Quality and Integrated Service Delivery: A Cross-Sectional Study of the Effects of Malaria and Antenatal Service Quality on Malaria Intervention Use in Sub-Saharan Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Ethical Considerations
2.3. Quality Score Development
2.4. Statistics
3. Results
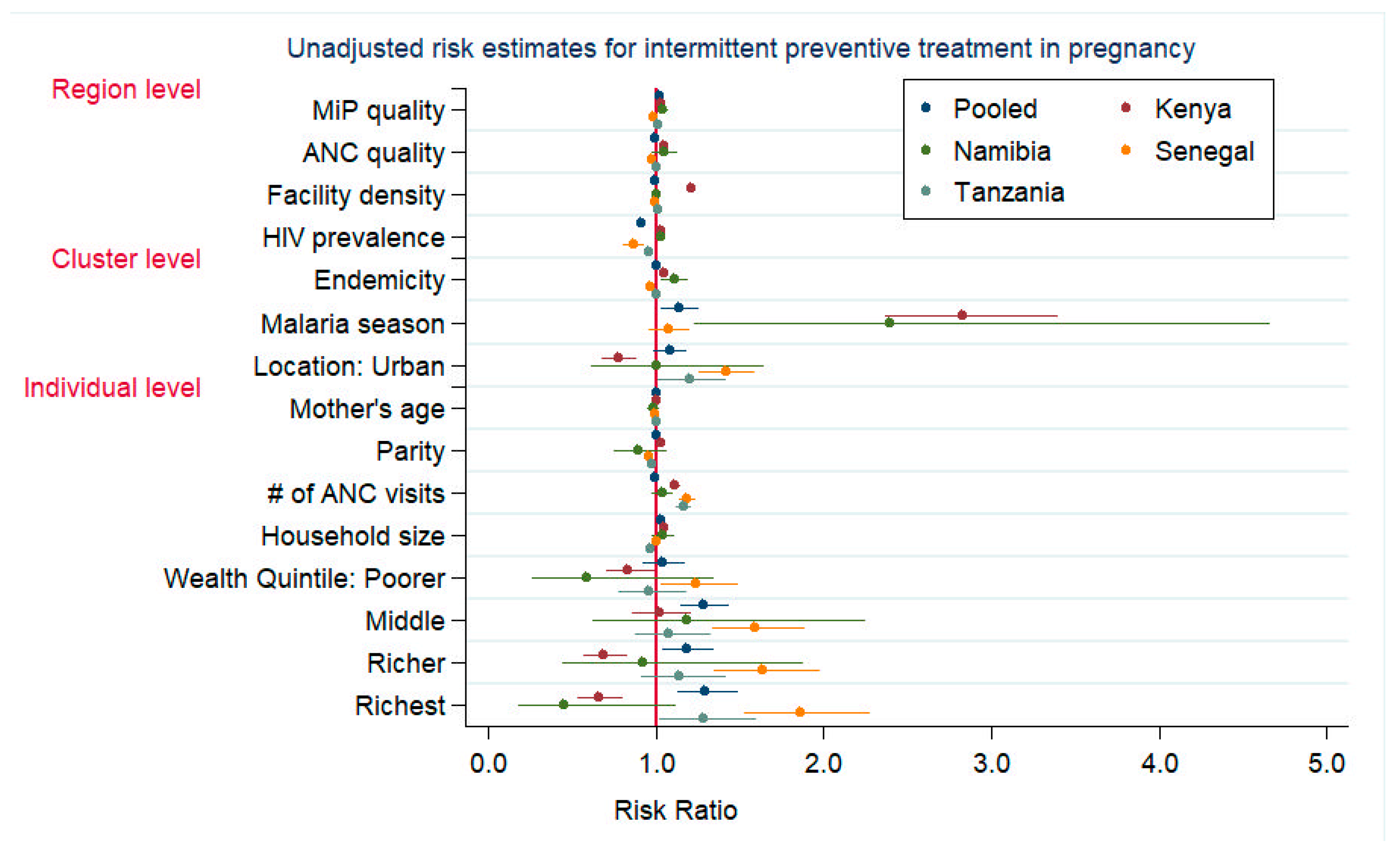
3.1. IPTp-2 Uptake in Pregnancy
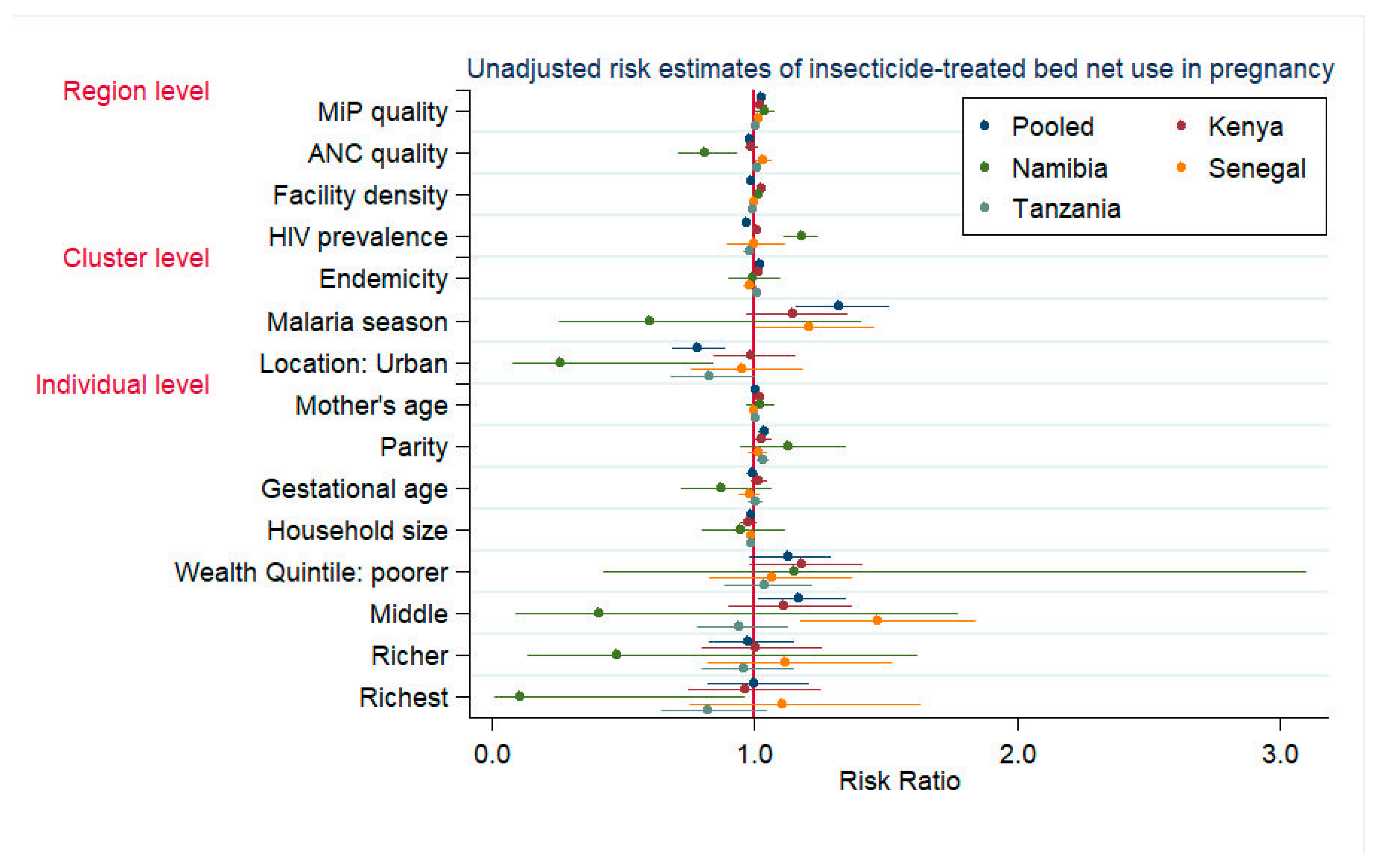
3.2. ITN Use in Pregnancy
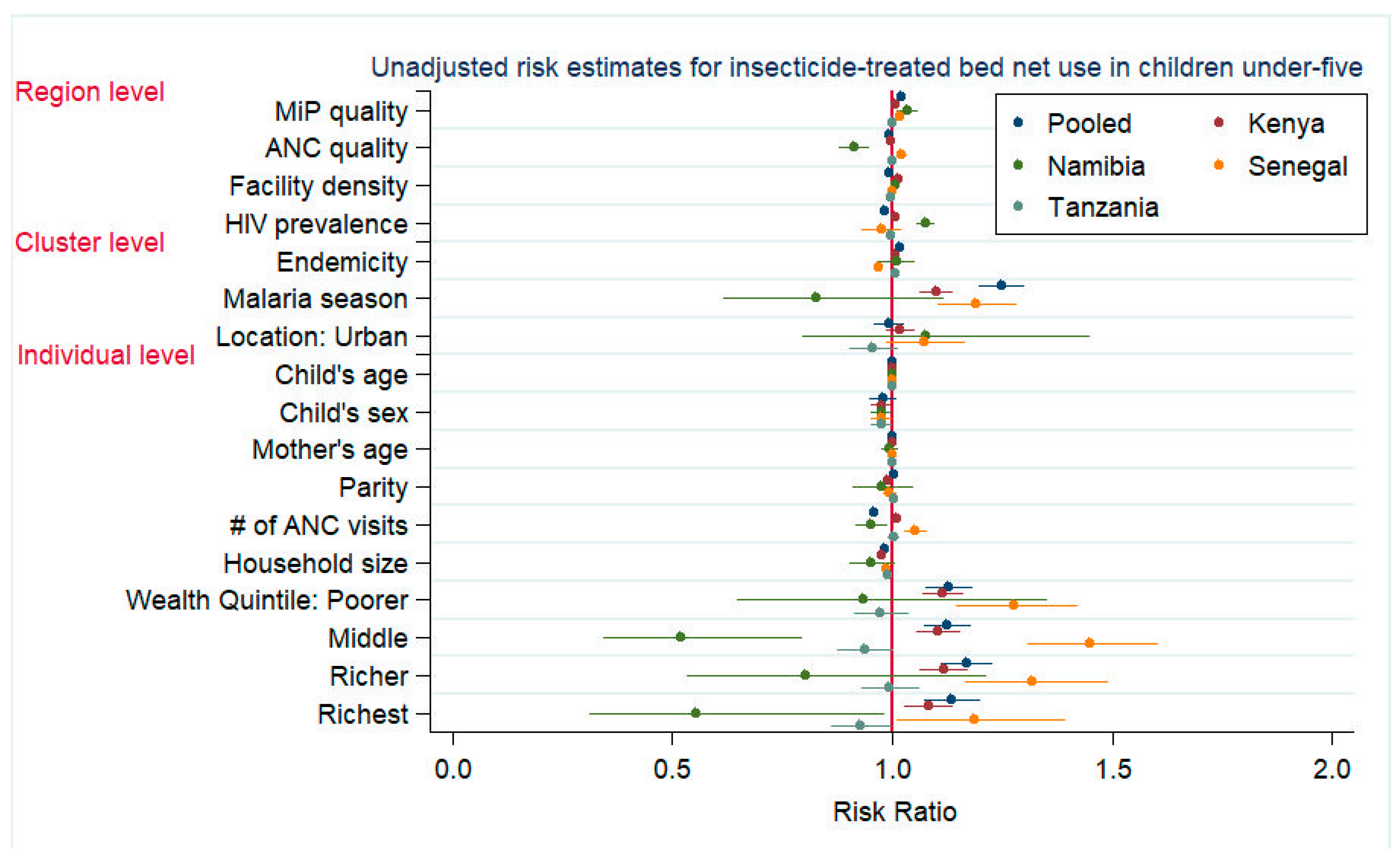
3.3. ITN Use in Children Under-Five
4. Discussion
4.1. Strengths and Limitations
4.2. Public Health Impact
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Hoffman, S.L.; Campbell, C.; White, N. Tropical Infectious Diseases: Principles, Pathogens and PracticeI, 3rd ed.; Weller, R.L., Guerrant, D.H., Walker, P.F., Eds.; W.B. Saunders: Edinburgh, UK, 2011; p. iv. [Google Scholar]
- World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019.
- The Global Malaria Action Plan: For a Malaria Free World; Roll Back Malaria Partnership: Geneva, Switzerland, 2008.
- Nosten, F.; Rogerson, S.; Beeson, J.; McGready, R.; Mutabingwa, T.; Brabin, B. Malaria in pregnancy and the endemicity spectrum: What can we learn? Trends Parasitol. 2004, 20, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.A.; Kyes, S.A. MicroReview: The role of Plasmodium falciparum var genes in malaria in pregnancy. Mol. Microbiol. 2004, 53, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Takem, E.N.; D’Alessandro, U. Malaria in pregnancy. Mediterr. J. Hematol. Infect. Dis. 2013, 5, e2013010. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, S.J.; Mwapasa, V.; Meshnick, S.R. Malaria in pregnancy: Linking immunity and pathogenesis to prevention. Am. J. Trop. Med. Hyg. 2007, 77, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chandrasiri, U.P.; Chua, C.L.L.; Umbers, A.J.; Chaluluka, E.; Glazier, J.D.; Rogerson, S.J.; Boeuf, P. Insight Into the Pathogenesis of Fetal Growth Restriction in Placental Malaria: Decreased Placental Glucose Transporter Isoform 1 Expression. J. Infect. Dis. 2014, 209, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.F. Tropical obstetrics and gynaecology. 1. Anaemia in pregnancy in tropical Africa. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 441–448. [Google Scholar] [CrossRef]
- Steketee, R.W.; Wirima, J.J.; Slutsker, L.; Heymann, D.L.; Breman, J.G. The problem of malaria and malaria control in pregnancy in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 1996, 55, 2–7. [Google Scholar] [CrossRef]
- World Malaria Report 2020: 20 Years of Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020.
