The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy
Abstract
:1. Introduction
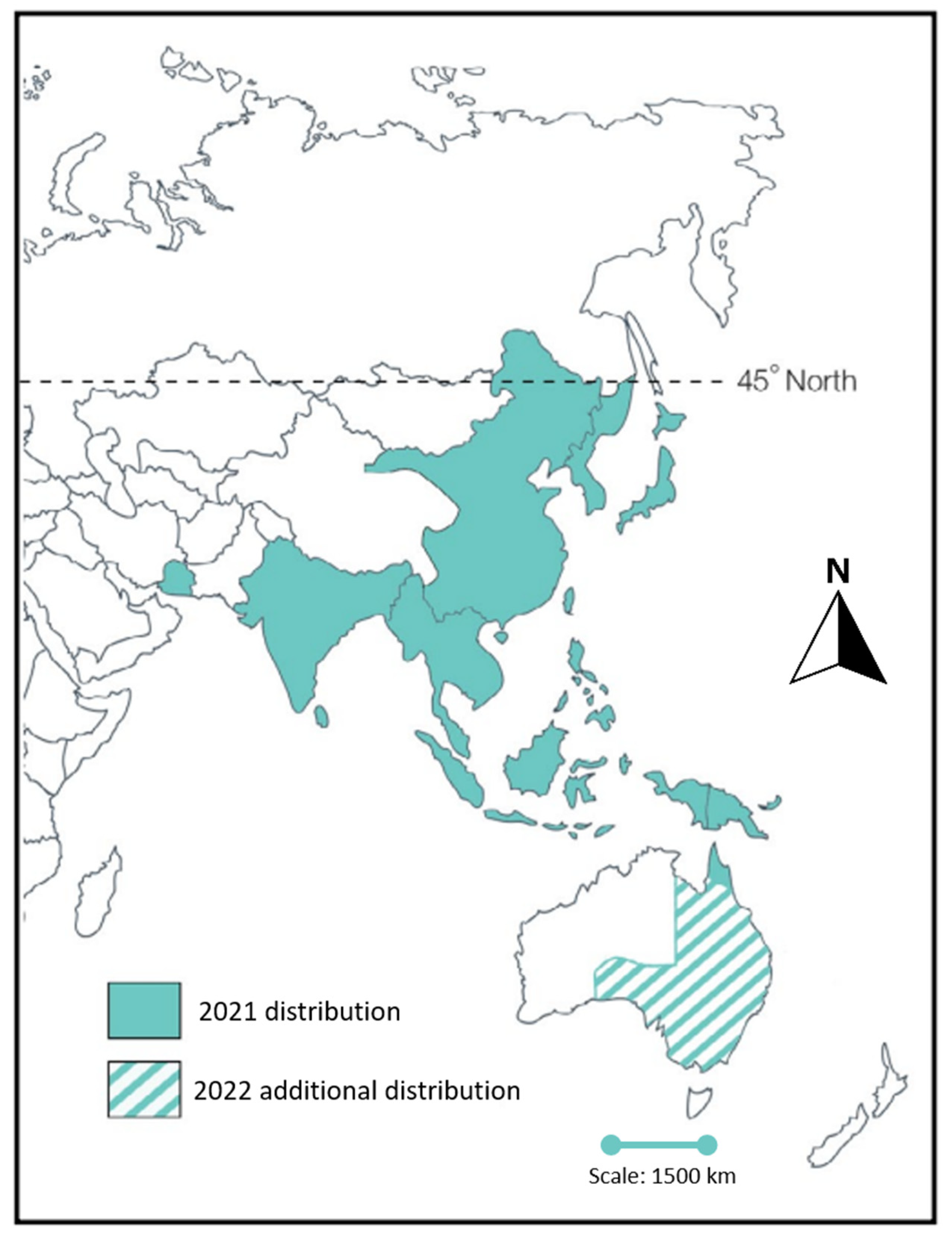
2. Emergence of Japanese Encephalitis Virus
3. Japanese Encephalitis Vaccines
4. Alternative Approaches for a More Economical and Effective Vaccine Delivery
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Japanese Encephalitis. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/japanese-encephalitis (accessed on 27 May 2022).
