Abstract
The hand-net is the standard method for capturing mosquitoes with sylvatic diurnal activity in disease outbreaks in Brazil. However, occupational risks and biases related to the collectors’ abilities and attractiveness are important limitations. In this study, we compared hand-nets with automatic traps (CDC) associated to CO2 and BG-Lure® in the Vassununga State Park, a Brazilian Savanna protection area. The collections carried out over 27 days on the ground and the forest canopy. A total of 1555 mosquitoes were obtained in 20 taxa. The diversity index ranged between 1.12 and 1.79 and the dominance index from 0.22 to 0.40. The dominant species on the ground was Aedes scapularis (46.0%), and in the canopy, Hg. janthinomys/capricornii (31.9%). Haemagogus leucocelaenus was rare (n = 2). The hand-net resulted in the greatest diversity and abundance of species in both strata, followed by the traps associated with CO2. A low degree of similarity was observed between the hand-net on the ground compared to the other capture methods. The use of BG-Lure® alone resulted in a low number of specimens. In conclusion, the hand-net is still the method of choice for collecting arbovirus vectors in the diurnal period, especially yellow fever vectors.
1. Introduction
In Brazil, many species of Culicidae (Diptera) are of medical interest [1,2] and much of the knowledge about their bioecology and epidemiological role is the result of research and entomological surveys associated with outbreaks of arboviruses [3,4,5,6,7]. In these studies, the mobile human attraction technique using capture hand-nets was widely used, often in entomological captures in the forest canopy, aimed at increasing the sample of mosquitoes that feed on the blood of birds and nonhuman primates, which are considered important hosts for several arboviruses [5,8,9]. For this reason, in Brazil, the hand-net was adopted as the standard technique by health surveillance services across the country, with canopy capture recommended as a complementary procedure [10].
However, in captures with humans beings, the attractants are subject to variations in individual performance and the attractiveness of each collector, which can imply a bias resulting in sample divergences [11]. Moreover, collections performed by individuals raises concerns about occupational risks [12,13], especially when working at heights in tree canopies, according to the specific safety protocols of each country.
Thus, there is a need for alternative techniques to replace the standard hand-net technique that can ensure representativeness in mosquito sampling. Service (1993) and Santos et al. (2021) point out that the selection of the appropriate method should take into account the specific characteristics of the natural history, biology, and ecology of the target species of potential vectors [14,15].
One of the alternative techniques is the use of automatic suction traps with a luminous attractant, commonly used to collect nocturnal species [13,16]. In Brazil, the association of a carbon dioxide source and other types of odor attractants (such as octenol and lactic acid) have been used to increase the sensitivity of this type of trap for daytime use [17,18,19,20,21]. Other studies were restricted to afternoon and nocturnal periods [22,23,24,25,26], or focused on the urban environment, for capturing Aedes aegypti and Culex quinquefasciatus [27,28,29].
Recently, our group began looking for alternative and/or complementary methods for daytime collections in accordance with forest stratification to carry out routine surveys in different biomes. In a previous study, we conducted a comparative evaluation of in-person capture using hand-nets and automatic traps with carbon dioxide (dry ice) and BG-Lure® as attractants on the ground and in the canopy stratum of an Atlantic Forest environmental reserve [21]. In the present study, we apply the same methodology used in the previous study for a comparative evaluation of these methods in the Cerrado biome.
2. Materials and Methods
2.1. Research Area
The Vassununga state park (Parque Estadual Vassununga—PEV) is a protected conservation area that was created to protect representative areas of the seasonal semideciduous forest (inland Atlantic Forest) and different “Cerrado” (Brazilian savanna) physiognomies. This park, made up of six discontinuous sectors with a total area of 2071.42 hectares, is located in the municipality of Santa Rita do Passa Quatro, São Paulo, Brazil [30] (Figure 1). The Pé-do-Gigante sector, chosen for this study, is the largest area in the Park (1212.92 ha) and contains the three types of savanna: forested savanna (“Cerradão”), arborized savanna (“Cerrado stricto sensu”), and savanna “Gramíneo-Lenhosa” (grassy-shrub), according to the classification adopted by Veloso et al. (1991) [31,32].
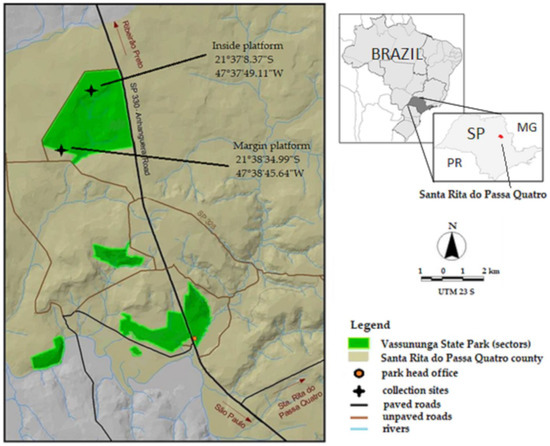
Figure 1.
Location of the study area in the savanna biome, Vassununga State Park. Source: Plano de Manejo PEV, 2009.
According to the Köppen climate classification, the climate of the Santa Rita do Passa Quatro region is Cwa: rainy in summer and dry in winter. The average annual temperature is 23.3 °C, with an average maximum temperature of approximately 26.0 °C in summer (December to February) and an average minimum temperature of approximately 19.0 °C in winter (June to August). According to the Park Management Plan [30], the park has an average annual rainfall of 1365.7 mm and potential evapotranspiration of 1160.61 mm.
Two sites in the area were selected for the collections: the forest margin and inside the forest (about 3 km apart). In each site, collections were carried out in the forest canopy and at ground level. For the sampling of mosquitoes at the edge of the forest, a platform was installed at the first branch ramification of the tree canopy, seven meters above the ground, accessed using ropes and climbing equipment by a trained capturer (File S1). This platform is in an area characterized as arborized savanna (“Cerrado ss”), a dense savanna with a predominantly arboreal vegetation subtype, with 50 to 70% coverage and an average height of five to eight meters. This physiognomy represents the highest and densest form of savanna. In the second sampling area, inside the forest, we used the platform of a meteorological tower. The capturer worked at a height of eight meters, corresponding to the average canopy height of the surrounding trees. This sector of the park corresponds to an area of dense savanna transitioning into “Cerradão”, with a predominance of trees in a closed canopy that approaches forest vegetation, with an average tree height of 10 to 12 m [30].