- Tadesse Boltena, M.; El-Khatib, Z.; Kebede, A.S.; Asamoah, B.O.; Yaw, A.S.C.; Kamara, K.; Constant Assogba, P.; Tadesse Boltena, A.; Adane, H.T.; Hailemeskel, E.; et al. Malaria and Helminthic Co-Infection during Pregnancy in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 5444. [Google Scholar] [CrossRef]
- Chico, R.M.; Mayaud, P.; Ariti, C.; Mabey, D.; Ronsmans, C.; Chandramohan, D. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: A systematic review. JAMA 2012, 307, 2079–2086. [Google Scholar] [CrossRef]
- Eisele, T.P.; Larsen, D.A.; Anglewicz, P.A.; Keating, J.; Yukich, J.; Bennett, A.; Hutchinson, P.; Steketee, R.W. Malaria prevention in pregnancy, birthweight, and neonatal mortality: A meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect. Dis. 2012, 12, 942–949. [Google Scholar] [CrossRef]
- World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- USAID’S Vision for Health Systems Strengthening: 2015–2019; USAID: Washington, DC, USA, 2015.
- Haws, R.A.; Thomas, A.L.; Bhutta, Z.A.; Darmstadt, G.L. Impact of packaged interventions on neonatal health: A review of the evidence. Health Policy Plan. 2007, 22, 193–215. [Google Scholar] [CrossRef] [PubMed]
- Hatt, L.; Johns, B.; Connor, C.; Meline, M.; Kukla, M.; Moat, K. Impact of Health Systems Strengthening on Health; Abt Associates: Bethesda, MD, USA, 2015. [Google Scholar]
- Dudley, L.; Garner, P. Strategies for integrating primary health services in low- and middle-income countries at the point of delivery. Cochrane Database Syst. Rev. 2011, 2011, CD003318. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.S.; Gavin, L.; Galavotti, C. The integration of family planning with other health services: A literature review. Int. Perspect. Sex. Reprod. Health 2010, 36, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Lindegren, M.L.; Kennedy, C.E.; Bain-Brickley, D.; Azman, H.; Creanga, A.A.; Butler, L.M.; Spaulding, A.B.; Horvath, T.; Kennedy, G.E. Integration of HIV/AIDS services with maternal, neonatal and child health, nutrition, and family planning services. Cochrane Database Syst. Rev. 2012, 9, CD010119. [Google Scholar] [CrossRef]
- Spaulding, A.B.; Brickley, D.B.; Kennedy, C.; Almers, L.; Packel, L.; Mirjahangir, J.; Kennedy, G.; Collins, L.; Osborne, K.; Mbizvo, M. Linking family planning with HIV/AIDS interventions: A systematic review of the evidence. AIDS 2009, 23 (Suppl. S1), S79–S88. [Google Scholar] [CrossRef]
- WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; World Health Organization: Geneva, Switzerland, 2016.
- Campbell, O.M.R.; Graham, W.J. Strategies for reducing maternal mortality: Getting on with what works. Lancet 2006, 368, 1284–1299. [Google Scholar] [CrossRef]
- Countdown to 2015 Decade Report (2000–2010): Taking Stock of Maternal, Newborn and Child Survival; World Health Organization, UNICEF: Geneva, Switzerland, 2010.
- Maheu-Giroux, M.; Castro, M.C. Factors affecting providers’ delivery of intermittent preventive treatment for malaria in pregnancy: A five-country analysis of national service provision assessment surveys. Malar. J. 2014, 13, 440. [Google Scholar] [CrossRef]
- Hill, J.; Kazembe, P. Reaching the Abuja target for intermittent preventive treatment of malaria in pregnancy in African women: A review of progress and operational challenges. Trop. Med. Int. Health 2006, 11, 409–418. [Google Scholar] [CrossRef]
- Gross, K.; Alba, S.; Schellenberg, J.; Kessy, F.; Mayumana, I.; Obrist, B. The combined effect of determinants on coverage of intermittent preventive treatment of malaria during pregnancy in the Kilombero Valley, Tanzania. Malar. J. 2011, 10, 140. [Google Scholar] [CrossRef]
- Anders, K.; Marchant, T.; Chambo, P.; Mapunda, P.; Reyburn, H. Timing of intermittent preventive treatment for malaria during pregnancy and the implications of current policy on early uptake in north-east Tanzania. Malar. J. 2008, 7, 79. [Google Scholar] [CrossRef]
- Marchant, T.; Nathan, R.; Jones, C.; Mponda, H.; Bruce, J.; Sedekia, Y.; Schellenberg, J.; Mshinda, H.; Hanson, K. Individual, facility and policy level influences on national coverage estimates for intermittent preventive treatment of malaria in pregnancy in Tanzania. Malar. J. 2008, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Amoran, O.E.; Ariba, A.A.; Iyaniwura, C.A. Determinants of intermittent preventive treatment of malaria during pregnancy (IPTp) utilization in a rural town in Western Nigeria. Reprod. Health 2012, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.E.; Larson, E.; Twum-Danso, N.A.Y. Time for a quality revolution in global health. Lancet Glob. Health 2016, 4, e594–e596. [Google Scholar] [CrossRef]
- DHS Program. Data. Available online: http://dhsprogram.com/Data/ (accessed on 1 August 2016).
- Malaria Atlas Project. Data. Available online: http://www.map.ox.ac.uk/ (accessed on 1 August 2016).
- The Spatial Distribution of Plasmodium Falciparum Malaria Endemicity Map in 2010 Globally. Available online: http://www.map.ox.ac.uk/browse-resources/endemicity/Pf_mean/world/ (accessed on 20 August 2015).
- Quality of Care: A Process for Making Strategic Choices in Health Systems; World Health Organization: Geneva, Switzerland, 2006.
- Donabedian, A. The quality of care. How can it be assessed? JAMA 1988, 260, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Madhavan, S.; Bauhoff, S. Levels and variations in the quality of facility-based antenatal care in Kenya: Evidence from the 2010 service provision assessment. Health Policy Plan. 2016, 31, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Division, H.S.; Systems, I. Service Availability and Readiness Assessment Reference Manual: An Annual Monitoring System for Service Delivery, Version 2.2; World Health Organization: Geneva, Switzerland, 2015.
