Trypanosoma cruzi Parasite Burdens of Several Triatomine Species in Colombia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information and T. cruzi Detection
2.2. T. cruzi Quantification and qPCR Standardization
2.3. Standard Calibration, Limit of Detection (LOD), and Parasite Burdens
2.4. Parasite Genotyping
2.5. Statistical Analysis
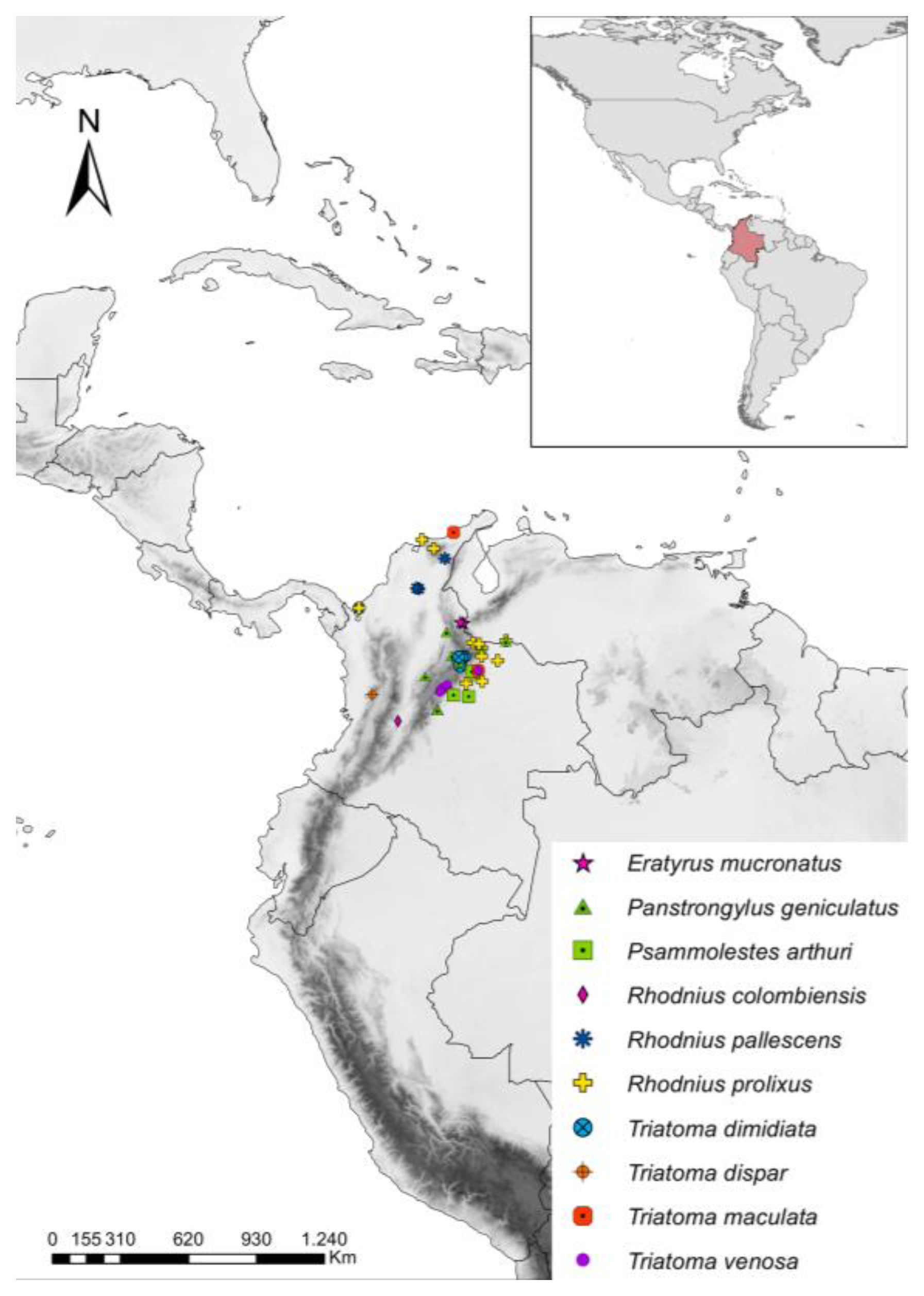
3. Results
3.1. Standard Curve and LOD
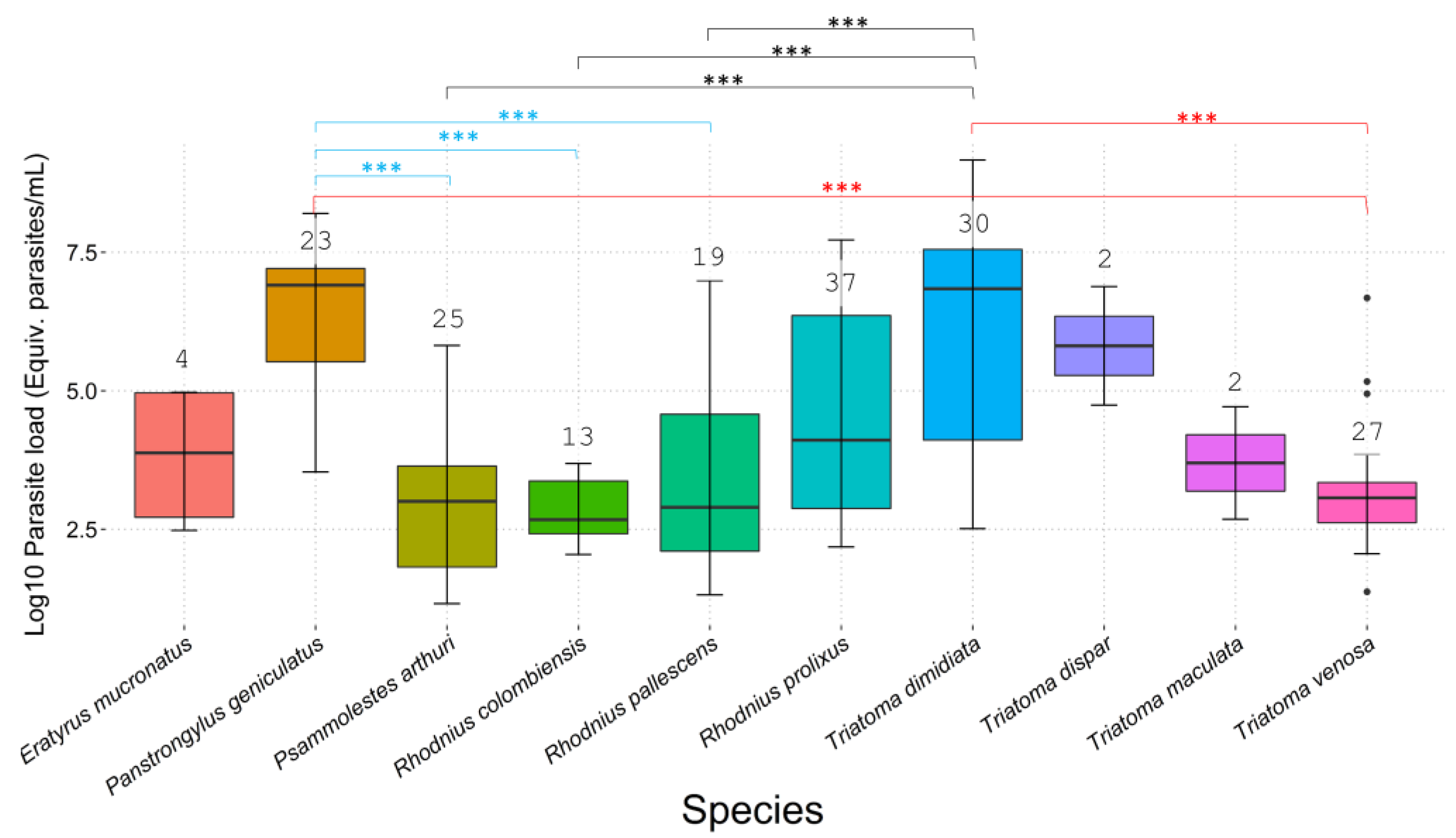
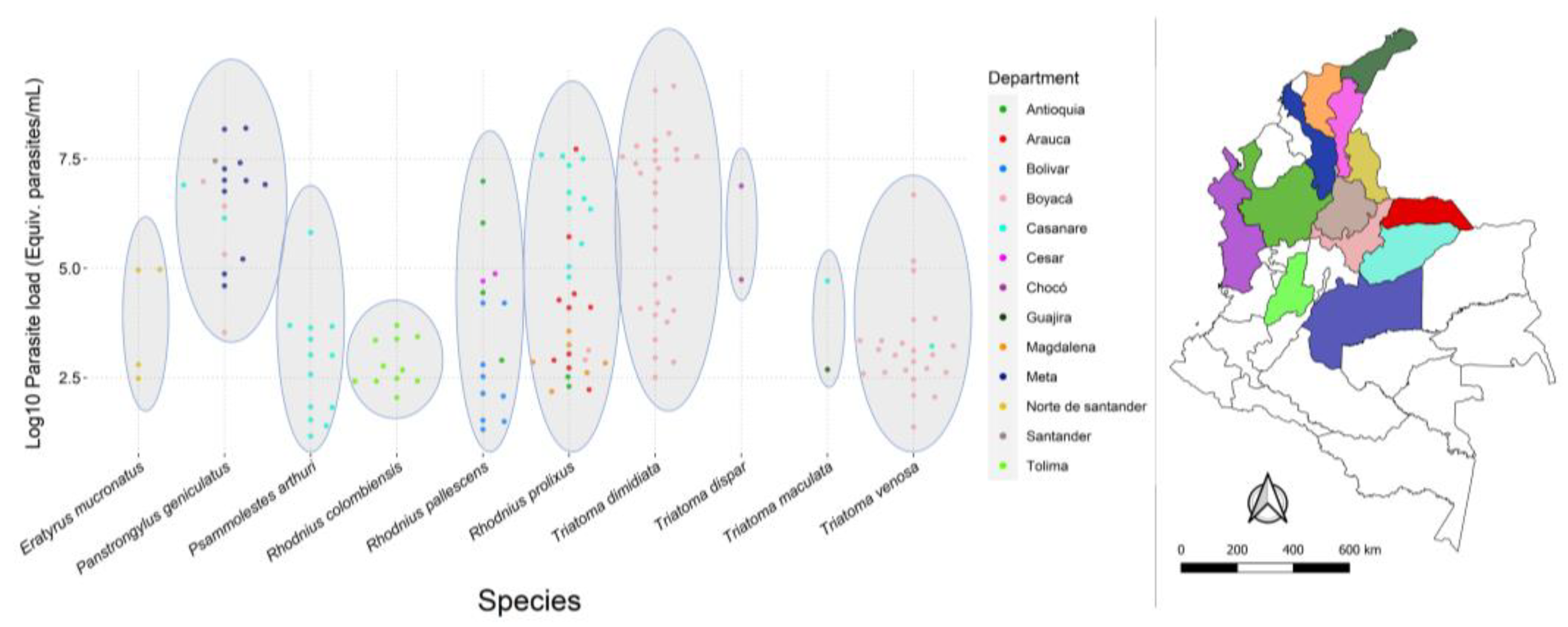
3.2. T. cruzi Infection, Parasite Loads, and DTUs
3.3. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandes de Paiva, V.; Belintani, T.; de Oliveira, J.; Galvão, C.; da Rosa, J.A. A review of the taxonomy and biology of Triatominae subspecies (Hemiptera: Reduviidae). Parasitol. Res. 2022, 121, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Chagas, C. Nova tripanozomiaze humana: Estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Memórias Inst. Oswaldo Cruz 1909, 1, 159–218. [Google Scholar] [CrossRef]
- Ramírez, J.D.; Hernández, C. Trypanosoma cruzi I: Towards the need of genetic subdivision? Part II. Acta Trop. 2018, 184, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Pereiro, A.C. Guidelines for the diagnosis and treatment of Chagas disease. Lancet 2019, 393, 1486–1487. [Google Scholar] [CrossRef] [PubMed]
- Rojas de Arias, R.; Monroy, C.; Guhl, F.; Sosa-Estani, S.; Santos, W.S.; Abad-Franch, F. Chagas disease control-surveillance in the americas: The multinational initiatives and the practical impossibility of interrupting vector-borne Trypanosoma cruzi transmission. Mem. Inst. Oswaldo Cruz 2021, 116, 130. [Google Scholar] [CrossRef] [PubMed]
- Gorla, D.; Noireau, F. Geographic distribution of Triatominae vectors in America. In American Trypanosomiasis Chagas Disease, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Guhl, F.; Aguilera, G.; Pinto, N.; Vergara, D. Actualización de la distribución geográfica y ecoepidemiología de la fauna de triatominos (Reduviidae: Triatominae) en Colombia. Biomédica 2007, 27, 143. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Hernández, C.; Herrera, G.; Cruz-Saavedra, L.; Higuera, A.; Arias-Giraldo, L.M.; Urbano, P.; Urbano, P.; Cuervo, A.; Teherán, A.; et al. Trypanosoma cruzi infection, discrete typing units and feeding sources among Psammolestes arthuri (Reduviidae: Triatominae) collected in eastern Colombia. Parasites Vectors 2019, 12, 3422. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Hernandez, C.; Cantillo-Barraza, O.; Muñoz, M.; Medina, M.; Medina-Alfonso, M.; Suescu, S.; Vega, L.; Castañeda, S.; Cruz-Saavedra, L.; et al. Estimating the genetic structure of Triatoma dimidiata (Hemiptera: Reduviidae) and the transmission dynamics of Trypanosoma cruzi, eastern Colombia in Boyaca. PLoS Negl. Trop. Dis. 2022, 16, e0010534. [Google Scholar] [CrossRef]
- Hernández, C.; Salazar, C.; Brochero, H.; Teherán, A.; Buitrago, L.S.; Vera, M.; Soto, H.; Florez-Rivadeneira, Z.; Ardila, S.; Parra-Henao, G.; et al. Untangling the transmission dynamics of primary and secondary vectors of Trypanosoma cruzi in Colombia: Parasite infection, feeding sources and discrete typing units. Parasites Vectors 2016, 9, 1907. [Google Scholar] [CrossRef]
- Medina, M.; Zuluaga, S.; Martínez, M.F.; Bermúdez, J.C.; Hernández, C.; Beltrán, V.; Velásquez-Ortiz, N.; Muñoz, M.; Ramírez, J.D.; Triana, O.; et al. Interrogating the transmission dynamics of Trypanosoma cruzi (Trypanosomatida, Trypanosomatidae) by Triatoma venosa (Hemiptera: Reduviidae) after the elimination of vector transmission by Rhodnius prolixus in Boyacá eastern Colombia. Front. Cell. Infect. Microbiol. 2022, 12, 99820. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Ramírez, J.D. Understanding the oral transmission of Trypanosoma cruzi as a veterinary and medical foodborne zoonosis. Res. Vet. Sci. 2020, 132, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Cantillo-Barraza, O.; Medina, M.; Zuluaga, S.; Blanco, M.I.; Caro, R.; Jaimes-Dueñez, J.; Beltrán, V.; Xavier, S.C.; Triana-Chavez, O. Distribution and natural infection status of synantrophic triatomines (Hemiptera: Reduviidae), vectors of Trypanosoma cruzi, reveals new epidemiological scenarios for chagas disease in the highlands of Colombia. PLoS Negl. Trop. Dis. 2021, 15, e0009574. [Google Scholar] [CrossRef] [PubMed]
