The Natural Alkaloid Tryptanthrin Induces Apoptosis-like Death in Leishmania spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Macrophage Viability Assay
2.3. Anti-Promastigote Assay
2.4. Mitochondrial Membrane Potential (ΔΨm)
2.5. Assay for Autophagy
2.6. Determination of Ergosterol Content in Promastigotes
2.7. Annexin V-FITC/PI Dual Staining
2.8. Macrophage Infection and Anti-Amastigote Assay
2.9. Selectivity Index
2.10. Prediction of ADMET by Computational Analysis
2.11. Statistical Analysis
3. Results
3.1. Tryptanthrin Cytotoxicity and Selectivity
3.2. Antileishmanial Activity
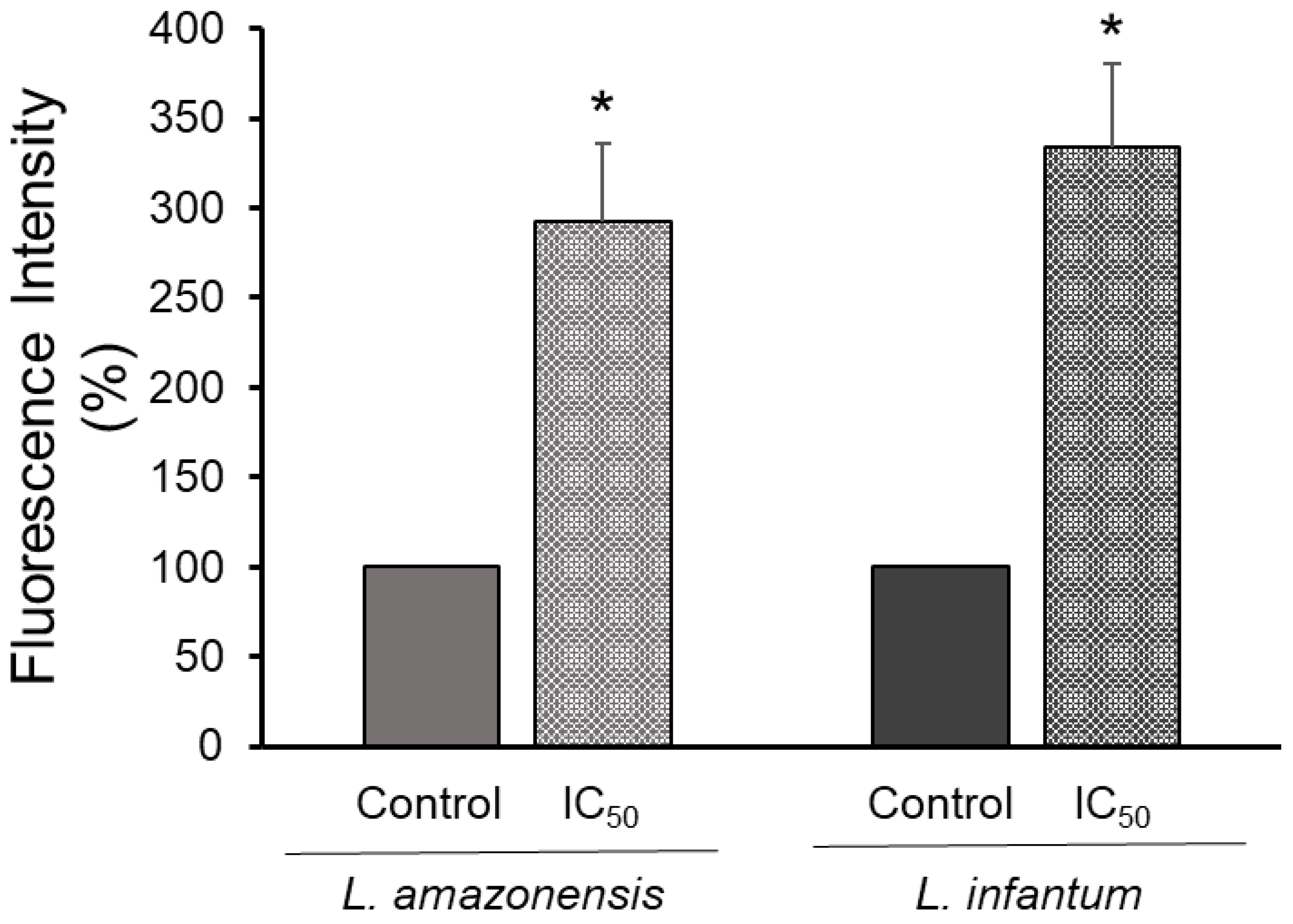
3.3. Inhibition of Ergosterol Biosynthesis
3.4. Mitochondrion Dysfunction
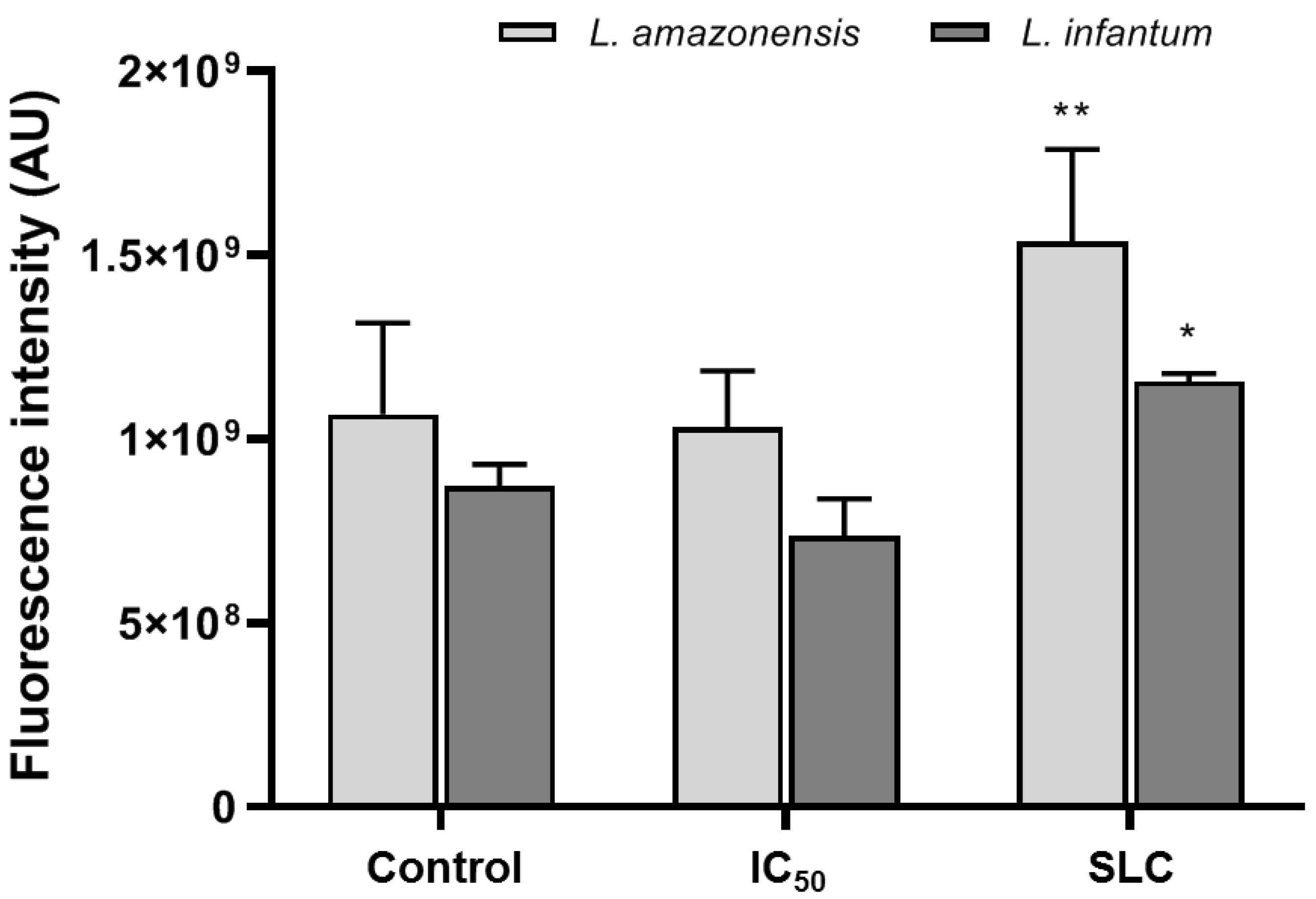
3.5. Autophagy Activity
3.6. Leishmania Apoptosis-like Death
3.7. ADMET Features of Tryptanthrin