- Yoder, P.S.; Nsabagasani, X.; Eckert, E.; Moran, A.; Yé, Y. Perspectives of health care providers on the provision of intermittent preventive treatment in pregnancy in health facilities in Malawi. BMC Health Serv. Res. 2015, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Cherlin, E.J.; Allam, A.A.; Linnander, E.L.; Wong, R.; El-Toukhy, E.; Sipsma, H.; Krumholz, H.M.; Curry, L.A.; Bradley, E.H. Inputs to quality: Supervision, management, and community involvement in health facilities in Egypt in 2004. BMC Health Serv. Res. 2011, 11, 282. [Google Scholar] [CrossRef]
- Kamal-Yanni, M.M.; Potet, J.; Saunders, P.M. Scaling-up malaria treatment: A review of the performance of different providers. Malar. J. 2012, 11, 414. [Google Scholar] [CrossRef]
- Steinhardt, L.C.; Chinkhumba, J.; Wolkon, A.; Luka, M.; Luhanga, M.; Sande, J.; Oyugi, J.; Ali, D.; Mathanga, D.; Skarbinski, J. Quality of Malaria Case Management in Malawi: Results from a Nationally Representative Health Facility Survey. PLoS ONE 2014, 9, e89050. [Google Scholar] [CrossRef]
- Moon, S.; Pérez Casas, C.; Kindermans, J.-M.; de Smet, M.; von Schoen-Angerer, T. Focusing on Quality Patient Care in the New Global Subsidy for Malaria Medicines. PLoS Med. 2009, 6, e1000106. [Google Scholar] [CrossRef]
- WHO Policy Brief for the Implementation of Intermittent Preventive Treatment of Malaria in Pregnancy Using Sulfadoxine-Pyrimethamine (IPTp-SP); World Health Organization: Geneva, Switzerland, 2014.
- Kalilani-Phiri, L.V.; Lungu, D.; Coghlan, R. Knowledge and malaria treatment practices using artemisinin combination therapy (ACT) in Malawi: Survey of health professionals. Malar. J. 2011, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Conrad, P.; Schmid, G.; Tientrebeogo, J.; Moses, A.; Kirenga, S.; Neuhann, F.; Müller, O.; Sarker, M. Compliance with focused antenatal care services: Do health workers in rural Burkina Faso, Uganda and Tanzania perform all ANC procedures? Trop. Med. Int. Health 2012, 17, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Successes and Challenges for Malaria in Pregnancy Programming: A Three-Country Analysis; Maternal and Child Health Integrated Program, USAID: Washington, DC, USA, 2012.
- Hill, J.; D’Mello-Guyett, L.; Hoyt, J.; van Eijk, A.M.; ter Kuile, F.O.; Webster, J. Women’s Access and Provider Practices for the Case Management of Malaria during Pregnancy: A Systematic Review and Meta-Analysis. PLoS Med. 2014, 11, e1001688. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Hoyt, J.; van Eijk, A.M.; D’Mello-Guyett, L.; ter Kuile, F.O.; Steketee, R.; Smith, H.; Webster, J. Factors Affecting the Delivery, Access, and Use of Interventions to Prevent Malaria in Pregnancy in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS Med. 2013, 10, e1001488. [Google Scholar] [CrossRef] [PubMed]
- Ouma, P.O.; Van Eijk, A.M.; Hamel, M.J.; Sikuku, E.; Odhiambo, F.; Munguti, K.; Ayisi, J.G.; Kager, P.A.; Slutsker, L. The effect of health care worker training on the use of intermittent preventive treatment for malaria in pregnancy in rural western Kenya. Trop. Med. Int. Health 2007, 12, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Kayentao, K.; Diarra, S.; Diawara, S.I.; Haiballa, A.A.; Doumbo, O.K.; Hill, J. A qualitative health systems effectiveness analysis of the prevention of malaria in pregnancy with intermittent preventive treatment and insecticide treated nets in Mali. PLoS ONE 2013, 8, e65437. [Google Scholar] [CrossRef]
- Hill, J.; Dellicour, S.; Bruce, J.; Ouma, P.; Smedley, J.; Otieno, P.; Ombock, M.; Kariuki, S.; Desai, M.; Hamel, M.J.; et al. Effectiveness of Antenatal Clinics to Deliver Intermittent Preventive Treatment and Insecticide Treated Nets for the Control of Malaria in Pregnancy in Kenya. PLoS ONE 2013, 8, e64913. [Google Scholar] [CrossRef]
- Bryce, J.; Gouws, E.; Adam, T.; Black, R.E.; Schellenberg, J.A.; Manzi, F.; Victora, C.G.; Habicht, J.-P. Improving quality and efficiency of facility-based child health care through Integrated Management of Childhood Illness in Tanzania. Health Policy Plan. 2005, 20, i69–i76. [Google Scholar] [CrossRef]
- Ejigu, T.; Woldie, M.; Kifle, Y. Quality of antenatal care services at public health facilities of Bahir-Dar special zone, Northwest Ethiopia. BMC Health Serv. Res. 2013, 13, 443. [Google Scholar] [CrossRef]
- Mubyazi, G.M.; Byskov, J.; Magnussen, P.; Bygbjerg, I.C.; Ijumba, J.N.; Marero, M.; Mboera, L.E.G.; Molteni, F.; Bloch, P. Health facility-based data on women receiving sulphadoxine-pyrimethamine during pregnancy in Tanzania: Lessons to learn from a cross-sectional survey in Mkuranga and Mufindi districts and other national survey reports. Reprod. Health 2014, 11, 6. [Google Scholar] [CrossRef]
- Pell, C.; Meñaca, A.; Afrah, N.A.; Manda-Taylor, L.; Chatio, S.; Were, F.; Hodgson, A.; Hamel, M.J.; Kalilani, L.; Tagbor, H.; et al. Prevention and management of malaria during pregnancy: Findings from a comparative qualitative study in Ghana, Kenya and Malawi. Malar. J. 2013, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Zou, G. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef] [PubMed]
- StataCorpLP. Stata Multilevel Mixed-Effects Reference Manual Release 14; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Analyzing Correlated (Clustered) Data. Available online: http://www.ats.ucla.edu/stat/stata/library/cpsu.htm (accessed on 9 December 2016).