- Blohm, L.; de Sousa, J.; Roschman-González, A.; Ferrer, E.; Morocoima, A.; Herrera, L. Domiciliation and sympatry of Triatoma maculata and Rhodnius prolixus, risk of Trypanosoma cruzi transmission in villages of Anzoátegui, Venezuela. J. Parasit. Dis. 2022, 46, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, M.; Bacigalupo, A.; Victoria, M.; Vergara, M.J. Acta Tropica Trypanosoma cruzi infection in the wild Chagas disease vector, Mepraia spinolai: Parasitic load, discrete typing units, and blood meal sources. Acta Trop. 2022, 229, 6356. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, N.; Lobbia, P.A.; Mougabure-Cueto, G. Effects of the infection with Trypanosoma cruzi on the feeding and excretion/defecation patterns of Triatoma infestans. Bull. Entomol. Res. 2020, 110, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Botto-Mahan, C.; Cattan, P.E.; Medel, R. Chagas disease parasite induces behavioural changes in the kissing bug Mepraia spinolai. Acta Trop. 2006, 98, 219–223. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, A.; Mandel, S. Natural infections and behavior of Trypanosoma rangeli and Trypanosoma cruzi in the vector Rhodnius prolixus in Colombia. J. Parasitol. 1969, 55, 846–852. [Google Scholar] [CrossRef]
- Fellet, M.R.; Lorenzo, M.G.; Elliot, S.L.; Carrasco, D.; Guarneri, A.A. Effects of infection by Trypanosoma cruzi and Trypanosoma rangeli on the reproductive performance of the vector Rhodnius prolixus. PLoS ONE 2014, 9, e105255. [Google Scholar] [CrossRef]
- Marliére, N.; Latorre-Estivalis, J.; Lorenzo, M.; Carrasco, D.; Alves-Silva, J.; Rodrigues, J.; Ferreira, L.D.L.; Lara, L.D.M.; Lowenberger, C.; Guarneri, A.A. Trypanosomes modify the behavior of their insect hosts: Effects on locomotion and on the expression of a related gene. PLoS Negl. Trop. Dis. 2015, 9, e0003973. [Google Scholar] [CrossRef]
- Pérez, G.; Muñoz-San Martín, C.; Chacón, F.; Bacigalupo, A.; Cattan, P.E.; Solís, R. Modification of the daily activity pattern of the diurnal triatomine Mepraia spinolai (Hemiptera: Reduviidae) Induced by Trypanosoma cruzi (Trypanosomatida: Trypanosomatidae) Infection. J. Med. Entomol. 2021, 58, 2474–2478. [Google Scholar] [CrossRef]
- Ramírez-González, M.G.; Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Gutiérrez-Cabrera, A.E.; Rojas-Ortega, E.; Córdoba-Aguilar, A. Zombie bugs? Manipulation of kissing bug behavior by the parasite Trypanosoma cruzi. Acta Trop. 2019, 200, 105177. [Google Scholar] [CrossRef] [PubMed]
