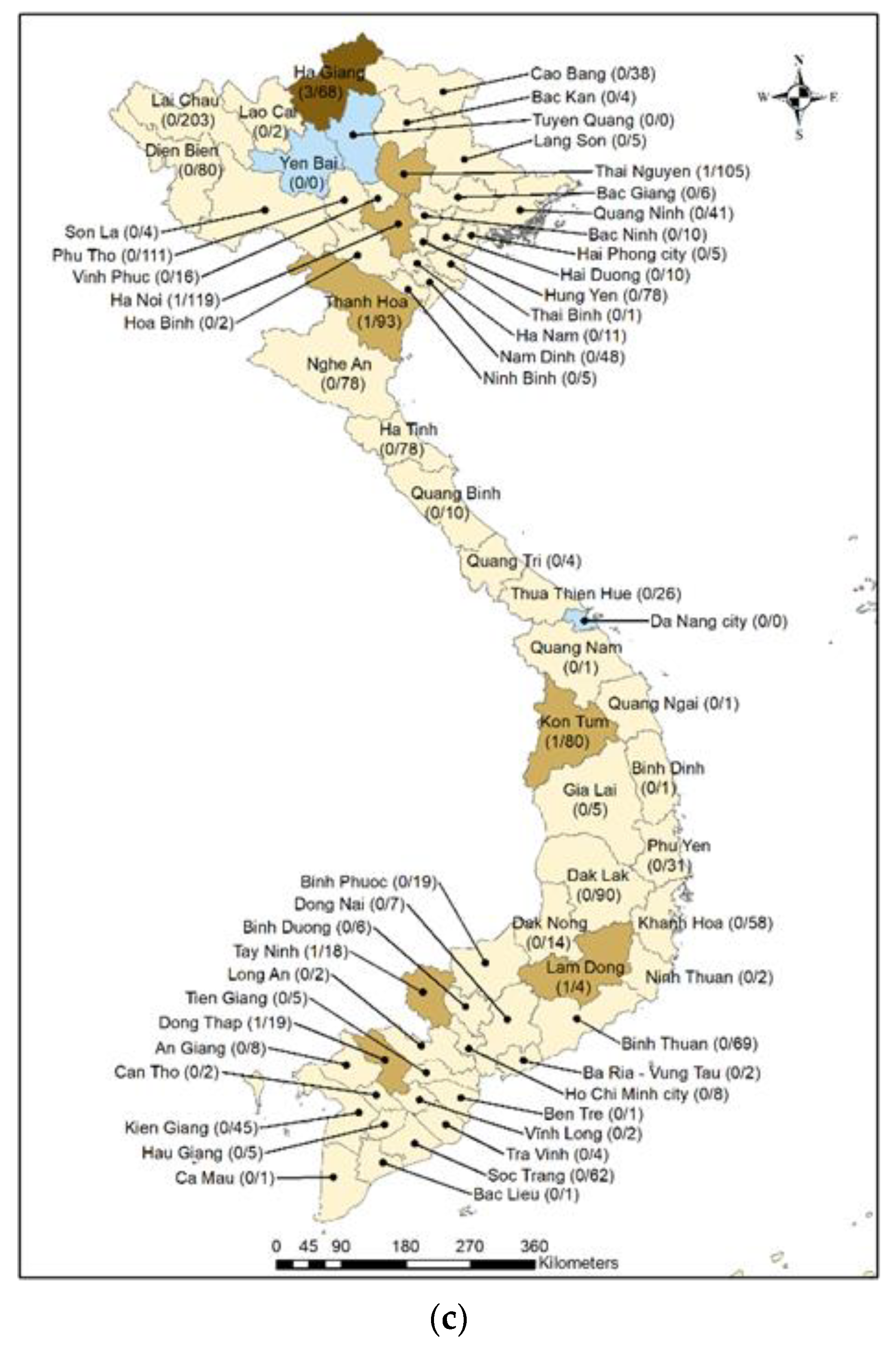
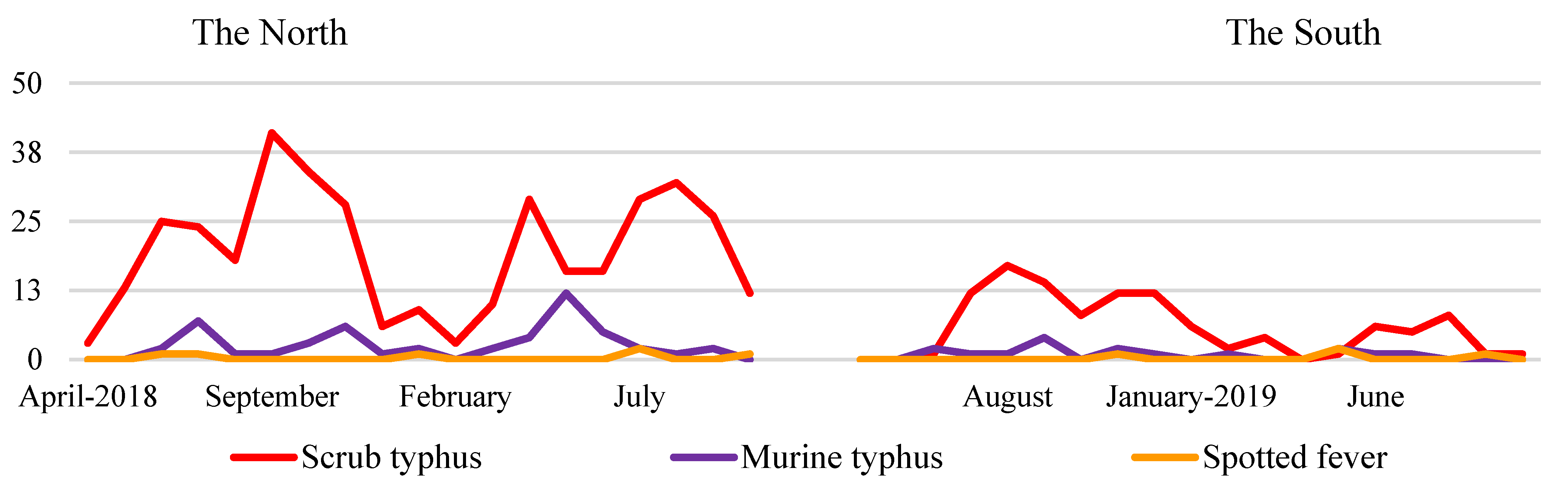
| Characteristics | Total | qPCR Negative | qPCR Positive | p-Value † | |
|---|---|---|---|---|---|
| Median (p25–p75) | |||||
| Age (n = 1834) | 39 (27–55) | 38 (27–54) | 42 (24–58) | 0.2883 ‡ | |
| n (%) | |||||
| Sex (n = 1831) | Female | 861 (47.0) | 554 (44.1) | 307 (53.4) | 0.000 |
| Male | 970 (53.0) | 702 (55.9) | 268 (46.6) | ||
| Residence (n = 1832) | Rural area | 1271 (69.4) | 813 (64.8) | 458 (79.2) | 0.000 |
| Urban area | 561 (30.6) | 441 (35.2) | 120 (20.8) | ||
| Occupation (n = 1843) | Farmer | 866 (47.2) | 550 (43.8) | 316 (54.7) | 0.000 |
| Non-farmer | 968 (52.8) | 706 (56.2) | 262 (45.3) | ||
| Origin (n = 1834) | Admitted from community | 1175 (64.1) | 858 (68.3) | 317 (54.8) | 0.000 |
| Transferred from another hospital or clinic | 659 (35.9) | 398 (31.7) | 261 (45.2) | ||
| Used antimicrobial drugs before admission (n = 1834) | Yes | 426 (23.2) | 264 (21.0) | 162 (28.0) | 0.001 |
| No | 1408 (76.8) | 992 (79.0) | 416 (72.0) | ||
| Characteristics | Scrub Typhus n = 484 | Murine Typhus n = 67 | Spotted Fever n = 10 | Total n = 561 | p Value § |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, median (IQR) (n = 560) | 43 (24–58) | 43 (31–54) | 28 (18–37) | 42.5 (24–58) | 0.344 ¶ |
| Male, (n, (%)), n = 560) | 206 (42.6) | 42 (63.6) | 8 (80.0) | 256 (45.7) | 0.001 |
| Female (n, (%), n = 560) | 278 (57.4) | 24 (36.4) | 2 (20.0) | 304 (54.3) | |
| Farmer, n = 561 | 283 (58.5) | 18 (26.9) | 5 (50.0) | 306 (54.6) | 0.000 |
| Residence in rural area, n = 561 | 402 (83.1) | 36 (53.7) | 6 (60.0) | 444 (79.1) | 0.000 |
| History | |||||
| Admitted from community, n = 561 | 264 (54.5) | 37 (55.2) | 5 (50.0) | 398 (54.6) | 1.000 |