- Royston, P.; Sauerbrei, W. Handling interactions in Stata, especially with continuous predictors. In Proceedings of the German Stata Users’ Meeting, Berlin, Germany, 1 June 2012. [Google Scholar]
- Moineddin, R.; Matheson, F.I.; Glazier, R.H. A simulation study of sample size for multilevel logistic regression models. BMC Med. Res. Methodol. 2007, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, C. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Green, S.B. How Many Subjects Does it Take to do a Regression Analysis? Multivar. Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef]
- Okell, L.C.; Paintain, L.S.; Webster, J.; Hanson, K.; Lines, J. From intervention to impact: Modelling the potential mortality impact achievable by different long-lasting, insecticide-treated net delivery strategies. Malar. J. 2012, 11, 327. [Google Scholar] [CrossRef]
- Roman, E.; Wallon, M.; Brieger, W.; Dickerson, A.; Rawlins, B.; Agarwal, K. Moving malaria in pregnancy programs from neglect to priority: Experience from Malawi, Senegal, and Zambia. Glob. Health Sci. Pract. 2014, 2, 55–71. [Google Scholar] [CrossRef][Green Version]
- Nganda, R.Y.; Drakeley, C.; Reyburn, H.; Marchant, T. Knowledge of malaria influences the use of insecticide treated nets but not intermittent presumptive treatment by pregnant women in Tanzania. Malar. J. 2004, 3, 42. [Google Scholar] [CrossRef][Green Version]
- Kibusi, S.M.; Kimunai, E.; Hines, C.S. Predictors for uptake of intermittent preventive treatment of malaria in pregnancy (IPTp) in Tanzania. BMC Public Health 2015, 15, 540. [Google Scholar] [CrossRef]
- Hill, J.; Kayentao, K.; Achieng, F.; Diarra, S.; Dellicour, S.; Diawara, S.I.; Hamel, M.J.; Ouma, P.; Desai, M.; Doumbo, O.K.; et al. Access and use of interventions to prevent and treat malaria among pregnant women in Kenya and Mali: A qualitative study. PLoS ONE 2015, 10, e0119848. [Google Scholar] [CrossRef] [PubMed]
- Odjidja, E.N.; Gatasi, G.; Duric, P. Delivery of integrated infectious disease control services under the new antenatal care guidelines: A service availability and readiness assessment of health facilities in Tanzania. BMC Health Serv. Res. 2019, 19, 153. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.E.; Jakubowski, A.; Rabkin, M.; Kimanga, D.O.; Kundu, F.; Lim, T.; Lumumba, V.; Oluoch, T.; Robinson, K.A.; El-Sadr, W. Association Between HIV Programs and Quality of Maternal Health Inputs and Processes in Kenya. Am. J. Public Health 2015, 105, S207–S210. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Pullum, T.; Ametepi, P. How Family Planning Supply and the Service Environment Affect Contraceptive Use: Findings from Four East African Countries; ICF International: Calverton, MD, USA, 2012. [Google Scholar]
- Chan, M.; Lake, A.; Hansen, K. The early years: Silent emergency or unique opportunity? Lancet 2016, 389, 11–13. [Google Scholar] [CrossRef]
- Mills, A. Health policy and systems research: Defining the terrain; identifying the methods. Health Policy Plan. 2012, 27, 1–7. [Google Scholar] [CrossRef]
- Thiam, S.; Kimotho, V.; Gatonga, P. Why are IPTp coverage targets so elusive in sub-Saharan Africa? A systematic review of health system barriers. Malar. J. 2013, 12, 353. [Google Scholar] [CrossRef]
- Millar, K. The Need for Malaria Integration in Maternal and Newborn Health; Maternal Health Task Force: Boston, MA, USA, 2014. [Google Scholar]
- Mazigo, H.D.; Obasy, E.; Mauka, W.; Manyiri, P.; Zinga, M.; Kweka, E.J.; Mnyone, L.L.; Heukelbach, J. Knowledge, Attitudes, and Practices about Malaria and Its Control in Rural Northwest Tanzania. Malar. Res. Treat. 2010, 2010, 794261. [Google Scholar] [CrossRef]
- Sultana, M.; Sheikh, N.; Mahumud, R.A.; Jahir, T.; Islam, Z.; Sarker, A.R. Prevalence and associated determinants of malaria parasites among Kenyan children. Trop. Med. Health 2017, 45, 25. [Google Scholar] [CrossRef]
- de Jongh, T.; Gurol-Urganci, I.; Allen, E.; Jiayue Zhu, N.; Atun, R. Barriers and enablers to integrating maternal and child health services to antenatal care in low and middle income countries. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 549–557. [Google Scholar] [CrossRef]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Atkinson, P.M.; Snow, R.W. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 2005, 3, 81–90. [Google Scholar] [CrossRef]
