Presence and Multi-Species Spatial Distribution of Oropouche Virus in Brazil within the One Health Framework
Abstract
1. Introduction
The One Health Framework
2. Materials and Methods
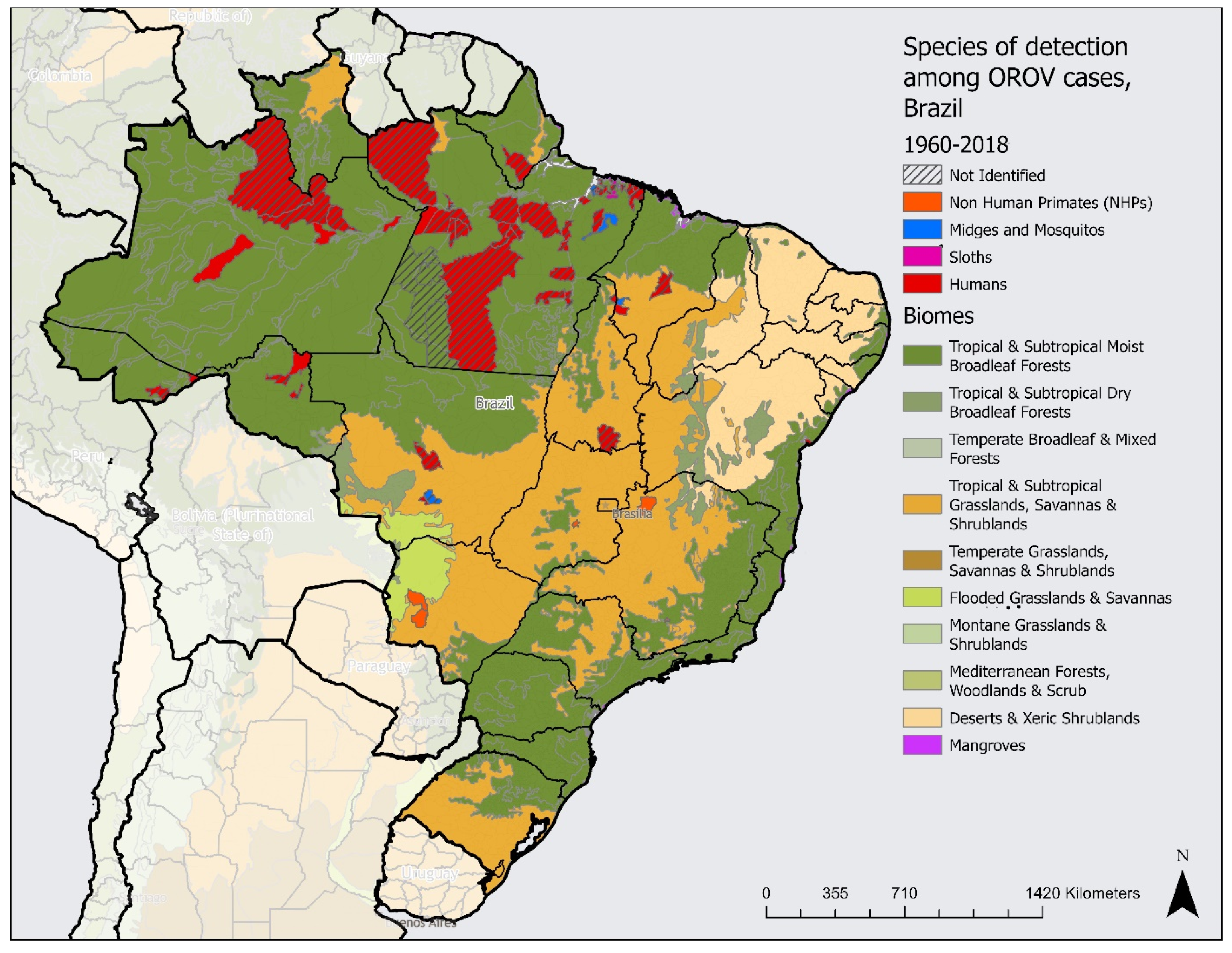
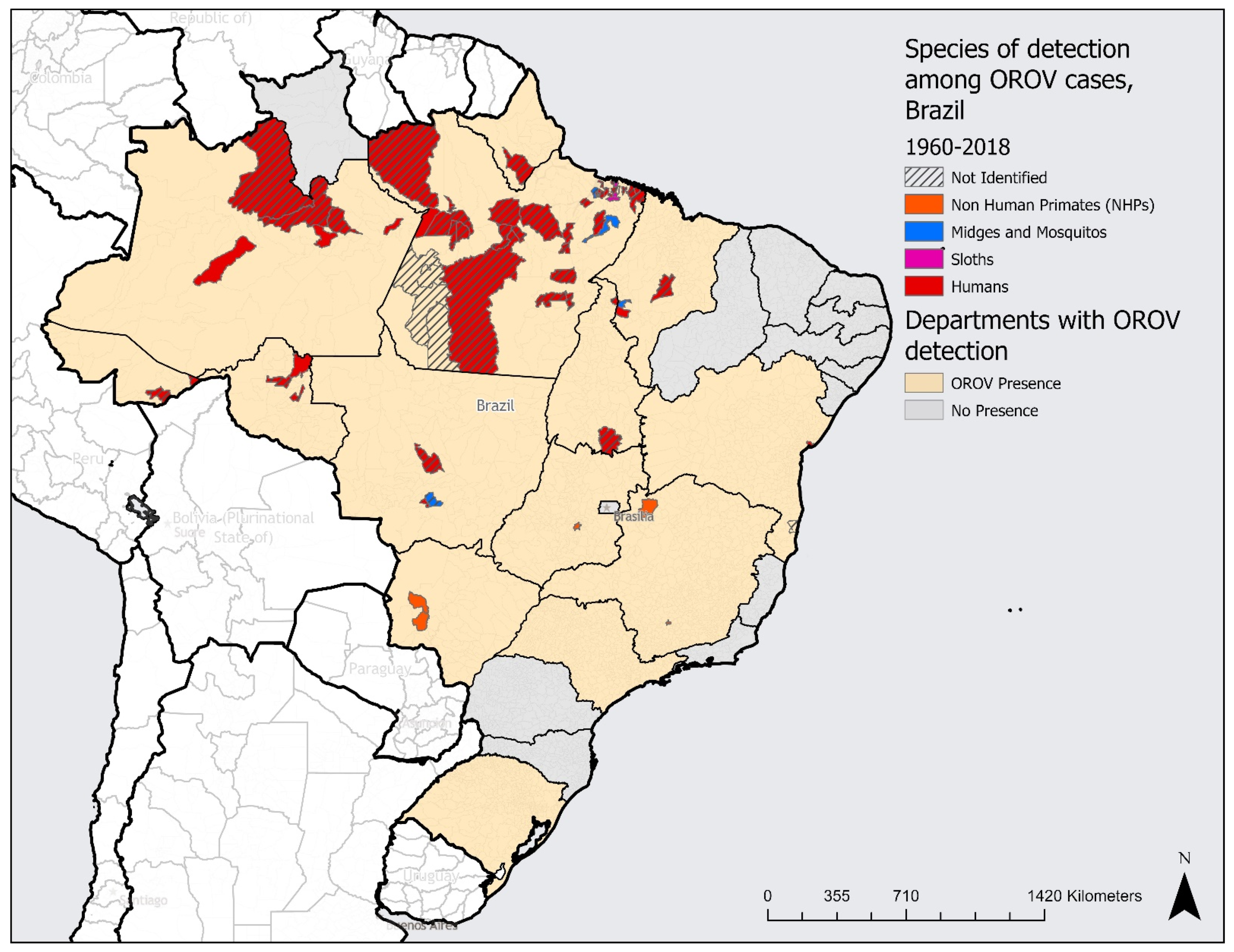
3. Results
4. Discussion
4.1. Drivers Associated with the Occurrence of Outbreaks
4.2. The Expansion of Cases in Brazil
4.3. Limitations to This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero-Alvarez, D.; Escobar, L.E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. 2018, 20, 135–146. [Google Scholar] [CrossRef]
- Pinherio, F.P.; Pinheiro, M.; Bensabeth, G.; Causey, O.R.; Shope, R. Epidemia de vírus Oropouche em Belém. Rev. Do Serviço Espec. De Saúde Pública 1962, 12, 13–23. [Google Scholar]
- Nunes, M.R.T.; Vasconcelos, H.B.; Medeiros, D.B.A.; Rodrigues, S.G.; Azevedo, R.S.S.; Chiang, J.O.; Martins, L.C.; Vasconcelos, P.F.C. Oropouche fever: A review of the epidemiological and molecular aspects in the Brazilian Amazon. Cad. Health Colet. Rio. J. 2007, 15, 303–318. [Google