- Quan, T.M.; Thao, T.T.N.; Duy, N.M.; Nhat, T.M.; Clapham, H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000–2015. eLife 2020, 9, e51027. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Shield, J.; Bailey, M.C.; Mackenzie, J.S.; Poidinger, M.; McCall, B.J.; Mills, P.J. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med. J. Aust. 1996, 165, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Lee, J.M.; Hills, S.L.; van den Hurk, A.F.; Pyke, A.T.; Johansen, C.A.; Mackenzie, J.S. Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 1999, 170, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Northern Territory Government. Japanese Encephalitis. 2021. Available online: https://nt.gov.au/wellbeing/health-conditions-treatments/viral/japanese-encephalitis (accessed on 27 May 2022).
- Australian Government—Department of Health. National Notifiable Diseases: Australia’s Notifiable Diseases Status: Annual Report of the National Notifiable Diseases Surveillance System. 2021. Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/cda-pubs-annlrpt-nndssar.htm (accessed on 27 May 2022).
- Christian, N. Australians Warned about Travelling to Bali Amid Spike in Japanese Encephalitis Cases. 2018. Available online: https://www.news.com.au/travel/travel-updates/warnings/australians-warned-about-travelling-to-bali-amid-spike-in-japanese-encephalitis-cases/news-story/e255ca32f524bcbdae9d1ee1f7e3a429 (accessed on 27 May 2022).
- Australian Government—Department of Health. Japanese Encephalitis. 2018. Available online: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/japanese-encephalitis (accessed on 27 May 2022).
- CSIRO. Expert Commentary: Japanese Encephalitis. 2022. Available online: https://www.csiro.au/en/news/News-releases/2022/Expert-commentary-Japanese-encephalitis (accessed on 27 May 2022).
- NSW Government—NSW Health. Public Health Alert—Mosquito Warning. 2022. Available online: https://www.health.nsw.gov.au/news/Pages/20220226_02.aspx (accessed on 27 May 2022).
- Australian Government—Department of Health. Japanese Encephalitis Virus (JEV). 2022. Available online: https://www.health.gov.au/health-alerts/japanese-encephalitis-virus-jev/about (accessed on 27 May 2022).
- Halstead, S.B.; Jacobson, J.; Dubischar-Kastner, K. Japanese encephalitis vaccines. In Vaccines, 6th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; pp. 312–351. [Google Scholar]
- Aaskov, J. Japanese Encephalitis in Australia Now and Forever? 2022. Available online: https://rstmh.org/news-blog/blogs/japanese-encephalitis-in-australia-now-and-forever (accessed on 27 May 2022).
- Faizah, A.N.; Kobayashi, D.; Maekawa, Y.; Amoa-Bosompem, M.; Fauziyah, S.; Mulyatno, K.C.; Subekti, S.; Rohmah, E.A.; Lusida, M.I.; Mori, Y.; et al. Identification and Isolation of Japanese Encephalitis Virus Genotype IV from Culex vishnui Collected in Bali, Indonesia in 2019. Am. J. Trop. Med. Hyg. 2021, 105, 813–817. [Google Scholar] [CrossRef]
- Mulvey, P.; Duong, V.; Boyer, S.; Burgess, G.; Williams, D.T.; Dussart, P.; Horwood, P.F. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens 2021, 10, 1534. [Google Scholar] [CrossRef]
- van den Hurk, A.; Ritchie, S.A.; Mackenzie, J.S. Ecology and Geographical Expansion of Japanese Encephalitis Virus. Annu. Rev. Entomol. 2009, 54, 17–35. [Google Scholar] [CrossRef] [Green Version]
- Whelan, P.I.; Jacups, S.P.; Melville, L.; Broom, A.; Currie, B.J.; Krause, V.L.; Brogan, B.; Smith, F.; Porigneaux, P. Rainfall and vector mosquito numbers as risk indicators for mosquito-borne disease in central Australia. Commun. Dis. Intell. Q. Rep. 2003, 27, 110–116. [Google Scholar]
