Antimicrobial Susceptibility Testing Patterns of Neisseria gonorrhoeae from Patients Attending Sexually Transmitted Infections Clinics in Six Regions in Tanzania
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Isolates
2.2. In Vitro Antimicrobial Susceptibility Testing
2.2.1. E-Test
2.2.2. Agar Dilution Method
2.3. Statistical Analysis
3. Results
Prevalence of Antimicrobial and Multi-Drug Resistance
4. Discussion
5. Conclusions and Recommendations
6. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bala, M. Characterization of profile of multidrug-resistant neisseria gonorrhoeae using old and new definitions in india over a decade: 2000–2009. Sex. Transm. Dis. 2011, 38, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Bala, M.; Singh, V.; Bhargava, A.; Kakran, M.; Joshi, N.C.; Bhatnagar, R. Gentamicin susceptibility among a sample of multidrug-resistant Neisseria gonorrhoeae isolates in India. Antimicrob. Agents Chemother. 2016, 60, 7518–7521. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.; Parti, R.; Thakur, S. Emergence of resistance and antimicrobial resistance mechanisms in Neisseria gonorrhoeae. Culture 2015, 35, 1–8. [Google Scholar]
- Workowski, K.A.; Bolan, G.A. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar] [PubMed]
- Rowley, J.; Toskin, I.; Ndowa, F. Global Incidence and Prevalence of Selected Curable Sexually Transmitted Infections-2008; World Health Organization: Geneva, Switzerland, 2012; pp. 1–28. [Google Scholar]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st Century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO Press: Geneva, Switzerland, 2015; pp. 1–28. Available online: https://apps.who.int/iris/handle/10665/193736 (accessed on 13 April 2022).
- Ndowa, F.; Christine, A. Global Action Plan to Control the Spread and Impact of Antimicrobial Resistance in Neisseria Gonorrhoeae; World Health Organization: Geneva, Switzerland, 2016; Available online: http://whqlibdoc.who.int/publications/2012/9789241503501_eng.pdf (accessed on 13 April 2022).
- Tapsall, J.W. Neisseria gonorrhoeae and emerging resistance to extended spectrum cephalosporins. Curr. Opin. Infect. Dis. 2009, 22, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.A.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef]