- Ameyaw, E.K.; Adde, K.S.; Dare, S.; Yaya, S. Rural-urban variation in insecticide-treated net utilization among pregnant women: Evidence from 2018 Nigeria Demographic and Health Survey. Malar. J. 2020, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Aberese-Ako, M.; Magnussen, P.; Ampofo, G.D.; Tagbor, H. Health system, socio-cultural, economic, environmental and individual factors influencing bed net use in the prevention of malaria in pregnancy in two Ghanaian regions. Malar. J. 2019, 18, 363. [Google Scholar] [CrossRef] [PubMed]
- Takken, W.; Lindsay, S. Increased Threat of Urban Malaria from Anopheles stephensi Mosquitoes, Africa. Emerg. Infect. Dis. 2019, 25, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- de Jongh, T.E.; Gurol-Urganci, I.; Allen, E.; Zhu, N.J.; Atun, R. Integration of antenatal care services with health programmes in low- and middle-income countries: Systematic review. J. Glob. Health 2016, 6, 010403. [Google Scholar] [CrossRef] [PubMed]

| World Health Organization Framework | |||||||
|---|---|---|---|---|---|---|---|
| Effectiveness | Efficiency | Accessibility | Acceptability/ Patient-Centeredness | Safety | Equity | ||
| Donabedian Framework | Structure |
|
|
|
|
| |
| Process |
|
|
|
| |||
| Outcome | |||||||
| Country | SPA Year | DHS, MAP and Pop. Year | N | Outcome | Outcome Prevalence 1 | MiP Quality 2 | ANC Quality 2 | Malaria Endemicity 2 | Facility Density 2 |
|---|---|---|---|---|---|---|---|---|---|
| Kenya | 2010 | 2014 | 7861 | IPTp-2 | 1409 (17.92) | 54.47 (53.32, 54.51) | 76.32 (74.56, 79.18) | 9.02 (4.50, 18.91) | 12.01 (6.41, 14.64) |
| 662 | ITN in pregnancy | 470 (71.00) | 54.47 (53.32, 54.51) | 76.32 (74.56, 79.18) | 11.03 (5.90, 21.23) | 12.01 (6.41, 15.54) | |||
| 13,870 | ITN in children under-5 | 10,073 (72.62) | 54.47 (53.32, 54.51) | 76.32 (74.56, 79.18) | 11.09 (5.99, 21.38) | 12.01 (6.41, 15.54) | |||
| Namibia | 2009 | 2013 | 1639 | IPTp-2 | 69 (4.21) | 30.18 (16.95, 35.64) | 73.05 (69.59, 74.35) | 5.17 (0.00,7.64) | 131.14 (86.12, 184.40) |
| 207 | ITN in pregnancy | 23 (11.11) | 35.64 (30.18, 38.87) | 74.24 (70.03, 77.79) | 6.55 (2.37, 7.86) | 96.38 (86.12, 184.40) | |||
| 1570 | ITN in children under-5 | 250 (15.92) | 33.45 (27.64, 37.13) | 73.86 (69.59, 76.10) | 6.24 (1.83, 7.79) | 130.61 (92.90, 184.40) | |||
| Senegal | 2014 | 2013 | 2682 | IPTp-2 | 1048 (39.08) | 44.51 (42.81, 47.05) | 73.56 (71.23, 76.64) | 2.54 (2.05, 3.69) | 24.60 (20.53, 32.14) |
| 729 | ITN in pregnancy | 400 (54.87) | 44.51 (42.81, 47.05) | 75.09 (71.23, 76.64) | 2.46 (1.96, 3.39) | 24.60 (20.53, 33.34) | |||
| 5602 | ITN in children under-5 | 3172 (56.62) | 46.38 (42.81, 50.00) | 73.56 (71.23, 76.64) | 2.46 (1.99, 3.39) | 24.60 (20.53, 33.34) | |||
| Tanzania | 2006 | 2010 | 2993 | IPTp-2 | 990 (33.08) | 42.26 (38.97, 46.33) | 57.83 (53.70, 63.26) | 7.13 (4.34, 13.68) | 9.30 (6.48, 13.91) |
| 780 | ITN in pregnancy | 515 (66.03) | 42.70 (38.97, 46.33) | 56.64 (53.70, 63.66) | 8.12 (4.39, 14.95) | 9.16 (6.48, 13.91) | |||
| 6175 | ITN in children under-5 | 4300 (69.64) | 42.26 (38.97, 46.33) | 57.83 (53.70, 63.66) | 7.40 (4.35, 14.29) | 9.30 (6.48, 13.91) | |||
| Pooled | -- | -- | 15,175 | IPTp-2 | 3516 (23.17) | 52.94 (41.94, 54.51) | 74.56 (70.03, 76.90) | 6.69 (2.84, 13.03) | 14.62 (9.02, 20.96) |
| 2378 | ITN in pregnancy | 1408 (59.21) | 46.33 (41.38, 53.32) | 73.37 (63.66, 76.64) | 6.16 (2.65, 12.55) | 15.54 (9.30, 28.05) | |||
| 27,217 | ITN in children under-5 | 17,795 (65.38) | 52.94 (42.81, 54.51) | 74.56 (69.82, 76.90) | 7.30 (3.07, 15.45) | 13.91 (9.13, 20.53) |
| Kenya (n = 7861) | Namibia (n = 1639) | Senegal (n = 2682) | Tanzania (n = 2993) | Pooled (n = 15,175) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Adjusted Model 1 | Measures of Association | ||||||||||
| Individual Level | Number of ANC visits | 1.136 | (1.100, 1.173) | -- | -- | 1.169 | (1.085, 1.259) | 1.147 | (1.103, 1.192) | 1.127 | (1.092, 1.163) |
| Mother’s education (none) | -- | -- | -- | -- | Ref | Ref | Ref | Ref | -- | -- | |
| Primary | -- | -- | -- | -- | 1.131 | (0.856, 1.493) | 1.164 | (0.999, 1.355) | -- | -- | |
| Secondary or higher | -- | -- | -- | -- | 0.832 | (0.712, 0.972) | 1.553 | (1.203, 2.005) | -- | -- | |
| Cluster Level | Residence location (urban) | 1.014 | (0.844, 1.219) | 1.482 | (1.015, 2.164) | 1.187 | (1.056, 1.335) | 1.093 | (0.889, 1.345) | 1.133 | (1.031, 1.244) |