2.2. Capture of Specimens
Entomological captures were carried out on three consecutive days per month in February, March, October, November, and December of 2020, and in January, February, April, and May of 2021. The pause in collections was due to COVID-19 pandemic restrictions. Two techniques were used:
2.2.1. Capture by Hand-Net
Mosquitoes were captured with hand-nets and sucked with a mouth aspirator, where they were retained until the end of the collection period (morning or afternoon) [10]. A detailed description of the equipment can be seen in the File S2. Captures were carried out from 9:00 to 12:00 am and 1:00 to 4:00 pm in both sectors simultaneously. In each sector, the collections were carried out by two collectors, one on the platform and another at ground level. On the ground, the collector covered approximately 1500 m of trails around each platform. The collectors rotated the stratum between 12:00 am and 1:00 pm when they also stored the specimens collected in the morning. At the end of the afternoon period, at 4:00 pm, the same procedure to store the samples was performed, which was important to avoid damage to the morphological structures and to be able to freeze the insects still alive. The insects collected were transferred to cryotubes and frozen alive in liquid nitrogen to preserve viral genetic material for future analysis.
2.2.2. Capture with Automatic Traps
CDC-type electric traps [33] were used with lights off and two chemical attractants: carbon dioxide (dry ice) and BG-Lure® (a commercial product), which is a chemical attractant composed of ammonia, L- lactic acid, and caproic acid. In each sector (forest margin and interior), four traps were installed close to the ground, between 1 and 1.5 min height, and another four in the tree canopy at a minimum height of 5 m, depending on the average height of the trees at the site. There were sixteen traps in total, with eight positioned at the forest edge and eight inside the forest around each platform, considered the central reference point (Figure 1). The traps were placed at points to the north, south, east, and west, at approximately 250 m from the platform, and each day the traps were rotated to alternate the position of the exposure of the attractants. One pair of traps (ground–canopy) was used with only CO2, another pair with only BG-Lure®, and two pairs with the two attractants together (in diametrically opposite positions). The electric traps were exposed during the same collection period as the hand-net method, and the samples were stored after the end of both the morning and afternoon periods using the same procedure.
2.3. Identification of Material
In the laboratory, the biological material frozen in nitrogen was transferred to a freezer at −80 °C until the identification of the genus and species on a cold table at −20 °C. Taxonomic keys were used for the morphological identification of Culicidae [1,2,34,35].
2.4. Data Analysis
The data were entered into an information system operating on a web server developed especially for the project.
For the estimation of species diversity, species accumulation rate and statistical abundance analysis were performed using the EstimateS version 9 statistics program [36]. Diversity (S) was determined by the number of species collected per month in each environment and stratum and by the capture method. The accumulation curve was calculated using the Coleman rarefaction method. The estimate of the true number of species was performed with the Chao1 estimator and a confidence interval of 95%.
To compare diversity among the different methods (technique–attractant–stratum), the Shannon (H) and Gini–Simpson (1-D) indices were calculated; with Simpson’s dominance (D) calculated using EstimateS v.9. To compare abundance among the different methods (technique–attractant–stratum), the Wilcoxon test was applied for the difference between the medians at a significance level of 95%. For this purpose, SPSS v.25 software was used.
For the cluster analysis, we used dendrograms based on Bray–Curtis similarity, which considers the abundance of specimens. For the pairwise analysis of the association between methods, we used Spearman’s correlation with the PAST version 4.05 statistics program [37].
3. Results
We collected a total of 1555 Culicidae specimens in 20 taxa, of which 17 species belonged to 8 genera. Only 24 specimens were males, corresponding to the genus Culex and to the species Aedes albopictus and Psorophora albigenu (Table S1). Eighty-five percent of the specimens were captured from the ground.
Aedes scapularis was the most abundant species, followed by Haemagogus janthinomys/capricornii, Psorophora albigenu, Sabethes albiprivus, and Ae. albopictus (Table 1). All species, except Limatus durhamii and Sabethes belisarioi, were more abundant at the ground level (n = 1302) than in the canopy (n = 229), including Hg. janthinomys/capricornii and Sa. albiprivus, which were the dominant species in the canopy. Haemagogus leucocelaenus was a rare species at ground level, with only two specimens collected (Table 1).

Table 1.
Number of Culicidae females collected in the “Pé-do-Gigante” sector of the Vassununga State Park, by forest stratum, technique, and attractant.
The hand-net capture technique obtained significantly higher yields than the automatic traps, both in the canopy and on the ground, accounting for 78.6% of the total of specimens collected in the canopy and 72.1% on the ground (Table 1 and Table S2). The traps using only CO2 and CO2 + BG-Lure® obtained an intermediate yield and showed no significant difference between them in relation to the same stratum (Table S2). Traps using exclusively the BG-Lure® attractant had the lowest yield in both strata (Table 1, Table S2 and Table S3).
Regarding the indicators for richness, diversity, and dominance (Table 2), we observed a qualitative similarity between the canopy and ground strata, as the differences between each method in the canopy had the same pattern on the ground. In addition, the values of the same method in the canopy were close to those on the ground. The differences were small between the values for absolute richness and abundance for the different techniques and attractants in each stratum and, in general, with greater richness on the ground compared to the canopy. The biggest difference was in the trap results associated with BG-Lure® in the canopy, where only one specimen was collected and, thus, presented very different indicators of the same method at ground level. Hand-net capture was the technique with greater richness and diversity and less dominance in both strata.

Table 2.
Indicators of abundance, richness, diversity, and dominance of Culicidae specimens collected in the “Pé-do-Gigante” sector of the Vassununga State Park, by forest strata, technique, and attractant. Next page.
Species accumulation curves show a tendency to anticipate stability for the canopy compared to the ground (Figure 2). This stability in the canopy added to the qualitative and quantitative results, as seen in Table 1.