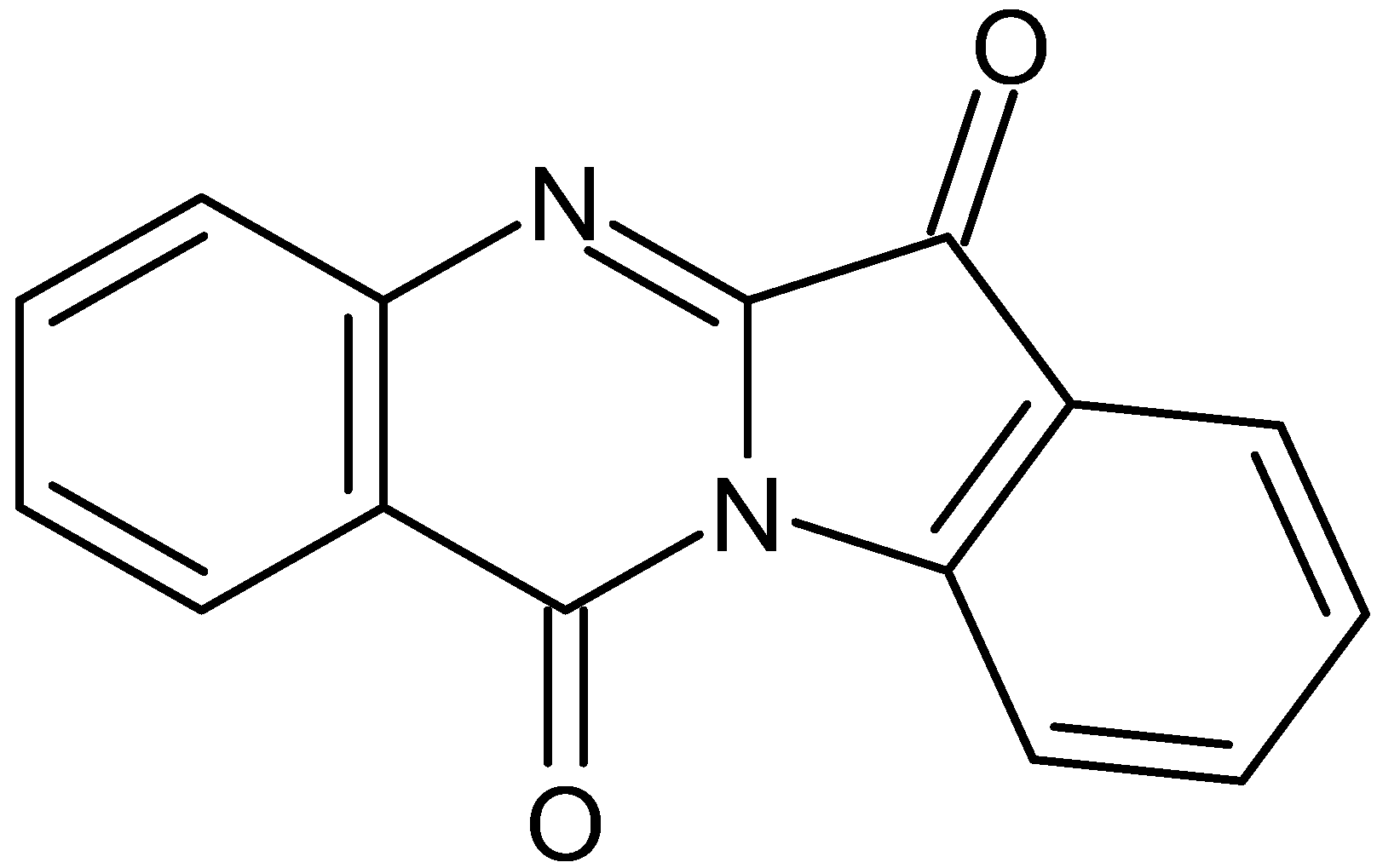
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bates, P.A. Revising Leishmania’s life cycle. Nat. Microbiol. 2018, 3, 529–530. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organization. Leishmaniasis—Status of Endemicity of Cutaneous Leishmaniasis. 2020. Available online: https://apps.who.int/neglected_diseases/ntddata/leishmaniasis/leishmaniasis.html (accessed on 20 January 2022).