- Chacón, F.; Muñoz-San Martín, C.; Bacigalupo, A.; Álvarez-Duhart, B.; Solís, R.; Cattan, P.E. Trypanosoma cruzi parasite load modulates the circadian activity pattern of Triatoma infestans. Insects 2022, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Montoya, G.; Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Vences-Blanco, M.O.; Rocha-Ortega, M.; Gutiérrez-Cabrera, A.E.; Rojas-Ortega, E.; Córdoba-Aguilar, A. The cost of being a killer’s accomplice: Trypanosoma cruzi impairs the fitness of kissing bugs. Parasitol. Res. 2019, 118, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Gurgel-Gonçalves, R.; Abad-Franch, F.; de Almeida, M.R.; Obara, M.T.; de Souza, R.D.C.M.; Batista, J.A.D.S.; Rocha, D.D.A. TriatoDex, an electronic identification key to the Triatominae (Hemiptera: Reduviidae), vectors of Chagas disease: Development, description, and performance. PLoS ONE 2021, 16, e0248628. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.C.; Cura, C.I.; Da, O.; Moreira, C.; Lages-Silva, E.; Juiz, N.; Velázquez, E.; Pavia, P.; Flores-Chávez, M.D.; Muñoz-Calderón, A.; et al. Analytical Validation of Quantitative Real-Time PCR Methods for Quantification of Trypanosoma cruzi DNA in Blood Samples from Chagas Disease Patients. J. Mol. Diagn. 2015, 17, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.; Vivas, A.; Montilla, M.; Hernández, C.; Flórez, C.; Parra, E.; Ramírez, J.D. Comparative study of the biological properties of Trypanosoma cruzi I genotypes in a murine experimental model. Infect. Genet. Evol. 2015, 29, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Duffy, T.; Cura, C.I.; Ramirez, J.C.; Abate, T.; Cayo, N.M.; Parrado, R.; Bello, Z.D.; Velazquez, E.B.; Muñoz-Calderon, A.; Juiz, N.A.; et al. Analytical Performance of a Multiplex Real-Time PCR Assay Using TaqMan Probes for Quantification of Trypanosoma cruzi Satellite DNA in Blood Samples. PLoS Negl. Trop. Dis. 2013, 7, e2000. [Google Scholar] [CrossRef]
- Diaz, S.; Panzera, F.; Jaramillo, O.N.; Perez, R.; Fernandez, R.; Vallejo, G.; Saldaña, A.; Calzada, J.E.; Triana, O.; Gómez-Palacio, A. Genetic, Cytogenetic and Morphological Trends in the Evolution of the Rhodnius (Triatominae: Rhodniini) Trans-Andean Group. PLoS ONE 2014, 9, e87493. [Google Scholar] [CrossRef]