- Ohnishi, M.; Golparian, D.; Shimuta, K.; Saika, T.; Hoshina, S.; Iwasaku, K.; Nakayama, S.I.; Kitawaki, J.; Unemo, M. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: Detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob. Agents Chemother. 2011, 55, 3538–3545. [Google Scholar] [CrossRef]
- Morse, S.A.; Johnson, S.R.; Biddle, J.W.; Roberts, M.C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob. Agents Chemother. 1986, 30, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Günther, D.; Düx, F.; Naumann, M.; Meyer, T.F.; Rudel, T. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 1999, 18, 339–352. [Google Scholar] [CrossRef]
- Cámara, J.; Serra, J.; Ayats, J.; Bastida, T.; Carnicer-Pont, D.; Andreu, A.; Ardanuy, C. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J. Antimicrob. Chemother. 2012, 67, 1858–1860. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.R.; Morse, S.A. Antibiotic resistance in Neisseria gonorrhoeae: Genetics and mechanisms of resistance. Sex. Transm. Dis. 1988, 15, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ison, C.A.; Martin, I.M. Susceptibility of gonococci isolated in London to therapeutic antibiotics: Establishment of a London surveillance programme. London Gonococcal Working Group. Sex. Transm. Infect. 1999, 75, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Shafer, W.M. Antibiotic resistance in Neisseria gonorrhoeae: Origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 2011, 1230, E19–E28. [Google Scholar] [CrossRef]
- Lewis, D.A. Antimicrobial-resistant gonorrhoea in Africa: An important public health threat in need of a regional gonococcal antimicrobial surveillance programme. S. Afr. J. Epidemiol. Infect. 2011, 26, 215–220. [Google Scholar] [CrossRef][Green Version]
- Moodley, P.; Sturm, A.W. Ciprofloxacin-resistant gonorrhoea in South Africa. Lancet 2005, 366, 1159. [Google Scholar] [CrossRef]
- Lewis, D.A.; Scott, L.; Slabbert, M.; Mhlongo, S.; van Zijl, A.; Sello, M.; du Plessis, N.; Radebe, F.; Wasserman, E. Escalation in the relative prevalence of ciprofloxacin-resistant gonorrhoea among men with urethral discharge in two South African cities: Association with HIV seropositive. Sex. Transm. Infect. 2008, 84, 352–355. [Google Scholar] [CrossRef]
- Fingerhuth, S.M.; Bonhoeffer, S.; Low, N.; Althaus, C.L. Antibiotic-Resistant Neisseria gonorrhoeae Spread Faster with More Treatment, Not More Sexual Partners. PLoS Pathog. 2016, 12, e1005611. [Google Scholar] [CrossRef]
