The Indispensability of Snail Control for Accelerating Schistosomiasis Elimination: Evidence from Zanzibar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Design
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. Basic Information
3.2. Regression Analysis of the Relationship between MDA and Prevalence
3.3. JRM Analysis of Trends in Prevalence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- King, C.H. Parasites and poverty: The case of schistosomiasis. Acta Trop. 2010, 113, 95–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utzinger, J.; N’Goran, E.K.; Caffrey, C.R.; Keiser, J. From innovation to application: Social–ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011, 120, S121–S137. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Wang, X.Y.; Juma, S.; Li, W.; Suleman, M.; Muhsin, M.A.; He, J.; Yang, K. Potential risk of colonization of Bulinus globosus in the mainland of China under climate change. Infect. Dis. Poverty 2022, 11, 52. [Google Scholar] [CrossRef]
- Chuah, C.; Gobert, G.N.; Latif, B.; Heo, C.C.; Leow, C.Y. Schistosomiasis in Malaysia: A review. Acta Trop. 2019, 190, 137–143. [Google Scholar] [CrossRef]
- McManus, D.P.; Loukas, A. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 2008, 21, 225–242. [Google Scholar] [CrossRef] [Green Version]
- Ross, A.G.P.; Bartley, P.B.; Sleigh, A.C.; Olds, G.R.; Li, Y.; Williams, G.M. Schistosomiasis. N. Engl. J. Med. 2002, 346, 1212–1220. [Google Scholar] [CrossRef] [Green Version]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Khurana, S.; Dubey, M.; Malla, N. Association of parasitic infections and cancers. Indian J. Med. Microbiol. 2005, 23, 74–79. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.F.; Yosery, A.; Narooz, S.; Esmat, G.; El Hak, S.; Nasif, S. Is Schistosoma mansoni replacing Schistosoma haematobium in the Fayoum? Am. J. Trop. Med. Hyg. 1993, 49, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Mansfield-Aders, W. Zanzibar protectorate: Annual Report on the Medical, Sanitary and Biological Divisions for the Year 1927. In Zanzibar: Sperman B; Government Printer: Zanzibar, Tanzania, 1927. [Google Scholar]
- McCarthy, D.D. Medical notes from Weti, Pemba. Trans. R. Soc. Trop. Med. Hyg. 1930, 23, 401–412. [Google Scholar] [CrossRef]
- Webbe, G. The transmission of Schistosoma haematobium in an area of Lake Province, Tanganyika. Bull. World Health Organ. 1962, 27, 59–85. [Google Scholar]
- Stothard, J.R.; Rollinson, D. Molecular characterization of Bulinus globosus and B. nasutus on Zanzibar, and an investigation of their roles in the epidemiology of Schistosoma haematobium. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 353–357. [Google Scholar] [CrossRef]
- Guidi, A.; Andolina, C.; Makame Ame, S.; Albonico, M.; Cioli, D.; Juma Haji, H. Praziquantel efficacy and long-term appraisal of schistosomiasis control in Pemba Island. Trop. Med. Int. Health 2010, 15, 614–618. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L. Praziquantel. Parasitol. Res. 2003, 90, S3–S9. [Google Scholar] [CrossRef]
- Bobyreva, N.S.; Korneeva, Y.A.; Degteva, G.N. Analysis of parasitological situation in nenets autonomous district. Gig. Sanit. 2016, 95, 157–162. [Google Scholar] [CrossRef]
- Civitello, D.J.; Fatima, H.; Johnson, L.R.; Nisbet, R.M.; Rohr, J.R. Bioenergetic theory predicts infection dynamics of human schistosomes in intermediate host snails across ecological gradients. Ecol. Lett. 2018, 21, 692–701. [Google Scholar] [CrossRef]
- Yasin, M.G.; Alim, M.A.; Anisuzzaman Ahasan, S.A.; Munsi, M.N.; Chowdhury, E.H.; Hatta, T.; Tsuji, N.; Mondal, M.M.H. Trematode infections in farm animals and their vector snails in Saint Martin’s Island, the southeastern offshore area of Bangladesh in the Bay of Bengal. J. Vet. Med. Sci. 2018, 80, 684–688. [Google Scholar] [CrossRef] [Green Version]
- King, C.H.; Sutherland, L.J.; Bertsch, D. Systematic Review and Meta-analysis of the Impact of Chemical-Based Mollusciciding for Control of Schistosoma mansoni and S. haematobium Transmission. PLoS Negl. Trop. Dis. 2015, 9, e0004290. [Google Scholar] [CrossRef]