- Hernández, C.; Cucunubá, Z.; Flórez, C.; Olivera, M.; Valencia, C.; Zambrano, P.; León, C.; Ramírez, J.D. Molecular Diagnosis of Chagas Disease in Colombia: Parasitic Loads and Discrete Typing Units in Patients from Acute and Chronic Phases. PLoS Negl. Trop. Dis. 2016, 10, e0004997. [Google Scholar] [CrossRef]
- Souto, R.P.; Fernandes, O.; Macedo, A.M.; Campbell, D.A.; Zingales, B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol. Biochem. Parasitol. 1996, 83, 141–152. [Google Scholar] [CrossRef]
- Hernández, C.; Vera, M.J.; Cucunubá, Z.; Flórez, C.; Cantillo, O.; Buitrago, L.S.; González, M.S.; Ardila, S.; Dueñas, L.Z.; Tovar, R.; et al. High-Resolution Molecular Typing of Trypanosoma cruzi in 2 Large Outbreaks of Acute Chagas Disease in Colombia. J. Infect. Dis. 2016, 214, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.D.; Montilla, M.; Cucunubá, Z.M.; Floréz, A.C.; Zambrano, P.; Guhl, F. Molecular Epidemiology of Human Oral Chagas Disease Outbreaks in Colombia. PLoS Negl. Trop. Dis. 2013, 7, e2041. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.D.; Cao, L.; Cruz-Saavedra, L.; Hernandez, C.; Castañeda, S.; Muñoz, M.; Ballesteros, N.; Banu, R.; Shrestha, P.; Cordon-Cardo, C.; et al. Pan-stage real-time PCR for quantitation of Trypanosoma cruzi parasitic loads in blood samples. Int. J. Infect. Dis. 2022, 122, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Proteccion Social; Organizacion Panamerica de Salud; Instituto Nacional de Salud. Protocolo de Rigilancia Entomologica y Control Vectorial de la Enfermedad de Chagas; Technical Report; Instituto Nacional de Salud: Madrid, Spain, 2020. (In Spanish)
- Moreira, O.C.; Verly, T.; Finamore-Araujo, P.; Gomes, S.A.O.; Lopes, C.M.; de Sousa, D.M.; Azevedo, L.R.; da Mota, F.F.; D’Avila-Levy, C.M.; Santos-Mallet, J.R.; et al. Development of conventional and real-time multiplex PCR-based assays for estimation of natural infection rates and Trypanosoma cruzi load in triatomine vectors. Parasites Vectors 2017, 10, 2343. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Barazarte, H.; Molina de Fernández, D. Primer registro de Eratyrus mucronatus Stål, 1959 (Hemiptera: Reduviidae) en el ambiente domiciliario en Venezuela. Entomotropica 2001, 16, 215–217. [Google Scholar]