- Buhalata, S.N.; Kwesigabo, G.; Sembuche, S.; Aboud, S.; Temu, M.M.; Changalucha, J.M. Genital tract infections in women attending sexually transmitted infection clinics in Mwanza, north-west Tanzania. S. Afr. J. Epidemiol. Infect. 2013, 28, 48–54. [Google Scholar] [CrossRef][Green Version]
- Goldstein, E.; Kirkcaldy, R.D.; Reshef, D.; Berman, S.; Weinstock, H.; Sabeti, P.; Del Rio, C.; Hall, G.; Hook, E.W.; Lipsitch, M. Factors related to increasing prevalence of resistance to ciprofloxacin and other antimicrobial drugs in Neisseria gonorrhoeae, United States. Emerg. Infect. Dis. 2012, 18, 1290–1297. [Google Scholar] [CrossRef]
- Robins-Brown, R.M.; Gaillard, M.R.C.; Koornhof, H.J.; Mauff, A.C. Penicillinase-producing Neisseria gonorrhoeae. S. Afr. Med. J. 1977, 51, 568. [Google Scholar]
- Hallet, A.F.; Appelbaum, P.C.; Cooper, R.; Mokgokong, S.; Monale, D. Penicillinase-producing Neisseria gonorrhoeae from South Africa. Lancet 1977, 1, 1205. [Google Scholar] [CrossRef]
- Mehta, S.D.; Maclean, I.; Ndinya-Achola, J.O.; Moses, S.; Martin, I.; Ronald, A.; Agunda, L.; Murugu, R.; Bailey, R.C.; Melendez, J.; et al. Emergence of quinolone resistance and cephalosporin MIC creep in Neisseria gonorrhoeae isolates from a cohort of young men in Kisumu, Kenya, 2002 to 2009. Antimicrob. Agents Chemother. 2011, 55, 3882–3888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Dyck, E.; Crabbe, F.; Nzila, N.; Bogaerts, J.; Munyabikali, J.P.; Ghys, P.; Diallo, M.; Laga, M. Increasing resistance of Neisseria gonorrheae in west and central Africa. Consequence on therapy of gonococcal infection. Sex. Transm. Dis. 1997, 24, 32–37. [Google Scholar] [CrossRef] [PubMed]
- West, B.; Changalucha, J.; Grosskurth, H.; Mayaud, P.; Gabone, R.M.; Ka-Gina, G.; Mabey, D. Antimicrobial susceptibility, auxotype and plasmid content of Neisseria gonorrhoeae in northern Tanzania: Emergence of high level plasmid mediated tetracycline resistance. Genitourin. Med. 1995, 71, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, E.; Karita, E.; Abdellati, S.; Dirk, V.H.; Ngabonziza, M.; Lafort, Y.; Laga, M. Antimicrobial susceptibilities of Neisseria gonorrhoeae in Kigali, Rwanda, and trends of resistance between 1986 and 2000. Sex. Transm. Dis. 2001, 28, 539–545. [Google Scholar] [CrossRef]
- Unemo, M.; Golparian, D.; Nicholas, R.; Ohnishi, M.; Gallay, A.; Sednaouie, P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: Novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 2012, 56, 1273–1280. [Google Scholar] [CrossRef]