Scholar]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; Martins, L.C.; Rodrigues, S.G.; Chiang, J.O.; Azevedo, R.D.S.D.S.; Da Rosa, A.P.T.; Vasconcelos, P.F.D.C. Oropouche Virus Isolation, Southeast Brazil. Emerg. Infect. Dis. 2005, 11, 1610–1613. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Chies, J.A.B. Zoonotic spillover: Understanding basic aspects for better prevention. Genet. Mol. Biol. 2021, 44, e20200355. [Google Scholar] [CrossRef]
- Tesh, R.B.; Vasconcelos, P.F.C. Sandfly Fever, Oropouche Fever, and Other Bunyavirus Infections. In Tropical Infectious Diseases, 3rd ed.; Guerrant, R.L., Walker, D.H., Weller, P.F., Eds.; Saunders: Philadelphia, PA, USA, 2011; Volume 72, pp. 481–482. [Google Scholar] [CrossRef]
- Pereira, T.N.; Virginio, F.; Souza, J.I.; Moreira, L.A. Emergent Arboviruses: A Review About Mayaro virus and Oropouche orthobunyavirus. Front. Trop. Dis. 2021, 2, 737436. [Google Scholar] [CrossRef]
- Frank, D. One world, one health, one medicine. Can. Vet. J. 2008, 49, 1063–1065. [Google Scholar]
- Kaplan, B.; Kahn, L.H.; Monath, T.P.; Woodall, J. ‘ONE HEALTH’ and parasitology. Parasites Vectors 2009, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- King, L.J. One health: Communicable diseases at human-animal interface. In Control of Communicable Diseases Manual, 20th ed.; Heymann, D.L., Ed.; American Public Health Association: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Schneider, M.C.; Munoz-Zanzi, C.; Min, K.; Aldighieri, S. “One Health” from Concept to Application in the Global World. Oxf. Res. Encycl. Glob. Public Health 2019. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- IBGE. Municipal Mesh: Product Access—2021. Available online: https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais/15774-malhas.html?=&t=acesso-ao-produto (accessed on 26 April 2022).
- IBGE. Brazil: Population. Available online: https://cidades.ibge.gov.br/brasil/panorama (accessed on 2 June 2022).
- IBGE. Sistema de Contas Regionais: Brasil 2019. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101873_informativo.pdf (accessed on 2 June 2022).