- Avendaño-Rangel, F.; Pefaur, J.; Lizano, E.; Aldana, E.; Velásques-Olivares, D.; Concepción, J.L. Eratyrus mucronatus (Hemiptera, Triatominae) domiciliado y alimentado con sangre humana y canina en el estado Mérida, Venezuela: Un riesgo potencial para la transmisión de la enfermedad de Chagas. Rev. Científica 2011, 21, 421–424. [Google Scholar]
- Florez, M.; Rojas, J.; Angulo, V. Biology of Eratyrus mucronatus (Hemiptera, Reduviidae, Triatominae) in laboratory conditions. Bol. Malariol. 2015, 55, 86–93. [Google Scholar]
- Cantillo-Barraza, O.; Chaverra, D.; Marcet, P.; Arboleda-Sánchez, S.; Triana-Chávez, O. Trypanosoma cruzi transmission in a Colombian Caribbean region suggests that secondary vectors play an important epidemiological role. Parasites Vectors 2014, 7, 381. [Google Scholar] [CrossRef]
- Cantillo-Barraza, O.; Garcés, E.; Gómez-Palacio, A.; Cortés, L.A.; Pereira, A.; Marcet, P.L.; Jansen, A.M.; Triana-Chávez, O. Eco-epidemiological study of an endemic Chagas disease region in northern Colombia reveals the importance of Triatoma maculata (Hemiptera: Reduviidae), dogs and Didelphis marsupialis in Trypanosoma cruzi maintenance. Parasites Vectors 2015, 8, 1100. [Google Scholar] [CrossRef]
- Peña, V.H.; García, A.; Gómez-Palacio, M.; Triana-Chávez, O.; Mejía-Jaramillo, A.M. Eco-Epidemiology of Chagas Disease in an Endemic Area of Colombia: Risk Factor Estimation, Trypanosoma cruzi Characterization and Identification of Blood-Meal Sources in Bugs. Am. J. Trop. Med. Hyg. 2014, 91, 1116–1124. [Google Scholar] [CrossRef]
- Rendón, L.M.; Guhl, F.; Cordovez, J.M.; Erazo, D. New scenarios of Trypanosoma cruzi transmission in the Orinoco region of Colombia. Mem. Inst. Oswaldo Cruz 2015, 110, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Vivas, R.; García, J.; Guhl, F.; Hernández, C.; Velásquez, N.; Ramírez, J.D.; Carranza, J.C.; Vallejo, G.A. Systematic review on the biology, ecology, genetic diversity and parasite transmission potential of Panstrongylus geniculatus (Latreille 1811) in Latin America. Mem. Inst. Oswaldo Cruz 2021, 116, e200528. [Google Scholar] [CrossRef] [PubMed]