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against Leishmania under current research. Expert Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e0006052. [Google Scholar] [CrossRef]
- Jao, C.W.; Lin, W.C.; Wu, Y.T.; Wu, P.L. Isolation, structure elucidation, and synthesis of cytotoxic tryptanthrin analogues from Phaius mishmensis. J. Nat. Prod. 2008, 71, 1275–1279. [Google Scholar] [CrossRef]
- Costa, D.C.M.; Azevedo, M.M.B.; Silva, D.O.E.; Romanos, M.T.V.; Souto-Padrón, T.C.B.S.; Alviano, C.S.; Alviano, D.S. In vitro anti-MRSA activity of Couroupita guianensis extract and its component tryptanthrin. Nat. Prod. Res. 2017, 31, 2077–2080. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.C.; Baek, H.Y.; Lee, K.D.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effects of tryptanthrin from Polygonum tinctorium Lour. in lipopolysaccharide-stimulated BV2 microglial cells. Arch. Pharmacal Res. 2018, 41, 419–430. [Google Scholar] [CrossRef]
- Garcellano, R.C.; Moinuddin, S.G.A.; Young, R.P.; Zhou, M.; Bowden, M.E.; Renslow, R.S.; Yesiltepe, Y.; Thomas, D.G.; Colby, S.M.; Chouinard, C.D.; et al. Isolation of tryptanthrin and reassessment of evidence for its isobaric isostere wrightiadione in plants of the Wrightia genus. J. Nat. Prod. 2018, 82, 440–448. [Google Scholar] [CrossRef]
- Jahng, Y. Progress in the studies on tryptanthrin, an alkaloid of history. Arch. Pharmacal Res. 2013, 36, 517–535. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lee, C.L.; Yen, H.R.; Chang, Y.S.; Lin, Y.P.; Huang, S.H.; Lin, C.W. Antiviral action of tryptanthrin isolated from Strobilanthes cusia leaf against human coronavirus NL63. Biomolecules 2020, 10, 366. [Google Scholar] [CrossRef]
- Narendrakumar, L.; Theresa, M.; Krishnankutty Chandrika, S.; Thomas, S. Tryptanthrin, a potential biofilm inhibitor against toxigenic Vibrio cholerae, modulating the global quorum sensing regulator, LuxO. Biofouling 2019, 35, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Hesse-Macabata, J.; Morgner, B.; Elsner, P.; Hipler, U.C.; Wiegand, C. Tryptanthrin promotes keratinocyte and fibroblast responses in vitro after infection with Trichophyton benhamiae DSM6916. Sci. Rep. 2020, 10, 1863. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.A.; Terryn, R.J., 3rd; Stewart, E.L.; Baum, J.C.; Novak, M.J. New insight into the action of tryptanthrins against Plasmodium falciparum: Pharmacophore identification via a novel submolecular QSAR descriptor. J. Mol. Graph. Model. 2018, 80, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.K.; Skanchy, D.J.; Jennings, B.; Hudson, T.H.; Brendle, J.J.; Werbovetz, K.A. Analysis of stereoelectronic properties, mechanism of action and pharmacophore of synthetic indolo[2,1-b]quinazoline-6,12-dione derivatives in relation to antileishmanial activity using quantum chemical, cyclic voltammetry and 3-D-QSAR CATALYST procedures. Bioorg. Med. Chem. 2002, 10, 1979–1989. [Google Scholar] [CrossRef]
- Garcia, A.R.; Oliveira, D.M.P.; Jesus, J.B.; Souza, A.M.T.; Sodero, A.C.R.; Vermelho, A.B.; Leal, I.C.R.; Souza, R.O.M.A.; Miranda, L.S.M.; Pinheiro, A.S.; et al. Identification of chalcone derivatives as inhibitors of Leishmania infantum arginase and promising antileishmanial agents. Front. Chem. 2021, 8, 624678. [Google Scholar] [CrossRef]