| Transferred from other hospital or clinic, n = 561 | 220 (45.6) | 30 (44.8) | 5 (50.0) | 255 (45.4) | |
| Received antimicrobial drugs before admission, n = 561 | 135 (27.9) | 20 (29.9) | 3 (30.0) | 158 (28.2) | 0.772 |
| Symptoms | |||||
| Headache, n = 561 | 448 (92.6) | 62 (92.5) | 10 (100) | 520 (92.7) | 1.000 |
| Myalgia, n = 561 | 250 (51.7) | 44 (65.7) | 9 (90.0) | 303 (54.0) | 0.036 |
| Retro-orbital pain, n = 560 | 105 (21.7) | 21 (31.3) | 2 (20.0) | 128 (22.9) | 0.088 |
| Sore throat, n = 561 | 117 (24.2) | 24 (35.8) | 3 (30.0) | 144 (25.7) | 0.098 |
| Cough, n = 560 | 220 (45.6) | 34 (50.8) | 5 (50.0) | 259 (46.3) | 0.435 |
| Nausea, n = 561 | 147 (30.4) | 23 (34.3) | 4 (40.0) | 174 (31.0) | 0.573 |
| Vomiting, n = 561 | 72 (14.9) | 11 (16.4) | 0 | 83 (14.8) | 0.717 |
| Abdominal pain, n = 561 | 95 (19.6) | 7 (10.5) | 0 | 102 (18.2) | 0.092 |
| Diarrhea, n = 560 | 59 (12.2) | 6 (9.0) | 0 | 65 (11.6) | 0.547 |
| Skin hyperemia, n = 560 | 124 (25.7) | 27 (40.3) | 0 | 151 (27.0) | 0.040 |
| Physical signs | |||||
| Congested skin, n = 561 | 273 (56.4) | 54 (80.6) | 4 (40.0) | 331 (59.0) | 0.000 |
| Conjunctivitis, n = 551 | 163 (33.7) | 36 (53.7) | 3 (10.0) | 202 (36.0) | 0.002 |
| Eschar, n = 561 | 368 (76.0) | 4 (6.0) | 1 (10.0) | 373 (66.5) | 0.000 |
| Rash, n = 561 | 118 (24.4) | 26 (38.8) | 0 | 144 (25.7) | 0.017 |
| Lymphadenopathy, n = 561 | 243 (50.2) | 7 (10.5) | 1 (10.0) | 251 (44.7) | 0.000 |
| Liver enlargement, n = 561 | 28 (5.8) | 0 | 2 (20.0) | 30 (5.4) | 0.037 |
| Spleen enlargement, n = 561 | 31 (6.4) | 0 | 1 (10.0) | 32 (5.7) | 0.024 |
| Subcutaneous hemorrhage, n = 560 | 13 (2.7) | 5 (7.5) | 0 | 18 (3.2) | 0.056 |