- Calzada, E.; Pineda, V.; Montalvo, E.; Alvarez, D.; Mari, A.N.A.; Santamari, A.; Bayard, V.; Cáceres, L.; Saldaña, A. Human Trypanosome Infection and the Presence of Intradomicile Rhodnius pallescens in the Western Border of the Panama Canal, Panama. Am. J. Trop. Med. Hyg. 2006, 74, 762–765. [Google Scholar] [CrossRef] [PubMed]
- Cantillo-Barraza, O.; Torres, J.; Hernández, C.; Hernández, C.; Romero, Y.; Zuluaga, S.; Correa-Cárdenas, C.A.; Herrera, G.; Rodríguez, O.; Alvarado, M.T.; et al. The potential risk of enzootic Trypanosoma cruzi transmission inside four training and re-training military battalions (BITER) in Colombia. Parasites Vectors 2021, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Roa, L.; Gaitán, X.; Granada, Y.; Clavijo, J.A.; Teixeira, M.; Montilla, M. Capacidad vectorial de Rhodnius colombiensis para transmitir Trypanosoma cruzi I y T. cruzi II. Rev. Asoc. Colomb. Cienc. Biol. Capacid. 2013, 25, 22–30. [Google Scholar]
- Velásquez-Ortiz, N.; Herrera, G.; Hernández, C.; Muñoz, M.; Ramírez, J.D. Discrete typing units of Trypanosoma cruzi: Geographical and biological distribution in the Americas. Sci. Data 2022, 9, 360. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.D.; Turriago, B.; Tapia-Calle, G.; Guhl, F. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet. Parasitol. 2013, 196, 216–219. [Google Scholar] [CrossRef]
- Abad-Franch, F.; Monteiro, F.A.; Gurgel, R.; Braga, F.; Dias, S.; Diotaiuti, L. Ecology, evolution, and the long-term surveillance of vector-borne Chagas disease: A multi-scale appraisal of the tribe Rhodniini (Triatominae). Acta Trop. 2009, 110, 159–177. [Google Scholar] [CrossRef]
- Moncayo, Á.; Silveira, A.C. Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem. Inst. Oswaldo Cruz 2009, 104, 17–30. [Google Scholar] [CrossRef]
- Luitgards-Moura, J.F.; Vargas, A.B.; Almeida, C.E.; Magno-Esperança, G.; Agapito-Souza, R.; Ramos, E.F.; Costa, J.; Tsouris, P.; Rosa-Freitas, M.G. A Triatoma maculata (Hemiptera, Reduviidae, Triatominae) population from Roraima, Amazon Region, Brazil, has some bionomic characteristics of a potential Chagas disease vector. Rev. Inst. Med. Trop. Sao Paulo 2005, 47, 131–137. [Google Scholar] [CrossRef]