- Lebedzeu, F.; Golparian, D.; Titov, L.; Pankratava, N.; Glazkova, S.; Shimanskaya, I.; Charniakova, N.; Lukyanau, A.; Domeika, M.; Unemo, M. Antimicrobial susceptibility/resistance and NG-MAST characterisation of in Belarus, Eastern Europe, 2010–2013. BMC Infect. Dis. 2015, 15, 29. [Google Scholar] [CrossRef][Green Version]
- Kubanova, A.; Kubanov, A.; Frigo, N.; Solomka, V.; Semina, V.; Vorobyev, D.; Khairullin, R.; Unemo, M. Russian gonococcal antimicrobial susceptibility programme (RU-GASP)—Resistance in Neisseria gonorrhoeae during 2009–2012 and NG-MAST genotypes in 2011 and 2012. BMC Infect. Dis. 2014, 14, 342. [Google Scholar] [CrossRef][Green Version]
- Unemo, M. Current and future antimicrobial treatment of gonorrhoea-the rapidly evolving Neisseria gonorrhoeae continues to challenge. BMC Infect. Dis. 2015, 15, 364. [Google Scholar] [CrossRef]
- Papp, J.R.; Abrams, A.J.; Nash, E.; Katz, A.R.; Kirkcaldy, R.D.; O’Connor, N.P.; O’Brien, P.S.; Harauchi, D.H.; Maningas, E.V.; Soge, O.O.; et al. Azithromycin resistance and decreased ceftriaxone susceptibility in Neisseria gonorrhoeae, Hawaii, USA. Emerg. Infect. Dis. 2017, 23, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Social Welfare. National Guidelines for Management of Sexually Transmitted and Reproductive Tract Infections, 1st ed.; Ministry of Health and Social Welfare: Dar es Salaam, Tanzania, 2007. [Google Scholar]
- Li, S.; Su, X.H.; Le, W.J.; Jiang, F.X.; Wang, B.X.; Rice, P.A. Antimicrobial susceptibility of Neisseria gonorrhoeae isolates from symptomatic men attending the Nanjing sexually transmitted diseases clinic (2011–2012): Genetic characteristics of isolates with reduced sensitivity to ceftriaxone. BMC Infect Dis. 2014, 14, 622. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tzelepi, E.; Avgerinou, H.; Kyriakis, K.P.; Tzouvelekis, L.S.; Flemetakis, A.; Kalogeropoulou, A.; Frangouli, E. Antimicrobial susceptibility and types of Neisseria gonorrhoeae in Greece. Data for the period 1990 to 1993. Sex. Transm. Dis. 1997, 24, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Beverly, A.; Bailey-Griffin, J.R.; Schwebke, J.R. InTray GC Medium versus Modified Thayer-Martin Agar Plates for Diagnosis of Gonorrhea from Endocervical Specimens. J. Clin. Microbiol. 2000, 38, 3825–3826. [Google Scholar] [CrossRef]