- Andrews, P.; Thyssen, J.; Lorke, D. The biology and toxicology of molluscicides, Bayluscide. Pharmacol. Ther. 1982, 19, 245–295. [Google Scholar] [CrossRef]
- Gray, D.J.; Li, Y.S.; Williams, G.M.; Zhao, Z.Y.; Harn, D.A.; Li, S.M.; Ren, M.Y.; Feng, Z.; Guo, F.Y.; Guo, J.G.; et al. A multi-component integrated approach for the elimination of schistosomiasis in the People’s Republic of China: Design and baseline results of a 4-year cluster-randomised intervention trial. Int. J. Parasitol. 2014, 44, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Seto, E.Y.W.; Remais, J.V.; Zhong, B.; Yang, C.; Hubbard, A.; Davis, G.M.; Gu, X.; Qiu, D.; Spear, R.C. Environmental effects on parasitic disease transmission exemplified by schistosomiasis in western China. Proc. Natl. Acad. Sci. USA 2007, 104, 7110–7115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knopp, S.; Mohammed, K.A.; Ali, S.M.; Khamis, I.S.; Ame, S.M.; Albonico, M.; Gouvras, A.; Fenwick, A.; Savioli, L.; Colley, D.G.; et al. Study and implementation of urogenital schistosomiasis elimination in Zanzibar (Unguja and Pemba islands) using an integrated multidisciplinary approach. BMC Public Health 2012, 12, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knopp, S.; Stothard, J.R.; Rollinson, D.; Mohammed, K.A.; Khamis, I.S.; Marti, H.; Utzinger, J. From morbidity control to transmission control: Time to change tactics against helminths on Unguja Island, Zanzibar. Acta Trop. 2013, 128, 412–422. [Google Scholar] [CrossRef]
- Wang, X.Y.; He, J.; Juma, S.; Kabole, F.; Guo, J.-G.; Dai, J.-R. Efficacy of China-made praziquantel for treatment of Schistosomiasis haematobium in Africa: A randomized controlled trial. PLoS Negl. Trop. Dis. 2019, 13, e0007238. [Google Scholar] [CrossRef] [Green Version]
- Ajari, E.E. China’s Input Ensured the Elimination of Schistosomiasis from Zanzibar’s Pemba Island. China Global South Project. 2020. Available online: https://chinaglobalsouth.com/analysis/chinas-input-ensured-the-elimination-of-schistosomiasis-from-zanzibars-pemba-island/ (accessed on 12 July 2022).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Li, H.Z.; Du, L.B. Application of Joinpoint regression model in cancer epidemiological time trend analysis. Chin. J. Prev. Med. 2020, 54, 5. [Google Scholar]
- Goatly, K.D.; Jordan, P. Schistosomiasis in Zanzibar and Pemba. East Afr. Med. J. 1965, 42, 1–9. [Google Scholar]
- Knopp, S.; Person, B.; Ame, S.M.; Mohammed, K.A.; Ali, S.M.; Khamis, I.S. Elimination of schistosomiasis transmission in Zanzibar: Baseline findings before the onset of a randomized intervention trial. PLoS Negl. Trop. Dis. 2013, 7, e2474. [Google Scholar] [CrossRef]
- Zanzibar. Revolutionary Government of Zanzibar. 2018. Available online: http://zanzibar.go.tz (accessed on 15 May 2022).
- Okoyo, C.; Simiyu, E.; Njenga, S.M.; Mwandawiro, C. Comparing the performance of circulating cathodic antigen and Kato-Katz techniques in evaluating Schistosoma mansoni infection in areas with low prevalence in selected counties of Kenya: A cross-sectional study. BMC Public Health 2018, 18, 478. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Yang, J.H. Effect of schistosomiasis japonica on the development of gastric and colorectal cancer. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2020, 32, 148–153. [Google Scholar]
- Zeng, S.Q.; Li, Y.; Liu, J.; Xie, S.L.; Fu, X.B.; Long, Q.S. Joinpoint regression model method and application for comparative analysis of trend change characteristics of two sets of sequence data. Chin. J. Health Stat. 2021, 38, 5. [Google Scholar]
- Xiao, Y.; Zhong, C.H.; Wei, F.H.; Dai, L.F.; Yang, J.J.; Chen, Y.Y. Epidemiological trends for human schistosomiasis prevalence in Hubei Province from 2004 to 2018 based on Joinpoint regression analysis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2022, 34, 122–127. [Google Scholar] [PubMed]