- Salgado, B.B.; Maués, F.C.J.; Pereira, R.L.; Chiang, J.O.; Freitas, M.N.O.; Ferreira, M.S.; Martins, L.C.; Vasconcelos, P.F.C.; Ganoza, C.; Lalwani, P. Prevalence of arbovirus antibodies in young healthy adult population in Brazil. Parasites Vectors 2021, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.O.; Azevedo, R.S.; Justino, M.C.A.; Matos, H.J.; Cabeça, H.L.S.; Silva, S.P.; Henriques, D.F.; Silva, E.V.P.; Andrade, G.S.S.; Vasconcelos, P.F.; et al. Neurological disease caused by Oropouche virus in northern Brazil: Should it be included in the scope of clinical neurological diseases? J. Neuro. Virol. 2021, 27, 626–630. [Google Scholar] [CrossRef]
- Nascimento, V.A.D.; Santos, J.H.A.; Monteiro, D.C.S.; Pessoa, K.P.; Cardoso, A.J.L.; de Souza, V.C.; Abdalla, L.F.; Naveca, F.G. Oropouche virus detection in saliva and urine. Memórias Inst. Oswaldo Cruz 2020, 115, e190338. [Google Scholar] [CrossRef]
- Fonseca, L.M.S.; Carvalho, R.H.; Bandeira, A.C.; Sardi, S.I.; Campos, G.S. Oropouche Virus Detection in Febrile Patients’ Saliva and Urine Samples in Salvador, Bahia, Brazil. Jpn. J. Infect. Dis. 2020, 73, 164–165. [Google Scholar] [CrossRef]
- Oliveira, E.; Raimunda Do Socorro Silva, A.; Coelho-Dos-Reis, J.G.; Lis Ribeiro Do Valle, A.; Ferreira, M.S.; Campi-Azevedo, A.C.; Costa-Silva, M.F.; Martins, L.C.; Chiang, J.O.; Teixeira-Carvalho, A.; et al. IFN-α as a time-sensitive biomarker during Oropouche virus infection in early and late seroconverters. Sci. Rep. 2019, 9, 17924. [Google Scholar] [CrossRef]
- Vernal, S.; Martini, C.C.R.; Fonseca, B.A.L. Oropouche Virus-Associated Aseptic Meningoencephalitis, Southeastern Brazil. Emerg. Infect. Dis. 2019, 25, 380–382. [Google Scholar] [CrossRef]
- Nunes, M.R.T.; de Souza, W.M.; Savji, N.; Figueiredo, M.L.; Cardoso, J.F.; da Silva, S.P.; Lima, C.P.D.S.D.; Vasconcelos, H.B.; Rodrigues, S.G.; Lipkin, W.I.; et al. Oropouche orthobunyavirus: Genetic characterization of full-length genomes and development of molecular methods to discriminate natural reassortments. Infect. Genet. Evol. 2018, 68, 16–22. [Google Scholar] [CrossRef]
- Naveca, F.G.; Nascimento, V.A.; Souza, V.C.; De Figueiredo, R. Human Orthobunyavirus Infections, Tefé, Amazonas, Brazil. PLoS Curr. 2018, 10. [Google Scholar] [CrossRef]
- Da Rosa, J.F.T.; De Souza, W.M.; De Paula Pinheiro, F.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche Virus: Clinical, Epidemiological, and Molecular Aspects of a Neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.D.S.; Zuchi, N.; de Souza, V.C.; Naveca, F.G.; Dos Santos, M.A.M.; Slhessarenko, R.D. Detection of Oropouche virus segment S in patients and inCulex quinquefasciatus in the state of Mato Grosso, Brazil. Memórias Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Tilston-Lunel, N.; Hughes, J.; Acrani, G.O.; da Silva, D.E.A.; Azevedo, R.S.S.; Rodrigues, S.G.; Vasconcelos, P.F.C.; Nunes, M.R.; Elliott, R.M. Genetic analysis of members of the species Oropouche virus and identification of a novel M segment sequence. J. Gen. Virol. 2015, 96, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.S.; Lessa, N.; Naveca, F.G.; Monte, R.L.; Braga, W.S.; Figueiredo, L.T.M.; Ramasawmy, R.; Mourão, M.P.G. Detection ofHerpesvirus, Enterovirus, and Arbovirusinfection in patients with suspected central nervous system viral infection in the Western Brazilian Amazon. J. Med. Virol. 2014, 86, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.M.; Andreotti, R.; De Almeida, P.S.; Marques, A.C.; Rodrigues, S.G.; Chiang, J.O.; Vasconcelos, P.F.D.C. Detection of arboviruses of public health interest in free-living New World primates (Sapajus spp.; Alouatta caraya) captured in Mato Grosso do Sul, Brazil. Rev. da Soc. Bras. de Med. Trop. 2013, 46, 684–690. [Google Scholar] [CrossRef]