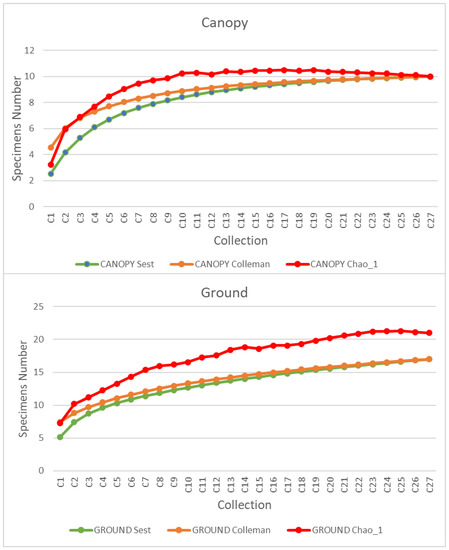
Figure 2.
Species accumulation curves for Culicidae specimens collected in canopy and ground strata of the arborized savanna in the “Pé-do-Gigante” sector of the Vassununga State Park. Note: C = collection.
The analysis of the pair-to-pair comparison of different methods (stratum–technique–attractant) showed significance values between practically all methods, with the exception of the trap using only the BG-Lure® installed in the canopy (with negative results) and the trap installed on the ground versus the traps with the CO2 + BG-Lure® 1 and 2 in the canopy (Table 3). The highest coefficients occur between the traps using CO2 and CO2 + BG-Lure® at the ground level and between the nets on the ground and in the canopy.

Table 3.
Spearman’s correlation coefficients (ρ) and statistical significance (p) values from the pair-to-pair comparison between different methods (stratum–technique–attractant) for mosquito collection in the “Pé-do-Gigante” sector of the Vassununga State Park.
The Bray–Curtis similarity analysis for the different methods for collecting mosquito species is used to verify clusters of those methods that are most similar in qualitative and quantitative terms of species. Figure 3a shows the similarity dendrogram based on the analysis of all species found. A cluster that stands out includes all the traps at ground level that used CO2 (exclusively or with BG-Lure®) and the hand-net in the canopy, which suggests that one method could replace the other or serve as a replica. At the other extreme, the ground-trap methods and traps using only BG-Lure® in the canopy have the fewest similarities, being isolated from the others. This, however, can be attributed to different reasons, the first being the method presented superior abundance and richness, and the latter being almost null.
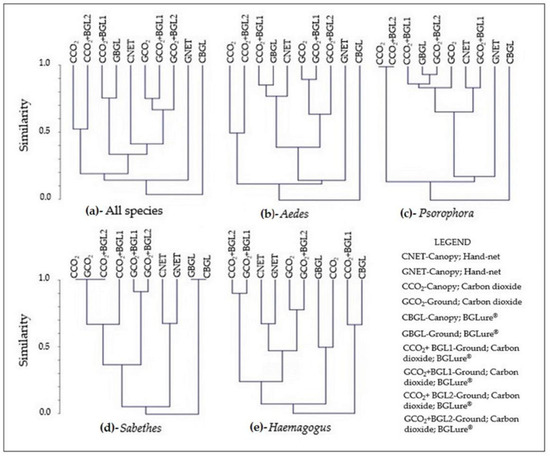
Figure 3.
Bray–Curtis similarity dendrogram for different methods (technique–attractant–stratum) for the collection of Culicidae specimens in the “Pé-do-Gigante” sector of the Vassununga State Park, according to all identified species (a) and, according to the four genera with the highest number of specimens: (b)—Aedes; (c)—Psorophora; (d)—Sabethes; (e)—Haemagogus.
The analysis considering each of the four most abundant genera in individualized dendrograms is presented in Figure 3b–e. The genus Psorophora presents a very similar large group comprising several methods; ground hand-net methods and traps with only BG-Lure® in the canopy appear in isolation, following a pattern similar to the dendrogram for all species, which also presents the group of traps with CO2 in the canopy. The genus Aedes also shows some variation of this pattern for the central group, with fewer similarities than Psorophora. In the case of the Haemagogus and Sabethes genera, both presented a group with great similarity composed of nets in the canopy and on the ground, suggesting the human attractant as an aggregator. However, Haemagogus seems to be the genus that expresses more standardized responses to methods, while Sabethes shows more diffuse behavior for technique –attractant–stratum combinations.
4. Discussion
Our study is the most recent survey of diurnal mosquitoes in the savanna biome, where species richness and abundance were studied according to forest stratification. The main objective was to evaluate alternative capture methods to the hand-net, using the attractants BG-Lure® and carbon dioxide in order to increase the effectiveness of automatic traps, especially for species of epidemiological interest that frequent the forest canopy. Thus, we sought to carry out collections in a favorable seasonal period, mainly for the species of the Aedini and Sabethini tribes [38,39,40,41,42,43].
Based on the results of the species accumulation curves for both strata, we verified that the sampling in the period was efficient, allowing for a good characterization of the diurnal mosquito fauna in the “Pé-do-Gigante” sector of the park. Thus, although we used a shorter collection period (27 days) than in other studies conducted in savanna areas (40–60 days) [3,44,45], we found similar species richness for the genera Aedes, Haemagogus, Psorophora, and Sabethes, with 14 species found in our study, and 14 to 17 in the others. It is also worth mentioning that these three studies were carried out in central Brazil, between latitudes 15.5 and 17.0°S, while our study, at latitude 21.5°S, represents an important record of this fauna in a region close to the southern limit of this biome in Brazil [46].
In addition, due to the methodology of simultaneous collection in the canopy and on the ground, a robust comparison between these two strata was possible. In other systematic studies [39,47] or specific entomological surveys associated with epidemic outbreaks [48,49,50] that also investigate the community of diurnal mosquitoes using the mobile human bait technique with hand-nets, collections were not carried out in the canopy. Thus, all the data on the abundance, richness, and dominance of species in the region were related to collections at the ground level. Our study provides pioneering results on the stratification of mosquito communities in the northwest region of the state of São Paulo.