- Rodrigues, I.A.; da Silva, B.A.; dos Santos, A.L.; Vermelho, A.B.; Alviano, C.S.; Dutra, P.M.; Rosa, M.S. A new experimental culture medium for cultivation of Leishmania amazonensis: Its efficacy for the continuous in vitro growth and differentiation of infective promastigote forms. Parasitol. Res. 2010, 106, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Proulx, M.E.; Désormeaux, A.; Marquis, J.F.; Olivier, M.; Bergeron, M.G. Treatment of visceral leishmaniasis with sterically stabilized liposomes containing camptothecin. Antimicrob. Agents Chemother. 2001, 45, 2623–2627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Machado, P.A.; Gomes, P.S.; Midlej, V.; Coimbra, E.S.; de Matos Guedes, H.L. PF-429242, a subtilisin inhibitor, is effective in vitro against Leishmania infantum. Front. Microbiol. 2021, 12, 583834. [Google Scholar] [CrossRef]
- Brasil, P.F.; de Freitas, J.A.; Barreto, A.L.S.; Adade, C.M.; Reis de Sá, L.F.; Constantino-Teles, P.; Toledo, F.T.; de Sousa, B.A.; Gonçalves, A.C.; Romanos, M.T.V.; et al. Antiproliferative and ultrastructural effects of phenethylamine derivatives on promastigotes and amastigotes of Leishmania (Leishmania) infantum chagasi. Parasitol. Int. 2017, 66, 47–55. [Google Scholar] [CrossRef]
- Niemann, A.; Baltes, J.; Elsasser, H.P. Fluorescence properties and staining behavior of monodansylpentane, a structural homologue of the lysosomotropic agent monodansylcadaverine. J. Histochem. Cytochem. 2001, 49, 177–185. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.S.; Amaral, A.C.F.; Lima, I.C.; Cruz, J.D.; Garcia, A.R.; Souza, H.A.S.; Adade, C.M.; Vermelho, A.B.; Alviano, C.S.; Alviano, D.S.; et al. β-Carboline-1-propionic acid alkaloid: Antileishmanial and cytotoxic effects. Rev. Bras. Farm. 2019, 29, 755–762. [Google Scholar] [CrossRef]
- Basmaciyan, L.; Casanova, M. Cell death in Leishmania. Parasite 2019, 26, 71. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.R.; Oliveira, D.M.P.; Amaral, A.C.F.; Jesus, J.B.; Rennó Sodero, A.C.; Souza, A.M.T.; Supuran, C.T.; Vermelho, A.B.; Rodrigues, I.A.; Pinheiro, A.S. Leishmania infantum arginase: Biochemical characterization and inhibition by naturally occurring phenolic substances. J. Enzym. Inhib. Med. Chem. 2019, 34, 1100–1109. [Google Scholar] [CrossRef]
- Passero, L.F.; Bonfim-Melo, A.; Corbett, C.E.; Laurenti, M.D.; Toyama, M.H.; de Toyama, D.O.; Romoff, P.; Fávero, O.A.; dos Grecco, S.S.; Zalewsky, C.A.; et al. Anti-leishmanial effects of purified compounds from aerial parts of Baccharis uncinella C. DC. (Asteraceae). Parasitol. Res. 2011, 108, 529–536. [Google Scholar] [CrossRef]
- Pereira, A.H.C.; Marcolino, L.M.C.; Pinto, J.G.; Ferreira-Strixino, J. Evaluation of the photodynamic therapy with curcumin on L. braziliensis and L. major Amastigotes. Antibiotics 2021, 10, 634. [Google Scholar] [CrossRef]
- Ashok, P.; Lathiya, H.; Murugesan, S. Manzamine alkaloids as antileishmanial agents: A review. Eur. J. Med. Chem. 2015, 97, 928–936. [Google Scholar] [CrossRef]
- Mishra, B.B.; Singh, R.K.; Srivastava, A.; Tripathi, V.J.; Tiwari, V.K. Fighting against leishmaniasis: Search of alkaloids as future true potential anti-leishmanial agents. Mini-Rev. Med. Chem. 2009, 9, 107–123. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Yang, G.Z.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Zhang, J.Y.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef]