- Bastos, M.D.S.; Figueiredo, L.T.M.; Naveca, F.G.; Monte, R.L.; Lessa, N.; De Figueiredo, R.M.P.; Gimaque, J.B.D.L.; João, G.P.; Ramasawmy, R.; Mourão, M.P.G. Identification of Oropouche Orthobunyavirus in the Cerebrospinal Fluid of Three Patients in the Amazonas, Brazil. Am. J. Trop. Med. Hyg. 2012, 86, 732–735. [Google Scholar] [CrossRef]
- Vasconcelos, H.B.; Nunes, M.R.; Casseb, L.M.; Carvalho, V.L.; da Silva, E.V.P.; Silva, M.; Casseb, S.M.; Vasconcelos, P.F. Molecular Epidemiology of Oropouche Virus, Brazil. Emerg. Infect. Dis. 2011, 17, 800–806. [Google Scholar] [CrossRef]
- Terzian, A.C.B.; Bronzoni, R.; Drumond, B.P.; Da Silva-Nunes, M.; da Silva, N.S.; Ferreira, M.U.; Sperança, M.A.; Nogueira, M.L. Sporadic Oropouche Infection, Acre, Brazil. Emerg. Infect. Dis. 2009, 15, 348–350. [Google Scholar] [CrossRef]
- Vasconcelos, H.B.; Azevedo, R.S.; Casseb, S.M.; Nunes-Neto, J.P.; Chiang, J.O.; Cantuária, P.C.; Segura, M.N.; Martins, L.C.; Monteiro, H.A.; Rodrigues, S.G.; et al. Oropouche fever epidemic in Northern Brazil: Epidemiology and molecular characterization of isolates. J. Clin. Virol. 2009, 44, 129–1333. [Google Scholar] [CrossRef]
- Azevedo, R.D.S.D.S.; Nunes, M.R.T.; Chiang, J.O.; Bensabath, G.; Vasconcelos, H.B.; Pinto, A.Y.D.N.; Martins, L.C.; Monteiro, H.A.D.O.; Rodrigues, S.G.; Vasconcelos, P.F.D.C. Reemergence of Oropouche Fever, Northern Brazil. Emerg. Infect. Dis. 2007, 13, 912–915. [Google Scholar] [CrossRef]
- De Figueiredo, R.M.; Thatcher, B.D.; de Lima, M.L.; Almeida, T.C.; Alecrim, W.D.; Guerra, M.V. Exanthematous diseases and the first epidemic of dengue to occur in Manaus, Amazonas State, Brazil, during 1998-1999. Rev. Soc. Bras. Med. Trop. 2004, 37, 476–479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreli, M.L.; Aquino, V.H.; Cruz, A.C.; Figueiredo, L.T. Diagnosis of Oropouche virus infection by RT-nested-PCR. J. Med. Virol. 2002, 66, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.F.; Wang, H.; Nunes, M.; Vasconcelos, P.F.; Weaver, S.C.; Shope, R.E.; Watts, D.M.; Tesh, R.B.; Barrett, A.D. Nucleotide sequences and phylogeny of the nucleocapsid gene of Oropouche virus. J. Gen. Virol. 2000, 81, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.D.S.; Cruz, L.C.D.T.A.D.; de Souza, V.J.; Neves, N.A.D.S.; de Souza, V.C.; Filho, L.C.F.; Lemos, P.D.S.; de Lima, C.P.S.; Naveca, F.G.; Atanaka, M.; et al. Insect-specific viruses and arboviruses in adult male culicids from Midwestern Brazil. Infect. Genet. Evol. 2020, 85, 104561. [Google Scholar] [CrossRef] [PubMed]
- Pauvolid-Corrêa, A.; Campos, Z.; Soares, R.; Nogueira, R.M.R.; Komar, N. Neutralizing antibodies for orthobunyaviruses in Pantanal, Brazil. PLoS Negl. Trop. Dis. 2017, 11, e0006014. [Google Scholar] [CrossRef]
- Lorenz, C.; Azevedo, T.S.; Virginio, F.; Aguiar, B.; Chiaravalloti-Neto, F.; Suesdek, L. Impact of environmental factors on neglected emerging arboviral diseases. PLOS Negl. Trop. Dis. 2017, 11, e0005959. [Google Scholar] [CrossRef]
- da Costa, V.G.; de Rezende Féres, V.C.; Saivish, M.V.; de Lima Gimaque, J.B.; Moreli, M.L. Silent emergence of Mayaro and Oropouche viruses in humans in Central Brazil. Int. J. Infect. Dis. 2017, 62, 84–85. [Google Scholar] [CrossRef]
- Dutra, H.; Caragata, E.; Moreira, L. The re-emerging arboviral threat: Hidden enemies: The emergence of obscure arboviral diseases, and the potential use of Wolbachia in their control. BioEssays 2016, 39, 1600175. [Google Scholar] [CrossRef]