Considering the general results from the two strata, we can observe the dominance of Aedes scapularis and the subdominance of Haemagogus janthinomys/capricornii, Psorophora albigenu, Sabethes albiprivus, and Ae. albopictus. In the comparison between strata, Ae. scapularis was dominant on the ground, while Hg. janthinomys/capricornii dominated in the canopy. Qualitatively, the list of species in the canopy and at ground level was very similar. In quantitative terms, practically all the species found in the canopy had a lower number of specimens compared to collections carried out on the ground, which was also observed in other studies carried out in the savanna [3,44,45].
This suggests that microclimatic conditions in the canopy may be less favorable than at ground level. We should emphasize that the collection points were in the “Cerrado ss” physiognomy, where the shading and size of the trees were less than in other physiognomies found in the park, such as the “Cerradão”, gallery forest, and semideciduous seasonal forest [31,51]. In this sense, the canopy was more subject to desiccation, affecting the intensity and maintenance of relative humidity, which is a key environmental factor for the main species found there [39,52,53].
The values of the indicators of diversity, uniformity, and dominance were similar for the same method between the canopy and the ground. The same was not observed for the richness and abundance indicators, which were higher in the soil. The Spearman’s correlation results corroborate this, since significant differences were found between the different methods in the same stratum and between strata. Contrarily, these indicators were different between the strata and methods in a similar study we carried out in the Atlantic Forest biome in Cantareira State Park [21].
However, in both studies, the hand-net was the technique with greater richness and diversity and lower dominance in both strata, while the indicators of traps using only BG-Lure® demonstrated inefficiency, a result also found for wild diurnal mosquitoes in Brazil by other researchers [19,54]. This shows that the exclusive use of BG-Lure® may be inefficient for wild neotropical species. The use of CO2 as an attractant demonstrated worse performance than its use together with BG-Lure®, a different result from that found in the Atlantic Forest [21] and probably due to the differences in specific environmental conditions in the savanna.
It is worth mentioning that there are few attractants available for commercial use, as can be seen in many studies carried out in Brazil to evaluate the performance of mosquito collection with automatic traps [19,21,22,23,24,25,27,28,29,54,55]. This suggests the need to develop in situ studies using olfactometry to discover different substances that present selective attractiveness for each species of interest, according to the biome where it will be used.
Among the most abundant species of mosquitoes collected in the “Pé-do-Gigante” sector, all of them showed epidemiological importance, especially for the transmission of yellow fever. Haemagogus janthinomys, Hg. leucocelaenus, and Sa. chloropterus are considered the main vector species in South America [56], the first two with the greatest range in Brazil [57,58]. In our study, we used the nomenclature Haemagogus janthinomys/capricornii because we were unable to determine which of the two species occurred at the site, since they are morphologically differentiated only by the male genitalia, and the investigated site is in the co-occurrence zone [2]. At any rate, whatever the species, it was the most abundant in the canopy compared to the other mosquito species, with a greater number of specimens at ground level than in the canopy. This result differs from several studies in which the species is cited as an acrodendrophile [19,45,52,59,60]. On the other hand, it adds to the results of other studies where variations in this pattern were observed [3,44,54].
The other species with epidemiological importance for yellow fever, which presented the greatest number of specimens, were Ae. scapularis, Ae. albopictus, Ps. albigenu, Sa. albiprivus, and Sa. glaucodaemon, and, to a lesser extent, Ae. serratus, Ps. ferox, and Hg. leucocelaenus. The rarity of Hg. leucocelaenus is noteworthy, since it is one of the dominant species in Cantareira State Park [21] and a vector for the transmission of the yellow fever virus in other areas of the Atlantic Forest [58,61,62]. Perhaps this species has no favorable habitat in the savanna, at least in the stricto sensu physiognomy investigated in this study. The assessment of its presence and abundance in enclaves of seasonal semideciduous forests, gallery forests, and in the physiognomy of the “Cerradão” deserves to be studied in order to better understand the details of the ecology of the species in this biome, since previous studies have not exactly focused on this [26,44,45,47,63].
An important result of this research is the assessment of the success of different techniques and attractants in the canopy and on the ground for capturing the genera Aedes, Haemagogus, Psorophora, and Sabethes, providing valuable data for arbovirus surveillance services in Brazil. In the similarity cluster analysis, the selectivity of hand-nets for the genera Haemagogus and Sabethes were evident in the dendrograms, while for the genera Aedes and Psorophora, the similarity between methods was more similar to the general dendrogram (Figure 3a all species), where the hand-net on the ground and the trap using only BG-Lure® in the canopy appear distant from the other methods, with the former having the highest relative yield and the latter the lowest relative yield.
In investigations of epidemics or epizootic outbreaks, the right place and time are crucial for obtaining good samples [10,64]. Thus, the use of the technique with the highest yield for the target species is very important. However, it is worth considering that the “ideal” collection technique is not always possible for surveillance services. Moreover, the use of automatic traps may be the only way to expand the space–time factor in routine and large-scale surveys [65,66]. Thus, if it is necessary to make use of these alternative techniques, this study, and that of Deus et al. (2022), may represent an important reference for the operational planning of field actions [21].
5. Conclusions
The results of this study highlight the benefits of using hand-nets over automatic traps with kairomones to capture diurnal mosquitoes, as they presented higher values of richness and abundance for species of epidemiological interest. Our research suggests that electric traps should be used to collect diurnal mosquitoes in the savanna when used with CO2 for species-richness studies of diurnal Culicidae. In the studied locality of “Cerrado ss”, collections at ground level showed greater abundance and species richness than the canopy. For surveys that require more abundance of specimens, it is necessary to consider the increase in effort in terms of the number of traps.
It is clear that there is still much to be explored concerning attractants, and it is important to encourage the development and testing of new kairomones associated with specific hosts, especially when the objective is to increase epidemiological and/or epizootic research based on the infectivity of mosquitoes and the genomic study of the etiologic agent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed7120446/s1, Table S1: Number of male specimens collected by genus and species, according to stratum (canopy and ground) and capture technique (treatment). Santa Rita do Passa Quatro, SP, Brazil.; Table S2: Results of the pairwise comparison between methods and strata by the Wilcoxon test; Table S3: Descriptive analysis results; File S1: Photos of Platforms and CDCs Installed; File S2: Description Collection Equipment: Hand-Net and Mouth Aspirator.