- van den Hurk, A.F.; Nisbet, D.J.; Hall, R.A.; Kay, B.H.; MacKenzie, J.S.; Ritchie, S.A. Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. J. Med. Entomol. 2003, 40, 82–90. [Google Scholar] [CrossRef] [Green Version]
- van den Hurk, A.F.; Pyke, A.T.; Mackenzie, J.S.; Hall-Mendelin, S.; Ritchie, S.A. Japanese Encephalitis Virus in Australia: From Known Known to Known Unknown. Trop. Med. Infect. Dis. 2019, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Hone, J. How many feral pigs in Australia? An update. Aust. J. Zool. 2019, 67, 215–220. [Google Scholar] [CrossRef]
- Williams, C.R.; Ritchie, S.A.; Whelan, P.I. Potential distribution of the Asian disease vector Culex gelidus Theobald (Diptera: Culicidae) in Australia and New Zealand: A prediction based on climate suitability. Aust. J. Entomol. 2005, 44, 425–430. [Google Scholar] [CrossRef]
- Lessard, B.D.; Kurucz, N.; Rodriguez, J.; Carter, J.; Hardy, C.M. Detection of the Japanese encephalitis vector mosquito Culex tritaeniorhynchus in Australia using molecular diagnostics and morphology. Parasites Vectors 2021, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- Furuya-Kanamori, L.; Xu, C.; Doi, S.A.R.; Clark, J.; Wangdi, K.; Mills, D.J.; Lau, C.L. Comparison of immunogenicity and safety of licensed Japanese encephalitis vaccines: A systematic review and network meta-analysis. Vaccine 2021, 39, 4429–4436. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Lau, C.; Leeb, A.; Mills, D.; Furuya-Kanamori, L. Safety profile comparison of chimeric live attenuated and Vero cell-derived inactivated Japanese encephalitis vaccines through an active surveillance system in Australia. Hum. Vaccines Immunother. 2022, 18, 573. [Google Scholar] [CrossRef]
- Chokephaibulkit, K.; Sirivichayakul, C.; Thisyakorn, U.; Pancharoen, C.; Boaz, M.; Bouckenooghe, A.; Feroldi, E. Long-term follow-up of Japanese encephalitis chimeric virus vaccine: Immune responses in children. Vaccine 2016, 34, 5664–5669. [Google Scholar] [CrossRef]
- Solomon, T.; Dung, N.M.; Kneen, R.; Gainsborough, M.; Vaughn, D.W.; Khanh, V.T. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry 2000, 68, 405–415. [Google Scholar] [CrossRef]
- Heffelfinger, J.D.; Li, X.; Batmunkh, N.; Grabovac, V.; Diorditsa, S.; Liyanage, J.B.; Pattamadilok, S.; Bahl, S.; Vannice, K.S.; Hyde, T.B.; et al. Japanese Encephalitis Surveillance and Immunization—Asia and Western Pacific Regions, 2016. Morb. Mortal. Wkly. Rep. 2017, 66, 579–583. [Google Scholar] [CrossRef]
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.L.; Furuya-Kanamori, L.; Soares-Magalhaes, R.; Devine, G. Japanese Encephalitis emergence in Australia: The potential population at risk. medRxiv 2022. [Google Scholar] [CrossRef]
- Bryan, J.H.; O’Donnell, M.S.; Berry, G.; Carvan, T. Dispersal of adult female Culex annulirostris in Griffith, New South Wales, Australia: A further study. J. Am. Mosq. Control Assoc. 1992, 8, 398–403. [Google Scholar]
- Mills, D.J.; Lau, C.L.; Furuya-Kanamori, L. Low uptake of Japanese encephalitis vaccination among Australian travellers. J. Travel Med. 2021, 28, taaa232. [Google Scholar] [CrossRef]
- Schaumburg, F.; De Pijper, C.A.; Grobusch, M.P. Intradermal travel vaccinations-when less means more. Travel Med. Infect. Dis. 2019, 28, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Australian Government—Therapeutic Goods Administration. Japanese Encephalitis Virus—Medicine Shortage Information. 2022. Available online: https://apps.tga.gov.au/shortages/search/Details/japanese-encephalitis-virus (accessed on 27 May 2022).