- Gibrail, M.M.; Fiaccadori, F.S.; Souza, M.; Almeida, T.N.V.; Chiang, J.O.; Martins, L.C.; Ferreira, M.S.; Cardoso, D.D.D.D.P. Detection of antibodies to Oropouche virus in non-human primates in Goiânia City, Goiás. Rev. Da Soc. Bras. De Med. Trop. 2016, 49, 357–360. [Google Scholar] [CrossRef]
- Mourão, M.P.G.; Bastos, M.D.S.; De Figueiredo, R.M.P.; Gimaque, J.B.D.L.; Alves, V.D.C.R.; Saraiva, M.D.G.G.; Figueiredo, M.L.G.; Ramasawmy, R.; Nogueira, M.; Figueiredo, L.T.M. Arboviral diseases in the Western Brazilian Amazon: A perspective and analysis from a tertiary health & research center in Manaus, State of Amazonas. Rev. da Soc. Bras. de Med. Trop. 2015, 48, 20–26. [Google Scholar] [CrossRef]
- Batista, P.M.; Andreotti, R.; Chiang, J.O.; Ferreira, M.S.; Vasconcelos, P.F.D.C. Seroepidemiological monitoring in sentinel animals and vectors of arbovirus surveillance in the State of Mato Grosso do Sul, Brazil. Rev. da Soc. Bras. de Med. Trop. 2012, 45, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.D.C.; De Paula, M.B.; Fernandes, A.; Dos Santos, E.; De Almeida, M.A.B.; Da Fonseca, D.F.; Sallum, M.A.M. New records and epidemiological potential of certain species of mosquito (Diptera, Culicidae) in the State of Rio Grande do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2010, 43, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.C.; Prazeres Ado, S.; Gama, E.C.; Lima, M.F.; Azevedo Rdo, S.; Casseb, L.M.; Nunes Neto, J.P.; Martins, L.C.; Chiang, J.O.; Rodrigues, S.G.; et al. Serological survey for arboviruses in Juruti, Para State, Brazil. Cad. Saude Publica 2009, 25, 2517–2523. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.T.; Brabosa, T.F.S.; Casseb, L.M.N.; Neto, J.P.N.; Segura, N.O.; Monteiro, H.A.O.; Pinto, E.V.; Casseb, S.M.; de Oliveira Chiang, J.; Martins, L.C.; et al. Arbovirus eco-epidemiology in the area affected by the Cuiaba-Santarem Highway (BR-163), Pará State, Brazil. Rev. Da Soc. Bras. De Med. Trop. 2009, 25, 2583–2602. [Google Scholar] [CrossRef]
- Mourão, M.P.G.; Bastos, M.S.; Gimaque, J.B.L.; Mota, B.R.; Souza, G.S.; Grimmer, G.H.N.; Galusso, E.S.; Arruda, E.; Figueiredo, L.T.M. Oropouche Fever Outbreak, Manaus, Brazil, 2007–2008. Emerg. Infect. Dis. 2009, 15, 2063–2064. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M. Emergent arboviruses in Brazil. Rev. Da Soc. Bras. De Med. Trop. 2007, 40, 224–229. [Google Scholar] [CrossRef]
- Mercer, D.R.; Castillo-Pizango, M.J. Changes in Relative Species Compositions of Biting Midges (Diptera: Ceratopogonidae) and an Outbreak of Oropouche Virus in Iquitos, Peru. J. Med. Èntomol. 2005, 42, 554–558. [Google Scholar] [CrossRef]
- Mercer, D.R.; Spinelli, G.R.; Watts, D.M.; Tesh, R.B. Biting Rates and Developmental Substrates for Biting Midges (Diptera: Ceratopogonidae) in Iquitos, Peru. J. Med. Entomol. 2003, 40, 807–812. [Google Scholar] [CrossRef]
- Weidmann, M.; Rudaz, V.; Nunes, M.R.T.; Vasconcelos, P.F.C.; Hufert, F.T. Rapid Detection of Human Pathogenic Orthobunyaviruses. J. Clin. Microbiol. 2003, 41, 3299–3305. [Google Scholar] [CrossRef]
- Schneider, M.C.; Najera, P.; Pereira, M.M.; Machado, G.; Dos Anjos, C.B.; Rodrigues, R.O.; Cavagni, G.M.; Munoz-Zanzi, C.; Corbellini, L.G.; Leone, M.; et al. Leptospirosis in Rio Grande do Sul, Brazil: An Ecosystem Approach in the Animal-Human Interface. PLOS Neglected Trop. Dis. 2015, 9, e0004095. [Google Scholar] [CrossRef]
- Dias, C.V. The Belém-Brasília Highway. Rev. Geográfica 1966, 65, 195–198. [Google Scholar]
- Academic. Rodovia Belém-Brasília. Available online: https://en-academic.com/dic.nsf/enwiki/1601565/ (accessed on 26 April 2022).