Author Contributions
Conceptualization, methodology, investigation, writing—original draft preparation, all the authors participated equally; validation, J.T.d.D., V.L.F.d.C.-N., E.S.B., M.P. and L.F.M.; formal analysis, V.L.F.d.C.-N.; resources, V.L.F.d.C.-N.; data curation, S.L.R. and V.L.F.d.C.-N.; writing—review and editing, J.T.d.D., L.F.M. and V.L.F.d.C.-N.; supervision, J.T.d.D. and V.L.F.d.C.-N.; project administration, V.L.F.d.C.-N.; funding acquisition, V.L.F.d.C.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP, Brazil grant number: Fapesp—2017/50345-5 and the Superintendência de Controle de Endemias—Sucen, Secretaria de Estado da Saúde de São Paulo, Brazil.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the teams that carried out the entomological captures and Agnaldo Nepomuceno, Regional Director of the Metropolitan Region of São Paulo, for his support. We are also grateful to Fabrício Pinheiro da Cunha at the Forestry Foundation of Secretaria de Infraestrutura e Meio Ambiente do Estado de São Paulo—SIMA/SP for logistics support and for granting permission to work in the park according to the license SIMA N° 260108—009.866/2019 and Instituto Chico Mendes de Conservação e Biodiversidade, authorization number—SISBIO 72494-1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Consoli, R.A.G.B.; de Oliveira, R.L. Principais Mosquitos de Importância Sanitária no Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994; ISBN 978-85-85676-03-2. Available online: https://static.scielo.org/scielobooks/th/pdf/consoli-9788575412909.pdf (accessed on 1 July 2022).
- Forattini, O.P. Culicidologia Médica: Identificação, Biologia, Epidemiologia; Edusp: São Paulo, Brasil, 2002; ISBN 13: 9788531406997. [Google Scholar]
- Pinheiro, F.P.; da Rosa, A.P.A.T.; Moraes, M.A.P. An epidemic of yellow fever in central Brazil, 1972–1973: II. Ecological studies. Am. J. Trop. Med. Hyg. 1981, 30, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.C.; da Rosa, A.P.A.T.; Dégalier, N.; da Rosa, J.F.S.T.; Pinheiro, F.P. Clinical and Ecoepidemiological Situation of Human Arboviruses in Brazilian Amazonia. Ciência e Cultura 1992, 44, 117–124. Available online: http://memoria.bn.br/docreader/DocReader.aspx?bib=003069&pagfis=59549 (accessed on 1 July 2022).
- Iversson, L.B. Current status of the eco-epidemiological knowledge on arboviruses pathogenic to humans in the Atlantic Forest region of the State of São Paulo. Rev. Inst. Med. Trop. Sao Paulo 1994, 36, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, P.F.; Rodrigues, S.G.; Degallier, N.; Moraes, M.A.; da Rosa, J.F.; da Rosa, E.S.; Mondet, B.; Barros, V.L.; da Rosa, A.P. An epidemic of sylvatic yellow fever in the southeast region of Maranhao state, Brazil, 1993–1994: Epidemiologic and entomologic findings. Am. J. Trop. Med. Hyg. 1997, 57, 132–137. [Google Scholar] [CrossRef]
- Rosa, A.P.D.A.T.D. A História a Arbovirologia No Instituto Evandro Chagas, Belém, Pará, Brasil, de 1954 a 1998. Rev. Pan Amaz. Saúde 2016, 7, 61–70. [Google Scholar] [CrossRef][Green Version]
- Dégallier, N.; Rosa, A.P.D.A.T.D.; Vasconcelos, P.F.D.C.; Rosa, E.S.T.D.; Rodrigues, S.G.; Sá Filho, G.C.; Rosa, J.F.S.T.D. New entomological and virological data on the vectors of sylvatic yellow fever in Brazil. Arthropod-Borne Virus Inf Exch 1993, 21–22. Available online: https://patuaback.iec.gov.br/server/api/core/bitstreams/63d9d538-fdf9-4b50-949a-c3cff5c8a0e1/content. (accessed on 1 July 2022).
- Vasconcelos, P.F.; Rosa, A.P.; Rodrigues, S.G.; Rosa, E.S.; Monteiro, H.A.; Cruz, A.C.; Barros, V.L.; Souza, M.R.; Rosa, J.F. Yellow fever in Pará state, Amazon region of Brazil, 1998–1999: Entomologic and epidemiologic findings. Emerg. Infect. Dis. 2001, 7, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Brasil, M.d.S. Guia de Vigilância de Epizootias em Primatas Não Humanos e Entomologia Aplicada à Vigilância Da Febre Amarela, 2nd ed.; Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância das Doenças Transmissíveis: Brasília (DF), Brasil, 2014; ISBN 978-85-334-2102-8. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_epizootias_primatas_entomologia.pdf (accessed on 1 July 2022).