| Mucous membrane hemorrhage, n = 561 | 5 (1.0) | 2 (3.0) | 0 | 7 (1.3) | 0.205 |
| Rales, n = 561 | 64 (13.2) | 8 (11.9) | 0 | 72 (12.8) | 1.000 |
| Laboratory test results at admission | |||||
| Erythrocytes, T/L, median (IQR), n = 417 | 4.4 (3.9–4.7) | 4.5 (4.2–4.9) | 4.4 (4.1–4.8) | 4.4 (4.0–4.7) | 0.027¶ |
| Leukocytes, T/L, median (IQR), n = 417 | 7.5 (5.6–10.3) | 6.5 (5.0–8.7) | 6.2 (4.2–7.7) | 7.4 (5.5–10.0) | 0.049¶ |
| Platelets, G/L, median (IQR), n = 415 | 100 (66–144) | 87.5 (65–133) | 177.5 (75–269.5) | 100 (66–144) | 0.303 ¶ |
| Platelet < 100 G/L, n = 415 | 187 (51.5) | 20 (45.5) | 6 (75.0) | 213 (51.3) | 0.524 |
| Alanine aminotransferase > 40 IU/L, n = 376 | 282 (85.2) | 36 (97.3) | 7 (87.5) | 325 (86.4) | 0.042 |
| Aspartate aminotransferase ST > 37 IU/L, n = 375 | 316 (95.8) | 35 (94.6) | 8 (100) | 359 (95.7) | 0.670 |
| Total bilirubin > 17 µmol/L, n = 82 | 18 (23.4) | 2 (18.2) | 0 | 20 (24.4) | 1.000 |
| Albumin < 32 g/L, n =111 | 54 (54.6) | 1 (8.3) | 0 | 55 (49.6) | 0.004 |
| Creatinine > 120 µmol/L, n = 352 | 19 (6.1) | 1 (3.0) | 0 | 20 (5.7) | 0.706 |
| Treatment | |||||
| One of three protocol-specific antibiotics (Doxycycline, Chloramphenicol and Azithromycin), n = 661 | 478 (98.8) | 62 (92.5) | 8 (80.0) | 548 (97.7) | - |
| Non-protocol antibiotics, n = 561 | 4 (0.8) | 4 (6.0) | 1 (10.0) | 9 (1.6) | |
| Other therapies, (n = 561) | 2 (0.4) | 1 (1.5) | 1 (10.0) | 4 (0.7) | |
| Respiratory support, n = 561 | 31 (6.4) | 4 (6.0) | 0 | 35 (6.2) | 1.000 |
| Albumin transfusion, n = 557 | 23 (4.8) | 3 (4.6) | 0 | 26 (4.7) | 1.000 |
| Blood purification, n = 555 | 3 (0.6) | 0 | 0 | 3 (0.5) | 1.000 |
| Outcomes | |||||
| Death, n = 561 | 5 (1.0) | 1 (1.5) | 0 | 6 (1.1) | 0.542 |