- Schneider, M.C.; Romijn, P.C.; Uieda, W.; Tamayo, H.; da Silva, D.F.; Belotto, A.; da Silva, J.B.; Leanes, L.F. Rabies transmitted by vampire bats to humans: An emerging zoonotic disease in Latin America? Rev. Panam. Salud. Publica 2009, 25, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Relief Web Brazil: Floods—2021 December. Available online: https://reliefweb.int/disaster/fl-2021-000204-bra (accessed on 26 April 2022).
- Yavarian, J.; Shafei-Jandaghi, N.Z.; Mokhtari-Azad, T. Possible viral infections in flood disasters: A review considering 2019 spring floods in Iran. Iran. J. Microbiol. 2019, 11, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of Temperate and Tropical Culicoides Midges: Knowledge Gaps and Consequences for Transmission ofCulicoides-Borne Viruses. Annu. Rev. Èntomol. 2015, 60, 373–392. [Google Scholar] [CrossRef]
- Garcia, G.A.; David, M.R.; Martins, A.J.; Maciel-De-Freitas, R.; Linss, J.G.B.; Araújo, S.C.; Lima, J.B.P.; Valle, D. The impact of insecticide applications on the dynamics of resistance: The case of four Aedes aegypti populations from different Brazilian regions. PLOS Negl. Trop. Dis. 2018, 12, e0006227. [Google Scholar] [CrossRef]
- The World Bank. Urban Population (% of Total Population)—Brazil. Available online: https://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS?locations=BR (accessed on 26 April 2022).
- Benevenuto, R.G.; Azevedo, I.C.C.; Caulfield, B. Assessing the spatial burden in health care accessibility of low-income families in rural Northeast Brazil. J. Transp. Health 2019, 14, 100595. [Google Scholar] [CrossRef]
- Mendonca, S.F.; Rocha, M.N.; Ferreira, F.V.; Leite, T.H.J.F.; Amadou, S.C.G.; Sucupira, P.H.F.; Marques, J.T.; Ferreira, A.G.A.; Moreira, L.A. Evaluation of Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus Mosquitoes Competence to Oropouche virus Infection. Viruses 2021, 13, 755. [Google Scholar] [CrossRef]


| Location * | Year | Case Count |
|---|---|---|
| Belem, Pará | 1961 | 11,000 |
| Braganca, Pará | 1967 | 6000 |
| Santarem, Pará | 1975 | 14,000 |
| Belem, Pará | 1979–1980 | >100,000 |
| Manaus, Amazonas | 1980–181 | 97,000 |
| Ariquemes, Rondonia | 1991 | 94,000 |
| Magalhaes Barata, Pará | 2006 | 17,000 |
| First Subnational Level (State) | Humans | NHPs | Midge and Mosquitoes | Sloths | Not Identified | Major Biomes | GDP per Capita |
|---|---|---|---|---|---|---|---|
| Acre | X | X | TSMBF | 1,772,241 | |||
| Amapa | X | X | TSMBF | 2,068,821 | |||
| Amazonas | X | X | TSMBF | 2,610,172 | |||
| Para | X | X | X | X | TSMBF | 2,073,460 | |
| Rondonia | X | X | TSMBF | 2,649,712 | |||
| Tocantins | X | X | TSGSS | 2,502,180 | |||
| North region | X | X | X | X | |||
| Bahia | X | X | DXS, TSGSS, & TSMBF | 1,971,621 | |||