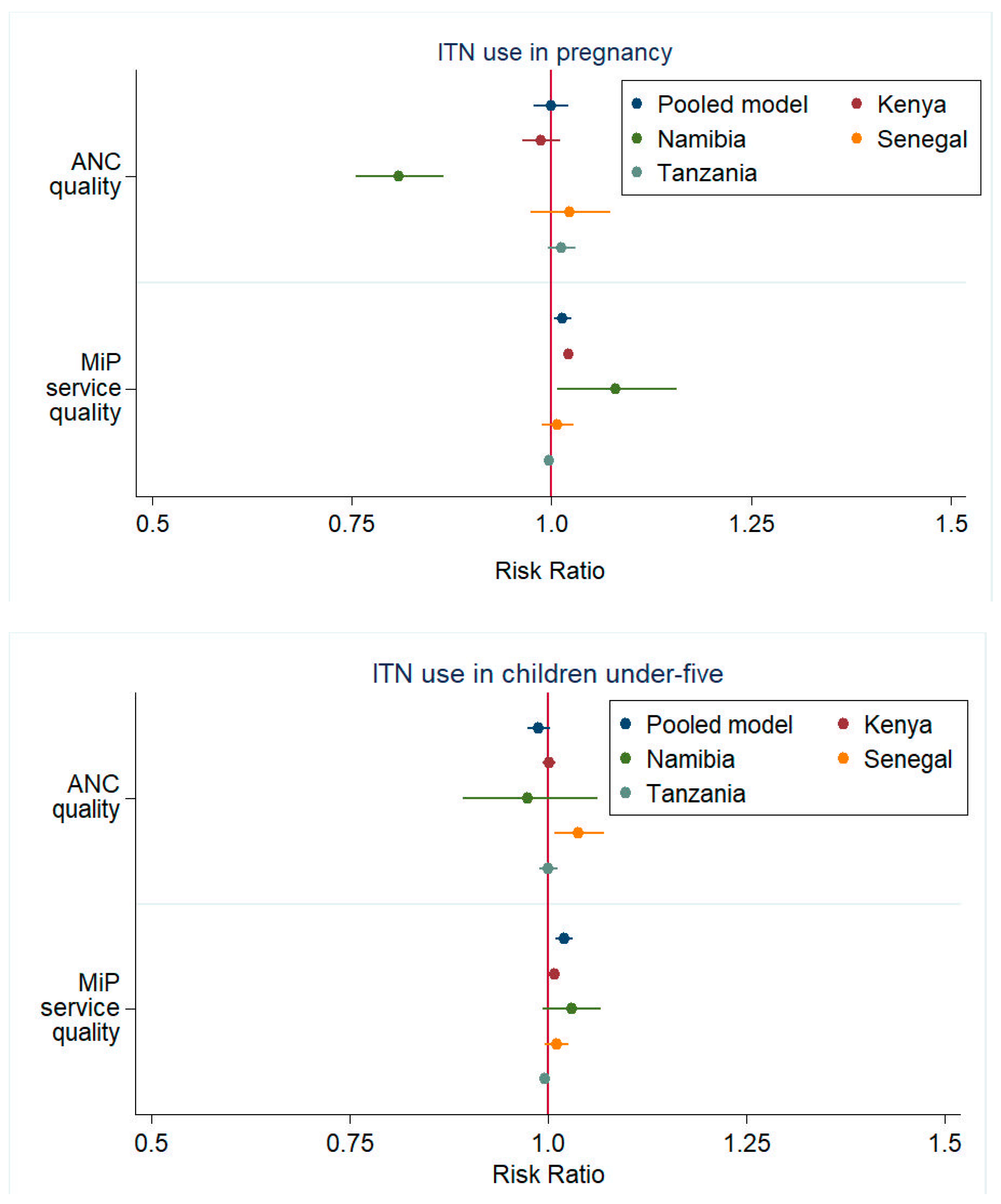
| Region Level | ANC quality 3 | 0.771 | (0.665, 0.892) | 0.935 | (0.807, 1.082) | 0.984 | (0.955, 1.015) | 0.984 | (0.970, 0.997) | 1.000 | (0.971, 1.031) |
| MiP quality 3 | 1.420 | (1.163, 1.733) | 1.080 | (1.037, 1.126) | 0.994 | (0.978, 1.010) | 1.026 | (1.016, 1.035) | 1.024 | (1.004, 1.045) | |
| Facility density 4 | 1.508 | (1.208, 1.883) | 0.993 | (0.986, 0.999) | -- | -- | 1.007 | (1.003, 1.011) | -- | -- | |
| HIV prevalence 5 | 1.028 | (0.928, 1.138) | -- | -- | -- | -- | 0.969 | (0.952, 0.987) | -- | -- | |
| ANC quality × MiP quality 6 | 0.939 | (0.893, 0.987) | -- | -- | -- | -- | 0.998 | (0.997, 0.999) | -- | -- | |
| Country Level | Country (Kenya) | -- | -- | -- | -- | -- | -- | -- | -- | Ref | Ref |
| Namibia | -- | -- | -- | -- | -- | -- | -- | -- | 0.437 | (0.148, 1.291) | |
| Senegal | -- | -- | -- | -- | -- | -- | -- | -- | 4.251 | (2.000, 9.036) | |
| Tanzania | -- | -- | -- | -- | -- | -- | -- | -- | 3.611 | (1.651, 7.897) | |
| Measures of Variation | |||||||||||
| Region level | 0.281 | (0.130, 0.607) | 0.255 | (0.105, 0.620) | 0.011 | (0.003, 0.045) | 0.011 | (0.002, 0.071) | 0.235 | (0.130, 0.427) | |
| Cluster level | 0.109 | (0.024, 0.494) | 0.064 | (0.000, 56,779.11) | -- | -- | 0.066 | (0.024, 0.180) | 0.070 | (0.033, 0.149) | |
| Model AIC | 58,891.522 | 607.330 | 3821.163 | 3852.737 | 15,921.980 | ||||||
| Empty Model 2 | Measures of Variation | ||||||||||
| Region level | 1.244 | (0.642, 2.410) | 0.458 | (0.233, 0.899) | 0.043 | (0.019, 0.099) | 0.052 | (0.024, 0.115) | 1.019 | (0.668, 1.557) | |
| Cluster level | 0.117 | (0.020, 0.681) | 0.121 | (0.000, 74.279) | 0.003 | (0.00, 1059.45) | 0.092 | (0.042, 0.199) | 0.094 | (0.050, 0.178) | |
| Model AIC | 5958.706 | 607.0791 | 3887.345 | 3899.29 | 16,142.320 | ||||||
| Kenya (n = 662) | Namibia (n = 586) | Senegal (n = 729) | Tanzania (n = 780) | Pooled (n = 2378) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Adjusted Model 1 | Measures of Association | ||||||||||
| Individual Level | Mother’s age (in years) | 1.019 | (1.014, 1.025) | -- | -- | -- | -- | -- | -- | -- | -- |
| Parity | -- | -- | -- | -- | -- | -- | 1.035 | (1.011, 1.060) | -- | -- | |
| Household size | -- | -- | -- | -- | -- | -- | 0.978 | (0.963, 0.992) | -- | -- | |
| Cluster Level | Malaria endemicity | 1.012 | (1.007, 1.018) | -- | -- | -- | -- | 1.009 | (1.003, 1.016) | 1.009 | (1.004, 1.014) |
| Residence location (urban) | 1.174 | (1.062, 1.298) | 0.393 | (0.178, 0.869) | 1.068 | (0.844, 1.352) | 0.825 | (0.686, 0.993) | 0.985 | (0.873, 1.112) | |
| Region Level | ANC quality 3 | 0.987 | (0.964, 1.011) | 0.809 | (0.755, 0.866) | 1.022 | (0.974, 1.073) | 1.013 | (0.996, 1.030) | 0.999 | (0.978, 1.021) |
| MiP quality 3 | 1.022 | (1.018, 1.026) | 1.080 | (1.007, 1.157) | 1.008 | (0.988, 1.028) | 0.997 | (0.992, 1.003) | 1.014 | (1.003, 1.025) | |
| Facility density 4 | 1.034 | (1.013, 1.054) | -- | -- | -- | -- | -- | -- | -- | -- | |
| ANC quality × residence location 5 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| ANC quality × MiP quality 5 | -- | -- | -- | -- | 1.006 | (1.001, 1.012) | -- | -- | 1.001 | (1.001, 1.002) | |
| Country Level | Country (Kenya) | -- | -- | -- | -- | -- | -- | -- | -- | Ref | Ref |
| Namibia | -- | -- | -- | -- | -- | -- | -- | -- | 0.230 | (0.110, 0.482) | |
| Senegal | -- | -- | -- | -- | -- | -- | -- | -- | 0.957 | (0.771, 1.188) | |
| Tanzania | -- | -- | -- | -- | -- | -- | -- | -- | 1.210 | (0.788, 1.858) | |
| Measures of Variation | |||||||||||
| Region level | 0.000 | (0.000, 0.000) | 0.000 | (0.000, 0.000) | 0.938 | (0.006, 0.219) | 0.000 | (0.000, 0.000) | 0.032 | (0.010, 0.103) | |
| Cluster level | -- | -- | -- | -- | 0.060 | (0.003, 1.130) | -- | -- | 0.025 | (0.001, 0.736) | |
| Model AIC | 1191.628 | 128.191 | 1096.993 | 1503.685 | 4840.075 | ||||||
| Empty Model 2 | Measures of Variation | ||||||||||
| Region level | 0.027 | (0.007, 0.109) | 0.761 | (0.169, 3.419) | 0.048 | (0.014, 0.163) | 0.000 | (0.000, 1.3 × 10155) | 0.048 | (0.171, 1.324) | |
| Cluster level | -- | -- | -- | -- | 0.063 | (0.004, 1.016) | -- | -- | 0.029 | (0.119, 0.692) | |
| Model AIC | 1204.449 | 140.769 | 1090.682 | 1506.818 | 2774.930 | ||||||