- Reiter, P.; Gubler, D. Surveillance and Control of Urban Dengue Vectors. In Dengue and Dengue Hemorrhagic Fever; Gubler, D.J., Kuno, G., Eds.; CAB International: London, UK, 1997; pp. 425–462. [Google Scholar]
- Lima, J.B.P.; Rosa-Freitas, M.G.; Rodovalho, C.M.; Santos, F.; Lourenço-de-Oliveira, R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches?—A review. Mem. Inst. Oswaldo Cruz 2014, 109, 685–705. [Google Scholar] [CrossRef]
- Achee, N.L.; Youngblood, L.; Bangs, M.J.; Lavery, J.V.; James, S. Considerations for the use of human participants in vector biology research: A tool for investigators and regulators. Vector Borne Zoonotic Dis. 2015, 15, 89–102. [Google Scholar] [CrossRef]
- Service, M.W. Mosquito Ecology: Field Sampling Methods; Springer Science & Business Media: Berlin, Germany, 1993; ISBN 978-94-011-1868-2. [Google Scholar]
- Santos, J.C.; de Almeida, W.R.; Fernandes, G.W. Arthropods: Why It Is So Crucial To Know Their Biodiversity? In Measuring Arthropod Biodiversity: A Handbook of Sampling Methods; Santos, J.C., Fernandes, G.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–11. ISBN 978-3-030-53226-0. [Google Scholar]
- Service, M.W. A critical review of procedures for sampling populations of adult mosquitoes. Bull. Entomol. Res. 1977, 67, 343–382. [Google Scholar] [CrossRef]
- Laporta, G.Z.; Sallum, M.A.M. Effect of CO2 and 1-Octen-3-Ol Attractants for estimating species richness and the abundance of diurnal mosquitoes in the southeastern atlantic forest, Brazil. Mem. Inst. Oswaldo Cruz 2011, 106, 279–284. [Google Scholar] [CrossRef]
- Barrio-Nuevo, K.M.; Cunha, M.S.; Luchs, A.; Fernandes, A.; Rocco, I.M.; Mucci, L.F.; de Souza, R.P.; Medeiros-Sousa, A.R.; Ceretti-Junior, W.; Marrelli, M.T. Detection of zika and dengue viruses in wild-caught mosquitoes collected during field surveillance in an environmental protection area in São Paulo, Brazil. PLoS ONE 2020, 15, e0227239. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; Hernandez-Acosta, E.; Valério, D.; Mendonça, C.; Costa, E.R.; Júnior, J.T.A.; Assunção, F.P.; Scarpassa, V.M.; Gordo, M.; Fé, N.F.; et al. The vertical stratification of potential bridge vectors of mosquito-borne viruses in a central amazonian forest bordering Manaus, Brazil. Sci. Rep. 2020, 10, 18254. [Google Scholar] [CrossRef] [PubMed]
- Alencar, J.; Melandri, V.; Silva, J.; Albuquerque, H.G.; Guimarães, A.E. Ecological characterization of mosquitoes (Diptera: Culicidae) in areas of the mato grosso pantanal, Mato Grosso State, Brazil. Trop. Zool. 2021, 34, 1–15. [Google Scholar] [CrossRef]
- de Deus, J.T.; Mucci, L.F.; Reginatto, S.L.; Pereira, M.; Bergo, E.S.; de Camargo-Neves, V.L.F. Evaluation of methods to collect diurnal culicidae (diptera) at canopy and ground strata, in the atlantic forest biome. Insects 2022, 13, 202. [Google Scholar] [CrossRef]
- Gama, R.A.; Da Silva, I.M.; Monteiro, H.A.D.O.; Eiras, A.E. Fauna of culicidae in rural areas of porto velho and the first record of Mansonia (Mansonia) flaveola (Coquillet, 1906), for the state of Rondônia, Brazil. Rev. Soc. Bras. Med. Trop. 2012, 45, 125–127. [Google Scholar] [CrossRef]
- de Sá, I.L.R.; Sallum, M.A.M. Comparison of automatic traps to capture mosquitoes (Diptera: Culicidae) in rural areas in the tropical Atlantic Rainforest. Mem. Inst. Oswaldo Cruz 2013, 108, 1014–1020. [Google Scholar] [CrossRef]
- Chaves, L.S.M.; Laporta, G.Z.; Sallum, M.A.M. Effectiveness of mosquito magnet in preserved area on the coastal atlantic rainforest: Implication for entomological surveillance. J. Med. Entomol. 2014, 51, 915–924. [Google Scholar] [CrossRef]
- Sant’Ana, D.C.; de Sa, I.L.R.; Sallum, M.A.M. Effectiveness of mosquito magnet® trap in rural areas in the southeastern tropical Atlantic forest. Mem. Inst. Oswaldo Cruz 2014, 109, 1021–1029. [Google Scholar] [CrossRef][Green Version]
- Pinheiro, G.G.; Rocha, M.N.; de Oliveira, M.A.; Moreira, L.A.; Filho, J.D.A. Detection of yellow fever virus in sylvatic mosquitoes during disease outbreaks of 2017–2018 in Minas Gerais state, Brazil. Insects 2019, 10, 136. [Google Scholar] [CrossRef]
- Kröckel, U.; Rose, A.; Eiras, A.E.; Geier, M. New Tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment. J. Am. Mosq. Control. Assoc. 2006, 22, 229–238. [Google Scholar] [CrossRef]
- de Ázara, T.M.F.; Degener, C.M.; Roque, R.A.; Ohly, J.J.; Geier, M.; Eiras, Á.E. The impact of CO2 on collection of Aedes aegypti (Linnaeus) and Culex quinquefasciatus Say by BG-Sentinel(r) traps in Manaus, Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Degener, C.M.; de Ázara, T.M.F.; Roque, R.A.; Codeço, C.T.; Nobre, A.A.; Ohly, J.J.; Geier, M.; Eiras, Á.E. Temporal Abundance of Aedes aegypti in Manaus, Brazil, Measured by Two Trap Types for Adult Mosquitoes. Mem. Inst. Oswaldo Cruz 2014, 109, 1030–1040. Available online: http://www.bioline.org.br/pdf?oc14155 (accessed on 1 July 2022). [CrossRef] [PubMed]
- São Paulo, S. Secretaria do Meio Ambiente do Estado de São Paulo Plano de Manejo pe Vassununga 2009. Secr. Meio. Ambiente. Estado. São Paulo. 2009, 309. Available online: http://arquivos.ambiente.sp.gov.br/consema/2011/11/oficio_consema_2011_092/Plano_de_Manejo.pdf (accessed on 1 July 2022).
- Pivello, V.R.; Bitencourt, M.D.; Mantovani, W.; Junior, N.D.M.; Batalha, M.A.; Shida, C.N. Proposta de Zoneamento Ecológico para a Reserva de Cerrado Pé-de-Gigante (Santa Rita do Passa Quatro, SP). Braz. J. Ecol. 1998, 18. Available online: http://ecologia.ib.usp.br/lepac/conservacao/Artigos/83_zoneamento_eco.pdf (accessed on 1 July 2022).