| Maranhao | X | X | X | TSMBF & TSGSS | 1,375,794 | ||
| Northeast region | X | X | X | ||||
| Minas Gerais | X | TSGSS & TSMBF | 3,079,404 | ||||
| Sao Paulo | X | TSGSS & TSMBF | 5,114,082 | ||||
| Southeast region | X | X | |||||
| Rio Grande do Sul | X | TSGSS | 4,240,609 | ||||
| South region | X | ||||||
| Goias | X | X | TSGSS | 2,973,240 | |||
| Mato Grosso | X | X | X | TSMBF & TSGSS | 4,078,732 | ||
| Mato Grosso do Sul | X | TSGSS | 3,848,283 | ||||
| Central–west region | X | X | X | X | |||
| Brazil | X | X | X | X | X | 3,516,170 |
| Location (State) | Population | Total Number of Municipalities in the State | Number of Municipalities with Evidence of Presence | Percentage of Municipalities with Evidence of Presence |
|---|---|---|---|---|
| Acre | 906,876 | 22 | 2 | 9.09 |
| Amapa | 877,613 | 16 | 1 | 6.25 |
| Amazonas | 4,269,995 | 62 | 6 | 9.68 |
| Pará | 8,777,124 | 144 | 39 | 27.08 |
| Rondonia | 1,815,278 | 52 | 3 | 5.77 |
| Tocantins | 1,607,363 | 139 | 2 | 1.43 |
| North region | 18,254,249 | 435 | 53 | 12.18 |
| Bahia | 14,985,284 | 417 | 2 | 0.48 |
| Maranhao | 7,153,262 | 217 | 3 | 1.38 |
| Northeast region | 22,138,546 | 634 | 5 | 0.79 |
| Minas Gerais | 21,411,923 | 853 | 2 | 0.23 |
| Southeast region | 21,411,923 | 853 | 2 | 0.23 |
| Goias | 7,206,589 | 246 | 1 | 0.41 |
| Mato Grosso | 3,567,234 | 141 | 3 | 2.13 |
| Mato Grosso do Sul | 2,839,188 | 77 | 3 | 3.90 |
| Central–west region | 13,613,011 | 464 | 7 | 1.51 |
| Brazil | 213,317,639 | 5,570 | 67 | 1.20 |
| Human | Animal | Environment |
|---|---|---|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciancalepore, S.; Schneider, M.C.; Kim, J.; Galan, D.I.; Riviere-Cinnamond, A. Presence and Multi-Species Spatial Distribution of Oropouche Virus in Brazil within the One Health Framework. Trop. Med. Infect. Dis. 2022, 7, 111. https://doi.org/10.3390/tropicalmed7060111
Sciancalepore S, Schneider MC, Kim J, Galan DI, Riviere-Cinnamond A. Presence and Multi-Species Spatial Distribution of Oropouche Virus in Brazil within the One Health Framework. Tropical Medicine and Infectious Disease. 2022; 7(6):111. https://doi.org/10.3390/tropicalmed7060111
Chicago/Turabian StyleSciancalepore, Sofia, Maria Cristina Schneider, Jisoo Kim, Deise I. Galan, and Ana Riviere-Cinnamond. 2022. "Presence and Multi-Species Spatial Distribution of Oropouche Virus in Brazil within the One Health Framework" Tropical Medicine and Infectious Disease 7, no. 6: 111. https://doi.org/10.3390/tropicalmed7060111
APA StyleSciancalepore, S., Schneider, M. C., Kim, J., Galan, D. I., & Riviere-Cinnamond, A. (2022). Presence and Multi-Species Spatial Distribution of Oropouche Virus in Brazil within the One Health Framework. Tropical Medicine and Infectious Disease, 7(6), 111. https://doi.org/10.3390/tropicalmed7060111