- Kambli, P.; Ajbani, K.; Sadani, M.; Nikam, C.; Shetty, A.; Udwadia, Z.; Rodwell, T.C.; Catanzaro, A.; Rodrigues, C. Correlating minimum inhibitory concentrations of ofloxacin and moxifloxacin with gyrA mutations using the genotype MTBDRsl assay. Tuberculosis 2015, 95, 137–141. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Patel, J.B.; Alder, J.; Bradford, P.A. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement. Clin. Lab. Stand. Inst. 2013, 33, 1–205. [Google Scholar]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing: Breakpoint Tables for Interpretation of MICs and Zone 520 Diameters; EUCAST: Växjö, Sweden, 2017; p. v7.1. [Google Scholar]
- Paula, A.; Costa-Lourenço, R.; dos Santos, K.T.; Moreira, B.M.; Fracalanzza, S.E.L.; Bonelli, R.R. Antimicrobial resistance in Neisseria gonorrhoeae: History, molecular mechanisms and epidemiological aspects of an emerging global threat. Braz. J. Microbiol. 2017, 48, 617–628. [Google Scholar]
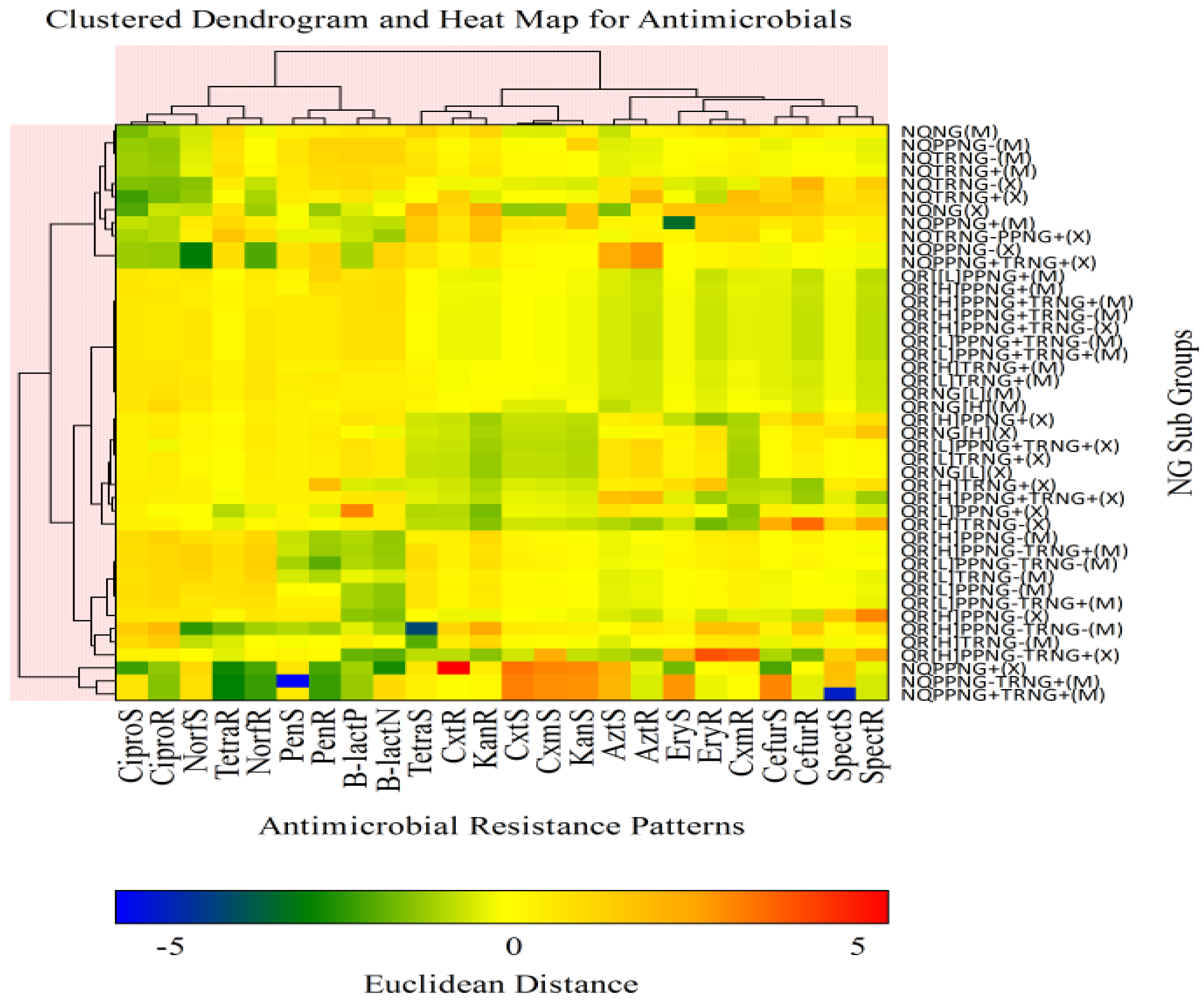
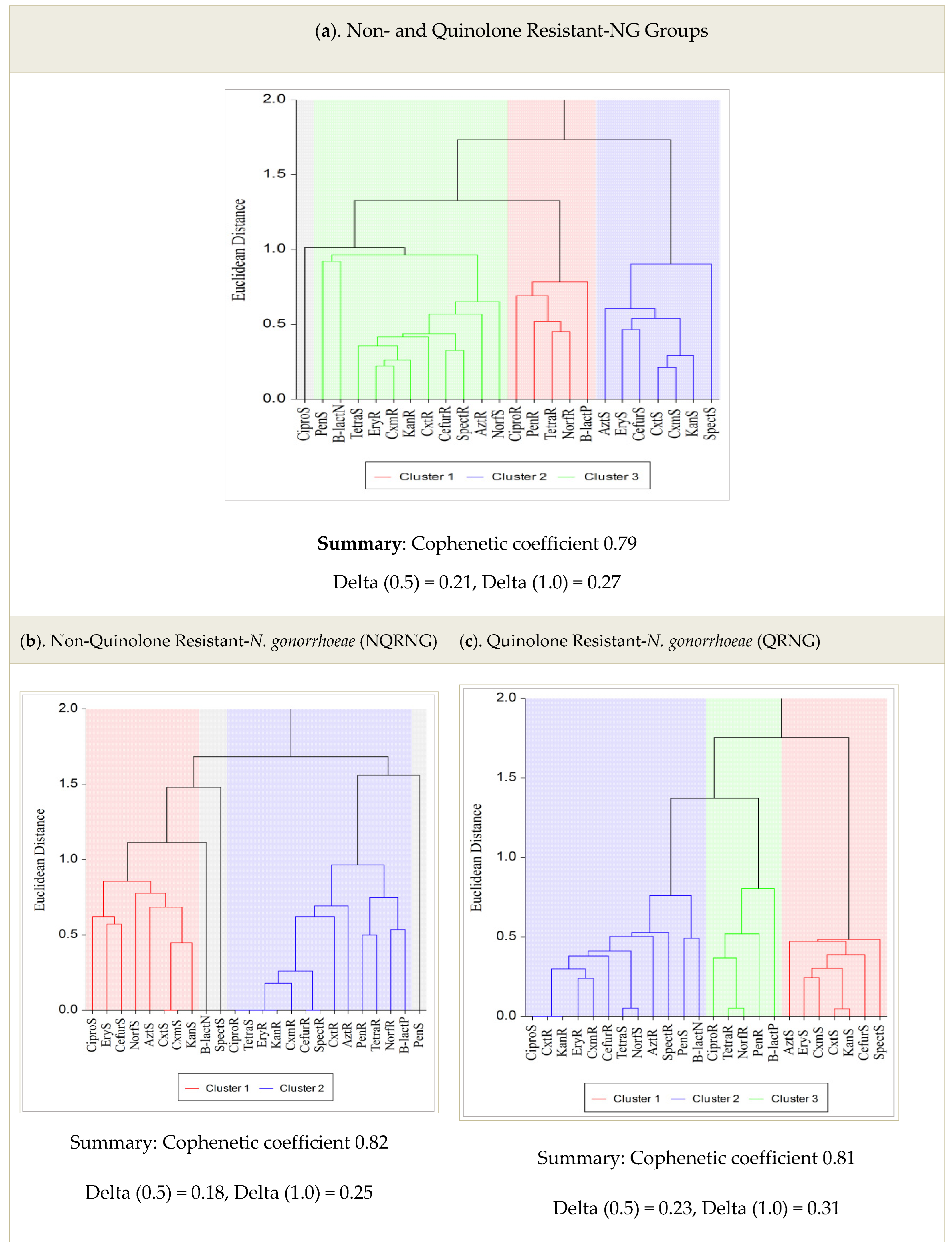
| Antimicrobial | Susceptible n (%) | Intermediate n (%) | Resistant n (%) |
|---|---|---|---|
| Ciprofloxacin | 20 (12.2) | 19 (11.5) | 125 (75.6) |
| Norfloxacin | 11 (7.8) | 14 (10.0) | 118 (84.3) |
| Tetracycline | 2 (1.4) | 1/140 (0.7) | 136 (97.1) |
| Penicillin | 9 (6. 4) | 16 (11.4) | 115 (82.2) |
| Ceftriaxone | 161 (98.2) | 1 (0.6) | 1 (0.6) |
| Cefixime | 160 (97.6) | 2 (1.2) | 2 (1.2) |
| Cefuroxime | 136 (97.1) | 2 (0.7) | 3 (2.1) |
| Azithromycin | 158 (96.3) | 2 (1.2) | 5 (3.1) |
| Erythromycin | 120 (85.7) | 18 (12.8) | 2 (1.4) |
| Spectinomycin | 135 (96.4) | 0 (0.0) | 5 (3.5) |
| Kanamycin | 137 (92.9) | 3 (2.1) | 0 (0.0) |
| Antimicrobial | MIC (µg/mL), Men (n = 112) | MIC (µg/mL), Women (n = 52) | Overall MIC (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | MIC50 | MIC90 | Mean (SD) | MIC50 | MIC90 | Mean (SD) | MIC50 | MIC90 | Range | |
| Ciprofloxacin | 5.1 (5.5) | 4.0 | 8.0 | 5.6 (7.3) | 4.0 | 8.0 | 5.3 (6.5) | 4.0 | 8.0 | 0.002–32 |
| Norfloxacin | 5.4 (9.6) | 2.0 | 4.0 | 4.8 (7.3) | 2.0 | 4.0 | 5.1 (8.1) | 2.0 | 4.0 | 0.002–32 |
| Tetracycline | 27.2 (9.9) | 32.0 | 32.0 | 27.7 (9.3) | 32.0 | 32.0 | 27.5 (10.4) | 32.0 | 32.0 | 0.016–32 |
| Penicillin | 21.1 (14.8) | 32.0 | 32.0 | 20.9 (14) | 32.0 | 32.0 | 21 (14.5) | 32.0 | 32.0 | 0.004–32 |
| Ceftriaxone | 0.009 (0.03) | 0.002 | 0.004 | 0.037 (0.17) | 0.002 | 0.004 | 0.023 (0.13) | 0.002 | 0.004 | 0.002–1.5 |