- Onambele, L.A.; Riepl, H.; Fischer, R.; Pradel, G.; Prokop, A.; Aminake, M.N. Synthesis and evaluation of the antiplasmodial activity of tryptanthrin derivatives. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Scovill, J.; Blank, E.; Konnick, M.; Nenortas, E.; Shapiro, T. Antitrypanosomal activities of tryptanthrins. Antimicrob. Agents Chemother. 2002, 46, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Kohno, K.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Tryptanthrin inhibits nitric oxide and prostaglandin E2 synthesis by murine macrophages. Eur. J. Pharmacol. 2000, 27, 197–204. [Google Scholar] [CrossRef]
- Xu, Y.; Quan, H.; Wang, Y.; Zhong, H.; Sun, J.; Xu, J.; Jia, N.; Jiang, Y. Requirement for ergosterol in berberine tolerance underlies synergism of fluconazole and berberine against fluconazole-resistant Candida albicans isolates. Front. Cell. Infect. Microbiol. 2017, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Shi, G.; Wang, T.; Wu, D.; Wang, C. Antiproliferation of berberine in combination with fluconazole from the perspectives of reactive oxygen species, ergosterol and drug efflux in a fluconazole-resistant Candida tropicalis isolate. Front. Microbiol. 2016, 7, 1516. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Jin, L.; Yang, C.; Zhu, Y.; Ye, X.; Li, X.; Zhang, B. Antifungal activity and potential mechanism of magnoflorine against Trichophyton rubrum. J. Antibiot. 2021, 74, 206–214. [Google Scholar] [CrossRef]
- Medina, J.M.; Rodrigues, J.C.; De Souza, W.; Atella, G.C.; Barrabin, H. Tomatidine promotes the inhibition of 24-alkylated sterol biosynthesis and mitochondrial dysfunction in Leishmania amazonensis promastigotes. Parasitology 2012, 139, 1253–1265. [Google Scholar] [CrossRef]
- Mukherjee, S.; Moitra, S.; Xu, W.; Hernandez, V.; Zhang, K. Sterol 14-α-demethylase is vital for mitochondrial functions and stress tolerance in Leishmania major. PLoS Pathog. 2020, 16, e1008810. [Google Scholar] [CrossRef]
- De Sarkar, S.; Sarkar, D.; Sarkar, A.; Dighal, A.; Staniek, K.; Gille, L.; Chatterjee, M. Berberine chloride mediates its antileishmanial activity by inhibiting Leishmania mitochondria. Parasitol. Res. 2019, 118, 335–345. [Google Scholar] [CrossRef]
- Sen, N.; Das, B.B.; Ganguly, A.; Mukherjee, T.; Tripathi, G.; Bandyopadhyay, S.; Rakshit, S.; Sem, T.; Majumder, H.K. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ. 2004, 11, 924–936. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Hu, F.; Li, Y.; Yang, Y.; Yan, J.; Kuang, C.; Yang, Q. Discovery of tryptanthrin derivatives as potent inhibitors of indoleamine 2, 3-dioxygenase with therapeutic activity in Lewis lung cancer (LLC) tumor-bearing mice. J. Med. Chem. 2013, 56, 8321–8331. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Shi, X.; Zhang, H.; Wang, S.; Sun, J.; Hua, W.; Miao, Q.; Zhao, Y.; Zhang, C. Proliferation-attenuating and apoptosis-inducing effects of tryptanthrin on human chronic myeloid leukemia k562 cell line in vitro. Int. J. Mol. Sci. 2011, 12, 3831–3845. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.T.; Chen, T.M.; Tseng, S.Y.; Chen, Y.H. Tryptanthrin inhibits MDR1 and reverses doxorubicin resistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2007, 358, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.M.; Ciccarelli, A.B.; Bollini, M.; Bruno, A.M.; Batlle, A.; Lombardo, M.E. Trypanocidal activity of thioamide-substituted imidazoquinolinone: Electrochemical properties and biological effects. Evid. Based Complement. Altern. Med. 2013, 2013, 945953. [Google Scholar] [CrossRef]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; Hooft van Huijsduijnen, R.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef]
- Ferreira, L.L.; Andricopulo, A.D. ADMET modeling approaches in drug discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef]