- UNICEF Supply Division. Japanese Encephalitis Virus—Medicine Shortage Information. 2021. Available online: https://www.unicef.org/supply/media/10381/file/Japanese-Encephalitis-Vaccine-Supply-Demand-Update-October2021.pdf (accessed on 27 May 2022).
- Fox, J.P.; Kossobudzki, S.L.; Cunha, J.F.D. Field studies on the immune response to 17D Yellow Fever virus: Relation to virus substrain, Dose, and route of inoculation. Am. J. Epidemiol. 1943, 38, 113–138. [Google Scholar] [CrossRef]
- Weller, T.H.; Cheever, F.S.; Enders, J.F. Immunologic Reactions Following the Intradermal Inoculation of Influenza A and B Vaccine. Proc. Soc. Exp. Biol. Med. 1948, 67, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, J.L.; De Pijper, C.A.; Garrido, H.M.G.; Daams, J.G.; Goorhuis, A.; Stijnis, C.; Schaumburg, F.; Grobusch, M.P. Fractional dose of intradermal compared to intramuscular and subcutaneous vaccination—A systematic review and meta-analysis. Travel Med. Infect. Dis. 2020, 37, 101868. [Google Scholar] [CrossRef]
- Australian Government—Department of Health. Rabies and Other Lyssaviruses. 2018. Available online: https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/rabies-and-other-lyssaviruses (accessed on 27 May 2022).
- World Health Organization. Rabies Vaccines: WHO Position Paper, April 2018 – Recommendations. Vaccine 2018, 36, 5500–5503. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Mills, D.J.; Lau, C.L. Could intradermal be an economical alternative route of administration for Japanese encephalitis vaccines? J. Travel Med. 2021, 28, taaa181. [Google Scholar] [CrossRef]
- Islam, N.; Xu, C.; Lau, C.L.; Mills, D.J.; Clark, J.; Devine, G.; Hugo, L.; Gyawali, N.; Thalib, L.; Furuya-Kanamori, L. Persistence of antibodies, boostability, and interchangeability of Japanese encephalitis vaccines: A systematic review and dose-response meta-analysis. Vaccine 2022, 40, 3546–3555. [Google Scholar] [CrossRef]
- Mills, D.J.; Lau, C.L.; Mills, C.; Furuya-Kanamori, L. Long-term persistence of antibodies and boostability after rabies intradermal pre-exposure prophylaxis. J. Travel Med. 2022, 29, taab188. [Google Scholar] [CrossRef]
- Roukens, A.H.E.; van Halem, K.; de Visser, A.W.; Visser, L.G. Long-Term Protection After Fractional-Dose Yellow Fever Vaccination: Follow-up Study of a Randomized, Controlled, Noninferiority Trial. Ann. Intern. Med. 2018, 169, 761–765. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuya-Kanamori, L.; Gyawali, N.; Mills, D.J.; Hugo, L.E.; Devine, G.J.; Lau, C.L. The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy. Trop. Med. Infect. Dis. 2022, 7, 85. https://doi.org/10.3390/tropicalmed7060085
Furuya-Kanamori L, Gyawali N, Mills DJ, Hugo LE, Devine GJ, Lau CL. The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy. Tropical Medicine and Infectious Disease. 2022; 7(6):85. https://doi.org/10.3390/tropicalmed7060085
Chicago/Turabian StyleFuruya-Kanamori, Luis, Narayan Gyawali, Deborah J. Mills, Leon E. Hugo, Gregor J. Devine, and Colleen L. Lau. 2022. "The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy" Tropical Medicine and Infectious Disease 7, no. 6: 85. https://doi.org/10.3390/tropicalmed7060085
APA StyleFuruya-Kanamori, L., Gyawali, N., Mills, D. J., Hugo, L. E., Devine, G. J., & Lau, C. L. (2022). The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy. Tropical Medicine and Infectious Disease, 7(6), 85. https://doi.org/10.3390/tropicalmed7060085