| No. days in hospital, n = 555 | 6 (5–8) | 7 (5–8) | 7 (5–7) | 6 (5–8) | 0.128 ¶ |
| Suffering from shock, n = 549 | 6 (1.2) | 1 (1.5) | 0 | 7 (1.3) | 0.595 |
| Afebrile ≤ 72 h after treatment began, n = 545 | 418 (88.2) | 47 (77.1) | 9 (90.0) | 474 (87.0) | 0.024 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trung, N.V.; Hoi, L.T.; Hoa, T.M.; Huong, D.T.; Huyen, M.T.; Tien, V.Q.; Mai, D.T.T.; Ha, N.T.T.; Kinh, N.V.; Farris, C.M.; et al. Systematic Surveillance of Rickettsial Diseases in 27 Hospitals from 26 Provinces throughout Vietnam. Trop. Med. Infect. Dis. 2022, 7, 88. https://doi.org/10.3390/tropicalmed7060088
Trung NV, Hoi LT, Hoa TM, Huong DT, Huyen MT, Tien VQ, Mai DTT, Ha NTT, Kinh NV, Farris CM, et al. Systematic Surveillance of Rickettsial Diseases in 27 Hospitals from 26 Provinces throughout Vietnam. Tropical Medicine and Infectious Disease. 2022; 7(6):88. https://doi.org/10.3390/tropicalmed7060088
Chicago/Turabian StyleTrung, Nguyen Vu, Le Thi Hoi, Tran Mai Hoa, Dang Thi Huong, Ma Thi Huyen, Vuong Quang Tien, Dao Thi Tuyet Mai, Nguyen Thi Thu Ha, Nguyen Van Kinh, Christina M. Farris, and et al. 2022. "Systematic Surveillance of Rickettsial Diseases in 27 Hospitals from 26 Provinces throughout Vietnam" Tropical Medicine and Infectious Disease 7, no. 6: 88. https://doi.org/10.3390/tropicalmed7060088
APA StyleTrung, N. V., Hoi, L. T., Hoa, T. M., Huong, D. T., Huyen, M. T., Tien, V. Q., Mai, D. T. T., Ha, N. T. T., Kinh, N. V., Farris, C. M., & Richards, A. L. (2022). Systematic Surveillance of Rickettsial Diseases in 27 Hospitals from 26 Provinces throughout Vietnam. Tropical Medicine and Infectious Disease, 7(6), 88. https://doi.org/10.3390/tropicalmed7060088