- Veloso, H.P.; Rangel Filho, A.L.R.; Lima, J.C.A. Classificação da Vegetação Brasileira, Adaptada a um Sistema Universal; Ministério da Economia, Fazenda e Planejamento, Fundação Instituto Brasileiro de Geografia e Estatística, Diretoria de Geociências, Departamento de Recursos Naturais e Estudos Ambientais: Rio de Janeiro, Brazil, 1991; ISBN 978-85-240-0384-4. Available online: http://www.botanicaamazonica.wiki.br/labotam/lib/exe/fetch.php?media=projetos:campinas:biblio:veloso_etal_1991.pdf (accessed on 1 July 2022).
- Sudia, W.D.; Chamberlain, R.W. Battery-operated light trap, an improved model. Mosq. News 1962, 22, 126–129. Available online: https://www.biodiversitylibrary.org/content/part/JAMCA/JAMCA_V04_N4_P536-538.pdf (accessed on 1 July 2022).
- Reinert, J.E. Revised list of abbreviations for genera and subgenera of culicidae (diptera) and notes on generic and subgeneric changes. J. Am. Mosq. Control. Assoc. 2001, 17, 51–55. Available online: https://www.biodiversitylibrary.org/content/part/JAMCA/JAMCA_V17_N1_P051-055.pdf (accessed on 1 July 2022).
- Lane, J. Neotropical Culicidae; EDUSP: São Paulo, Brazil, 1953; Volume 2. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples 2013. Available online: http://purl.oclc.org/estimates (accessed on 1 July 2022).
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 1 July 2022).
- Guimarães, A.É.; Arlé, M. Mosquitos no parque nacional da serra dos órgãos, estado do rio de janeiro, Brasil: I- distribuição estacional. Mem. Inst. Oswaldo Cruz 1984, 79, 309–323. [Google Scholar] [CrossRef]
- Forattini, O.P.; Gomes, A.D.C. Biting activity of Aedes scapularis (Rondani) and Haemagogus mosquitoes in southern Brazil (Diptera: Culicidae). Rev. Saúde. Pública. 1988, 22, 84–93. [Google Scholar] [CrossRef]
- Gomes, A.D.C.; Torres, M.A.N.; de Paula, M.B.; Fernandes, A.; Marassá, A.M.; Consales, C.A.; Fonseca, D.F. Ecologia de Haemagogus e Sabethes (Diptera: Culicidae) em áreas epizoóticas do vírus da febre amarela, rio grande do sul, Brasil. Epidemiol. E Serviços. Saúde. 2010, 19, 101–113. [Google Scholar] [CrossRef]
- Silva, J.D.S.; Pacheco, J.B.; Alencar, J.; Guimarães, A.E. Biodiversity and influence of climatic factors on mosquitoes (Diptera: Culicidae) around the Peixe Angical hydroelectric scheme in the state of Tocantins, Brazil. Mem. Inst. Oswaldo Cruz 2010, 105, 155–162. [Google Scholar] [CrossRef]
- Alencar, J.; Pacheco, J.B.; Silva, J.D.S.; Silva, S.O.F.; Guimarães, A.E. Influence of climatic factors on Psorophora (Janthinosoma) albigenu in Pantanal Landscape, Mato Grosso State, Brazil. J. Am. Mosq. Control. Assoc. 2018, 34, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Couto-Lima, D.; Andreazzi, C.S.; Leite, P.J.; Bersot, M.I.L.; Alencar, J.; Lourenço-de-Oliveira, R. Seasonal population dynamics of the primary yellow fever vector Haemagogus leucocelaenus (Dyar & Shannon) (Diptera: Culicidae) is mainly influenced by temperature in the atlantic forest, Southeast Brazil. Mem. Inst. Oswaldo Cruz. 2020, 115. [Google Scholar] [CrossRef]
- Carvalho, M.E.S.D.; Naves, H.A.M.; Carneiro, E.; de Miranda, M.F. Distribuição vertical de mosquitos dos gêneros Haemagogus e Sabehtes, em zona urbana de Goiânia-Goiás-Brasil. Rev. Patol. Trop. J. Trop. Pathol. 1997, 26. [Google Scholar] [CrossRef]
- Lira-Vieira, A.R.; Gurgel-Gonçalves, R.; Moreira, I.M.; Yoshizawa, M.A.C.; Coutinho, M.L.; Prado, P.S.; de Souza, J.L.; Chaib, A.J.D.M.; Moreira, J.S.; de Castro, C.N. Ecological aspects of mosquitoes (Diptera: Culicidae) in the gallery forest of Brasilia National Park, Brazil, with an emphasis on potential vectors of yellow fever. Rev. Soc. Bras. Med. Trop. 2013, 46, 566–574. [Google Scholar] [CrossRef]
- Werneck, F.P.; Nogueira, C.; Colli, G.R.; Sites, J.W., Jr.; Costa, G.C. Climatic stability in the Brazilian Cerrado: Implications for biogeographical connections of south american savannas, species richness and conservation in a biodiversity hotspot. J. Biogeogr. 2012, 39, 1695–1706. [Google Scholar] [CrossRef]
- Camargo-Neves, V.L.F.; Poletto, D.W.; Rodas, L.A.C.; Pacchioli, M.; Cardoso, R.P.; Scandar, S.A.S.; Sampaio, S.M.P.; Koyanagui, P.H.; Botti, M.V.; Mucci, L.F.; et al. Entomological investigation of a sylvatic yellow fever area in São Paulo State, Brazil. Cad Saude Públ. 2005, 21, 1278–1286. [Google Scholar] [CrossRef]
- Moreno, E.S.; Rocco, I.M.; Bergo, E.; Brasil, R.A.; Siciliano, M.M.; Suzuki, A.; Silveira, V.R.; Bisordi, I.; de Souza, R.P. Reemergence of yellow fever: Detection of transmission in the State of São Paulo, Brazil, 2008. Rev. Soc. Bras. Med. Trop. 2011, 44, 290–296. [Google Scholar] [CrossRef]
- Cunha, M.S.; da Costa, A.C.; de Azevedo Fernandes, N.C.C.; Guerra, J.M.; dos Santos, F.C.P.; Nogueira, J.S.; D’Agostino, L.G.; Komninakis, S.V.; Witkin, S.S.; Ressio, R.A.; et al. Epizootics Due to Yellow Fever Virus in São Paulo State, Brazil: Viral dissemination to new areas (2016–2017). Sci. Rep. 2019, 9, 5474. [Google Scholar] [CrossRef]
- Cunha, M.S.; Tubaki, R.M.; de Menezes, R.M.T.; Pereira, M.; Caleiro, G.S.; Coelho, E.; del Castillo Saad, L.; de Azevedo Fernandes, N.C.C.; Guerra, J.M.; Nogueira, J.S. Possible Non-sylvatic transmission of yellow fever between non-human primates in São Paulo City, Brazil, 2017–2018. Sci. Rep. 2020, 10, 15751. [Google Scholar] [CrossRef]
- Fidelis, A.T.; de Godoy, S.A.P. Estrutura de um cerrado strico sensu na Gleba Cerrado Pé-de-Gigante, Santa Rita do Passa Quatro, SP. Acta Bot. Bras. 2003, 17, 531–539. [Google Scholar] [CrossRef]
- Marcondes, C.B.; Alencar, J. Revisão de mosquitos Haemagogus Williston (Diptera: Culicidae) do Brasil. Rev. Biomed. 2010, 21, 18. Available online: http://www.revbiomed.uady.mx/pdf/rb102139.pdf (accessed on 1 July 2022).