| Kenya (n = 13,870) | Namibia (n = 1570) | Senegal (n = 3729) | Tanzania (n = 6175) | Pooled (n = 27,217) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Adjusted Model 1 | Measures of Association | ||||||||||
| Individual Level | Mother’s education (none) | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Primary | -- | -- | -- | -- | -- | -- | -- | -- | 1.030 | (0.993, 1.068) | |
| Secondary or higher | -- | -- | -- | -- | -- | -- | -- | -- | 1.131 | (1.063, 1.204) | |
| Number of ANC visits | -- | -- | -- | -- | 1.025 | (1.000, 1.051) | -- | -- | -- | -- | |
| Household size | -- | -- | -- | -- | -- | -- | 0.981 | (0.969, 0.994) | 0.981 | (0.976, 0.986) | |
| Cluster Level | Malaria endemicity | 1.003 | (1.000, 1.007) | -- | -- | -- | -- | 1.005 | (1.002, 1.009) | 1.005 | (1.001, 1.009) |
| Survey timing | 1.044 | (1.003, 1.087) | -- | -- | -- | -- | -- | -- | -- | -- | |
| Residence location (urban) | 1.108 | (1.071, 1.146) | 1.293 | (0.964, 1.734) | 1.208 | (1.096, 1.331) | 1.003 | (0.963, 1.045) | 1.113 | (1.056, 1.174) | |
| Region Level | ANC quality 3 | 0.996 | (0.988, 1.004) | 0.974 | (0.893, 1.062) | 1.026 | (0.991, 1.062) | 0.998 | (0.985, 1.010) | 0.988 | (0.975, 1.002) |
| MiP quality 3 | 1.024 | (1.015, 1.033) | 1.029 | (0.993, 1.067) | 1.019 | (1.001, 1.037) | 1.001 | (0.995, 1.007) | 1.021 | (1.010, 1.032) | |
| Facility density 4 | 1.020 | (1.017, 1.023) | -- | -- | -- | -- | -- | -- | -- | -- | |
| HIV prevalence 5 | -- | -- | 1.081 | (1.031, 1.133) | -- | -- | -- | -- | -- | -- | |
| Endemicity × MiP quality 6 | 1.001 | (1.001, 1.002) | -- | -- | -- | -- | -- | -- | -- | -- | |
| ANC quality × location 6 | 0.988 | (0.981, 0.994) | -- | -- | 0.944 | (0.924, 0.964) | -- | -- | -- | -- | |
| ANC quality × MiP quality 6 | -- | -- | -- | -- | -- | -- | -- | -- | 1.001 | (1.001, 1.002) | |
| Country Level | Country (Kenya) | -- | -- | -- | -- | -- | -- | -- | -- | Ref | Ref |
| Namibia | -- | -- | -- | -- | -- | -- | -- | -- | 0.279 | (0.174, 0.448) | |
| Senegal | -- | -- | -- | -- | -- | -- | -- | -- | 1.113 | (0.899, 1.379) | |
| Tanzania | -- | -- | -- | -- | -- | -- | -- | -- | 1.051 | (0.780, 1.418) | |
| Measures of Variation | |||||||||||
| Region level | 0.000 | (0.000, 0.000) | 0.106 | (0.031, 0.362) | 0.035 | (0.011, 0.110) | 0.015 | (0.008, 0.026) | 0.063 | (0.034, 0.119) | |
| Cluster level | -- | -- | 0.236 | (0.043, 1.303) | 0.062 | (0.025, 0.154) | -- | -- | 0.023 | (0.008, 0.066) | |
| Model AIC | 25,880.360 | 1255.409 | 5719.808 | 11,985.670 | 42,520.680 | ||||||
| Empty Model 2 | Measures of Variation | ||||||||||
| Region level | 0.005 | (0.003, 0.012) | 0.524 | (0.220, 1.247) | 0.052 | (0.024, 0.114) | 0.018 | (0.011, 0.031) | 0.652 | (0.340, 1.250) | |
| Cluster level | -- | -- | 0.223 | (0.042, 1.168) | 0.149 | (0.087, 0.254) | -- | -- | 0.028 | (0.010, 0.073) | |
| Model AIC | 25,927.490 | 1263.894 | 8458.662 | 12,005.910 | 42,803.670 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.H.; Mancuso, J.D.; Koehlmoos, T.; Stewart, V.A.; Bennett, J.W.; Olsen, C. Quality and Integrated Service Delivery: A Cross-Sectional Study of the Effects of Malaria and Antenatal Service Quality on Malaria Intervention Use in Sub-Saharan Africa. Trop. Med. Infect. Dis. 2022, 7, 363. https://doi.org/10.3390/tropicalmed7110363
Lee EH, Mancuso JD, Koehlmoos T, Stewart VA, Bennett JW, Olsen C. Quality and Integrated Service Delivery: A Cross-Sectional Study of the Effects of Malaria and Antenatal Service Quality on Malaria Intervention Use in Sub-Saharan Africa. Tropical Medicine and Infectious Disease. 2022; 7(11):363. https://doi.org/10.3390/tropicalmed7110363
Chicago/Turabian StyleLee, Elizabeth H., James D. Mancuso, Tracey Koehlmoos, V. Ann Stewart, Jason W. Bennett, and Cara Olsen. 2022. "Quality and Integrated Service Delivery: A Cross-Sectional Study of the Effects of Malaria and Antenatal Service Quality on Malaria Intervention Use in Sub-Saharan Africa" Tropical Medicine and Infectious Disease 7, no. 11: 363. https://doi.org/10.3390/tropicalmed7110363
APA StyleLee, E. H., Mancuso, J. D., Koehlmoos, T., Stewart, V. A., Bennett, J. W., & Olsen, C. (2022). Quality and Integrated Service Delivery: A Cross-Sectional Study of the Effects of Malaria and Antenatal Service Quality on Malaria Intervention Use in Sub-Saharan Africa. Tropical Medicine and Infectious Disease, 7(11), 363. https://doi.org/10.3390/tropicalmed7110363