- Vaca-Moyano, F.; Enríquez, S.; Arrivillaga-Henríquez, J.; Villacrés-Guevara, E.; Araujo, P.; Benítez-Ortíz, W. Geographical distribution update of Triatoma dispar (Hemiptera: Reduviidae: Triatominae) in Ecuador. Rev. Colomb. Entomol. 2017, 43, 255–261. [Google Scholar] [CrossRef]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors—Occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Maddrell, S.H. Excretion in the Blood-Sucking Bug, Rhodnius prolixus Stal. II. The Normal Course of Diuresis and the Effect of Temperature. J. Exp. Biol. 1964, 41, 163–176. [Google Scholar] [CrossRef]
- Oliveira, M.F.; Silva, J.R.; Dansa-Petretski, M.; de Souza, W.; Lins, U. Haem detoxification by an insect. Nature 1999, 400, 517–518. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Ortiz, N.; Hernández, C.; Cantillo-Barraza, O.; Ballesteros, N.; Cruz-Saavedra, L.; Herrera, G.; Buitrago, L.S.; Soto, H.; Medina, M.; Palacio, J.; et al. Trypanosoma cruzi Parasite Burdens of Several Triatomine Species in Colombia. Trop. Med. Infect. Dis. 2022, 7, 445. https://doi.org/10.3390/tropicalmed7120445
Velásquez-Ortiz N, Hernández C, Cantillo-Barraza O, Ballesteros N, Cruz-Saavedra L, Herrera G, Buitrago LS, Soto H, Medina M, Palacio J, et al. Trypanosoma cruzi Parasite Burdens of Several Triatomine Species in Colombia. Tropical Medicine and Infectious Disease. 2022; 7(12):445. https://doi.org/10.3390/tropicalmed7120445
Chicago/Turabian StyleVelásquez-Ortiz, Natalia, Carolina Hernández, Omar Cantillo-Barraza, Nathalia Ballesteros, Lissa Cruz-Saavedra, Giovanny Herrera, Luz Stella Buitrago, Hugo Soto, Manuel Medina, Jatney Palacio, and et al. 2022. "Trypanosoma cruzi Parasite Burdens of Several Triatomine Species in Colombia" Tropical Medicine and Infectious Disease 7, no. 12: 445. https://doi.org/10.3390/tropicalmed7120445
APA StyleVelásquez-Ortiz, N., Hernández, C., Cantillo-Barraza, O., Ballesteros, N., Cruz-Saavedra, L., Herrera, G., Buitrago, L. S., Soto, H., Medina, M., Palacio, J., González, M. S., Cuervo, A., Vallejo, G., Zuleta Dueñas, L., Urbano, P., Muñoz, M., & Ramírez, J. D. (2022). Trypanosoma cruzi Parasite Burdens of Several Triatomine Species in Colombia. Tropical Medicine and Infectious Disease, 7(12), 445. https://doi.org/10.3390/tropicalmed7120445