- Alencar, J.; Dégallier, N.; Hannart, A.; Silva, J.D.S.; Pacheco, J.B.; Guimarães, A.E. Circadian and Seasonal Preferences for Hematophagy among Haemagogus capricornii, Hg. janthinomys, and Hg. leucocelaenus (Diptera: Culicidae) in Different Regions of Brazil. J. Vector Ecol. 2008, 33, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Hendy, A.; Valério, D.; Fé, N.F.; Hernandez-Acosta, E.; Mendonça, C.; Andrade, E.; Pedrosa, I.; Costa, E.R.; Júnior, J.T.A.; Assunção, F.P.; et al. Microclimate and the vertical stratification of potential bridge vectors of mosquito-borne viruses captured by nets and ovitraps in a central amazonian forest bordering Manaus, Brazil. Sci. Rep. 2021, 11, 21129. [Google Scholar] [CrossRef] [PubMed]
- Câmara, D.C.P.; Codeço, C.T.; Ayllón, T.; Nobre, A.A.; Azevedo, R.C.; Ferreira, D.F.; da Silva Pinel, C.; Rocha, G.P.; Honório, N.A. Entomological surveillance of Aedes mosquitoes: Comparison of different collection methods in an endemic area in RIO de Janeiro, Brazil. Trop. Med. Infect. Dis. 2022, 7, 114. [Google Scholar] [CrossRef]
- Cano, M.E.; Marti, G.A.; Alencar, J.; Silva, S.O.F.; Micieli, M.V. Categorization by score of mosquito species (Diptera: Culicidae) related to yellow fever epizootics in Argentina. J. Med. Entomol. 2022, 59, 1766–1777. [Google Scholar] [CrossRef]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow fever. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- de Abreu, F.V.S.; Ribeiro, I.P.; Ferreira-de-Brito, A.; dos Santos, A.A.C.; de Miranda, R.M.; Bonelly, I.D.S.; Neves, M.S.A.S.; Bersot, M.I.; dos Santos, T.P.; Gomes, M.Q.; et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019, 8, 218–231. [Google Scholar] [CrossRef]
- Pinto, C.S.; Confalonieri, U.E.; Mascarenhas, B.M. Ecology of Haemagogus sp. and Sabethes sp.(Diptera: Culicidae) in relation to the microclimates of the Caxiuanã national forest, Pará, Brazil. Mem. Inst. Oswaldo Cruz 2009, 104, 592–598. [Google Scholar] [CrossRef]
- Confalonieri, U.E.C.; Neto, C.C. Diversity of mosquito vectors (Diptera: Culicidae) in Caxiuanã, Pará, Brazil. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 741273. [Google Scholar] [CrossRef][Green Version]
- Cardoso, J.D.C.; de Almeida, M.A.; dos Santos, E.; da Fonseca, D.F.; Sallum, M.A.; Noll, C.A.; Monteiro, H.A.D.O.; Cruz, A.C.; Carvalho, V.L.; Pinto, E.V.; et al. Yellow fever virus in Haemagogus leucocelaenus and Aedes serratus Mosquitoes, southern Brazil, 2008. Emerg. Infect. Dis. 2010, 16, 1918. [Google Scholar] [CrossRef]
- De Souza, R.P.; Petrella, S.; Coimbra, T.L.M.; Maeda, A.Y.; Rocco, I.M.; Bisordi, I.; Silveira, V.R.; Pereira, L.E.; Suzuki, A.; Silva, S.J.D.S.; et al. Isolation of yellow fever virus (YFV) from naturally infectied Haemagogus (Conopostegus) leucocelaenus (diptera, cukicudae) in São Paulo State, Brazil, 2009. Rev. Inst. Med. Trop. São Paulo 2011, 53, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Obara, M.T.; Monteiro, H.; de Paula, M.B.; Gomes, A.D.C.; Yoshizawa, M.A.C.; Lira, A.R.; Boffil, M.I.R.; de Carvalho, M.D.S.L. Infecção Natural de Haemagogus janthinomys e Haemagogus leucocelaenus pelo vírus da febre amarela no distrito federal, Brasil, 2007-2008. Epidemiol. E Serviços Saúde 2012, 21, 457–463. [Google Scholar] [CrossRef]
- Brasil, M.D.S. Guia de Vigilância do Culex Quinquefasciatus; Normas e Manuais Técnicos, 3rd ed.; Ministério da Saúde: Brasília, Brazil, 2011. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_culex_quinquefasciatus.pdf (accessed on 1 July 2022).
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P.J. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef] [PubMed]
- Tabbabi, A.; Daaboub, J. First investigation of deltamethrin pyrethroid susceptibility and resistance status of Anopheles labranchiae (Falleroni, 1926), potential malaria vector in Tunisia. Asian Pac. J. Trop. Biomed. 2017, 7, 1067–1070. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).