| Cefixime | 0.022 (0.058) | 0.016 | 0.016 | 0.067 (0.241) | 0.016 | 0.016 | 0.047 (0.18) | 0.016 | 0.016 | 0.016–2 |
| Cefuroxime | 0.005 (0.004) | 0.002 | 0.008 | 0.11 (0.43) | 0.008 | 0.008 | 0.064 (0.32) | 0.008 | 0.008 | 0.002–2 |
| Azithromycin | 3.9 (30.7) | 0.125 | 0.25 | 0.24 (0.27) | 0.125 | 0.19 | 1.9 (19.6) | 0.19 | 0.25 | 0.002–256 |
| Erythromycin | 0.08 (0.15) | 0.032 | 0.063 | 0.23 (0.57) | 0.063 | 0.25 | 0.18 (0.44) | 0.063 | 0.125 | 0.002–4 |
| Spectinomycin | 17.8 (11.1) | 16 | 32 | 35.5 (54.7) | 16 | 32 | 29.8 (46.9) | 16 | 32 | 1–256 |
| Kanamycin | 7.3 (6.5) | 8.0 | 8.0 | 10.6 (13.9) | 8.0 | 16 | 8.9 (11.2) | 8.0 | 8.0 | 0.25–64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboud, S.; Buhalata, S.N.; Onduru, O.G.; Chiduo, M.G.; Kwesigabo, G.P.; Mshana, S.E.; Manjurano, A.M.; Temu, M.M.; Kishamawe, C.; Changalucha, J.M. Antimicrobial Susceptibility Testing Patterns of Neisseria gonorrhoeae from Patients Attending Sexually Transmitted Infections Clinics in Six Regions in Tanzania. Trop. Med. Infect. Dis. 2022, 7, 89. https://doi.org/10.3390/tropicalmed7060089
Aboud S, Buhalata SN, Onduru OG, Chiduo MG, Kwesigabo GP, Mshana SE, Manjurano AM, Temu MM, Kishamawe C, Changalucha JM. Antimicrobial Susceptibility Testing Patterns of Neisseria gonorrhoeae from Patients Attending Sexually Transmitted Infections Clinics in Six Regions in Tanzania. Tropical Medicine and Infectious Disease. 2022; 7(6):89. https://doi.org/10.3390/tropicalmed7060089
Chicago/Turabian StyleAboud, Said, Simon N. Buhalata, Onduru G. Onduru, Mercy G. Chiduo, Gideon P. Kwesigabo, Stephen E. Mshana, Alphaxard M. Manjurano, Mansuet M. Temu, Coleman Kishamawe, and John M. Changalucha. 2022. "Antimicrobial Susceptibility Testing Patterns of Neisseria gonorrhoeae from Patients Attending Sexually Transmitted Infections Clinics in Six Regions in Tanzania" Tropical Medicine and Infectious Disease 7, no. 6: 89. https://doi.org/10.3390/tropicalmed7060089
APA StyleAboud, S., Buhalata, S. N., Onduru, O. G., Chiduo, M. G., Kwesigabo, G. P., Mshana, S. E., Manjurano, A. M., Temu, M. M., Kishamawe, C., & Changalucha, J. M. (2022). Antimicrobial Susceptibility Testing Patterns of Neisseria gonorrhoeae from Patients Attending Sexually Transmitted Infections Clinics in Six Regions in Tanzania. Tropical Medicine and Infectious Disease, 7(6), 89. https://doi.org/10.3390/tropicalmed7060089