- Rahimi-Moghaddam, P.; Ebrahimi, S.A.; Ourmazdi, H.; Selseleh, M.; Karjalian, M.; Haj-Hassani, G.; Alimohammadian, M.H.; Mahmoudian, M.; Shafiei, M. In vitro and in vivo activities of Peganum harmala extract against Leishmania major. J. Res. Med. Sci. 2011, 16, 1032–1039. [Google Scholar]
- Di Giorgio, C.; Delmas, F.; Ollivier, E.; Elias, R.; Balansard, G.; Timon-David, P. In vitro activity of the beta-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp. Parasitol. 2004, 106, 67–74. [Google Scholar] [CrossRef]
- Linani, A.; Benarous, K.; Bou-Salah, L.; Yousfi, M. Hispidin, Harmaline, and Harmine as potent inhibitors of bovine xanthine oxidase: Gout treatment, in vitro, ADMET prediction, and SAR studies. Bioorg. Chem. 2021, 112, 104937. [Google Scholar] [CrossRef]

| Drugs | MØ | L. amazonensis | L. infantum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CC50 (µM) | MLCPro (µM) | SLCPro (µM) | IC50Pro (µM) | IC50Ama (µM) | SI | MLCPro (µM) | SLCPro (µM) | IC50Pro (µM) | IC50Ama (µM) | SI | |
| Tryp | 465 ± 31.05 | 126 | 63 | 11 ± 1.06 | 75 ± 11.63 | 6.2 | 126 | 63 | 8.0 ± 2.60 | 115 ± 2.79 | 4.0 |
| Amph B | 5.0 ± 0.89 | n.d. | n.d. | 0.68 ± 0.03 | 0.37 ± 0.14 | 13.5 | n.d. | n.d. | 0.86 ± 0.36 | 1.11 ± 0.21 | 4.5 |
| Drugs | L. amazonensis | L. infantum | ||||||
|---|---|---|---|---|---|---|---|---|
| PI | AV | PI/AV | Total Apoptosis (AV + PI/AV) | PI | AV | PI/AV | Total Apoptosis (AV + PI/AV) | |
| Tryp | 0.79 ± 0.07 | 24.73 ± 5.94 | 1.35 ± 0.51 | 26.08 | 0.06 ± 0.02 | 22.90 ± 0.42 | 2.80 ± 0.20 | 25.70 |
| Amph B | 0.09 ± 0.04 | 15.98 ± 2.81 | 0.88 ± 0.02 | 16.86 | 0.09 ± 0.03 | 17.06 ± 7.53 | 4.81 ± 1.11 | 21.87 |
| Control | 0.23 ± 0.04 | 0.25 ± 0.13 | 2.5 ± 0.31 | 2.98 | 0.11 ± 0.02 | 1.42 ± 025 | 0.64 ± 0.32 | 2.06 |
| Drug | ADME | Druglikeness | Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SP a (log Kp, cm/h) | HIA b (%) | PPB c (%) | MDCK d (nm/s) | CYP e Inhibitor | CYP f Substrate | BBB g [Brain]/[Blood] | Rule of 5 h | Ames Test i | CarR j | CarM j | hERG k | |
| Tryp | −3.8 | 97.4 | 85.8 | 166.2 | non | CYP3A4 (weakly) | 1.94 | Suitable | Mutagen | Positive | Positive | Medium risk |
| MTF | −0.80 | 98.1 | 86.2 | 43.4 | CYP2D6 | CYP3A4 | 0.13 | Suitable | Mutagen | Positive | Negative | Low risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.R.; Silva-Luiz, Y.P.G.; Alviano, C.S.; Alviano, D.S.; Vermelho, A.B.; Rodrigues, I.A. The Natural Alkaloid Tryptanthrin Induces Apoptosis-like Death in Leishmania spp. Trop. Med. Infect. Dis. 2022, 7, 112. https://doi.org/10.3390/tropicalmed7060112
Garcia AR, Silva-Luiz YPG, Alviano CS, Alviano DS, Vermelho AB, Rodrigues IA. The Natural Alkaloid Tryptanthrin Induces Apoptosis-like Death in Leishmania spp. Tropical Medicine and Infectious Disease. 2022; 7(6):112. https://doi.org/10.3390/tropicalmed7060112
Chicago/Turabian StyleGarcia, Andreza R., Yasmin P. G. Silva-Luiz, Celuta S. Alviano, Daniela S. Alviano, Alane B. Vermelho, and Igor A. Rodrigues. 2022. "The Natural Alkaloid Tryptanthrin Induces Apoptosis-like Death in Leishmania spp." Tropical Medicine and Infectious Disease 7, no. 6: 112. https://doi.org/10.3390/tropicalmed7060112
APA StyleGarcia, A. R., Silva-Luiz, Y. P. G., Alviano, C. S., Alviano, D. S., Vermelho, A. B., & Rodrigues, I. A. (2022). The Natural Alkaloid Tryptanthrin Induces Apoptosis-like Death in Leishmania spp. Tropical Medicine and Infectious Disease, 7(6), 112. https://doi.org/10.3390/tropicalmed7060112