- Cui, Y.; Mubarik, S.; Li, R.; Nawsherwan Yu, C. Trend dynamics of thyroid cancer incidence among China and the U.S. adult population from 1990 to 2017: A joinpoint and age-period-cohort analysis. BMC Public Health 2021, 21, 624. [Google Scholar]
- Yv, K.Z. A Case Study on Carrying out Regression Analysis by Means of SPSS. J. Chongqing Univ. Technol. (Nat. Sci.) 2002, 2, 29–34. [Google Scholar]
- Chen, Y. Spatial Autocorrelation Approaches to Testing Residuals from Least Squares Regression. PLoS ONE 2016, 11, e0146865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, L.; Sylivester, Y.D.; Zerefa, M.D.; Maru, M.; Allan, F.; Zewge, F.; Emery, A.M.; Kinung’Hi, S.; Templeton, M.R. Chlorination of Schistosoma mansoni cercariae. PLoS Negl. Trop. Dis. 2020, 14, e0008665. [Google Scholar] [CrossRef]
- Mbereko, A.; Chimbari, M.J.; Manyangadze, T.; Mukaratirwa, S. Knowledge and perceptions of schistosomiasis, a water-borne disease, in two semi-arid rural areas of South Africa (Ndumo) and Zimbabwe (Ntalale). Food Waterborne Parasitol. 2020, 21, e00091. [Google Scholar] [CrossRef]
- Knopp, S.; Person, B.; Ame, S.M.; Ali, S.M.; Muhsin, J.; Juma, S.; Khamis, I.S.; Rabone, M.; Blair, L.; Fenwick, A.; et al. Praziquantel coverage in schools and communities targeted for the elimination of urogenital schistosomiasis in Zanzibar: A cross-sectional survey. Parasit Vectors 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. WHO Guideline on Control and Elimination of Human Schistosomiasis. 2022. Available online: https://www.who.int/publications/i/item/9789240041608?msclkid=66e125e4aa9911eca97802b2699b0852 (accessed on 20 June 2022).
- Kloos, H. Human behavior, health education and schistosomiasis control: A review. Soc. Sci. Med. 1995, 40, 1497–1511. [Google Scholar] [CrossRef]
- Vincenzi, R.; Neto, J.S.; Fonseca, E.A.; Pugliese, V.; Leite, K.R.M.; Benavides, M.R.; Cândido, H.L.; Porta, G.; Miura, I.K.; Pugliese, R.; et al. Schistosoma mansoni infection in the liver graft: The impact on donor and recipient outcomes after transplantation. Liver Transpl. 2011, 17, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Kapungu, N.N.; Li, X.; Nhachi, C.; Masimirembwa, C.; Thelingwani, R.S. In vitro and in vivo human metabolism and pharmacokinetics of S- and R-praziquantel. Pharmacol. Res. Perspect. 2020, 8, e00618. [Google Scholar] [CrossRef]
- Guo, J.G. History and current situation of comprehensive management of schistosomiasis in China. Chin. J. Prev. Med. 2006, 40, 4. [Google Scholar]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Lafferty, K.D.; Kuris, A.M.; Hsieh, M.H.; De Leo, G.A. To Reduce the Global Burden of Human Schistosomiasis, Use ‘Old Fashioned’ Snail Control. Trends Parasitol. 2018, 34, 23–40. [Google Scholar] [CrossRef]
- Sokolow, S.H.; Wood, C.L.; Jones, I.J.; Swartz, S.J.; Lopez, M.; Hsieh, M.H.; Lafferty, K.D.; Kuris, A.M.; Rickards, C.; De Leo, G.A. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl. Trop. Dis. 2016, 10, e0004794. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.H.; Shen, J.X.; Liu, Z.H. A retrospective research of osteoporotic diagnosis standards in China. Chin. J. Osteoporos. 2004, 10, 255–262. [Google Scholar]
- Liu, R.; Dong, H.-F.; Jiang, M.-S. The new national integrated strategy emphasizing infection sources control for schistosomiasis control in China has made remarkable achievements. Parasitol. Res. 2013, 112, 1483–1491. [Google Scholar] [CrossRef]
- Wu, L.-L.; Hu, H.-H.; Zhang, X.; Zhou, X.-N.; Jia, T.-W.; Wang, C.; Hong, Z.; Xu, J. Cost-effectiveness analysis of the integrated control strategy for schistosomiasis japonica in a lake region of China: A case study. Infect. Dis. Poverty 2021, 10, 79. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, Y.B.; Song, X.X.; Li, S.Z.; Zhong, B.; Wang, T.P.; Bergquist, R.; Zhou, X.N.; Jiang, Q.W. Chapter Nine—Integrated Control Strategy of Schistosomiasis in The People’s Republic of China: Projects Involving Agriculture, Water Conservancy, Forestry, Sanitation and Environmental Modification. Adv. Parasitol. 2016, 92, 237–268. [Google Scholar] [PubMed]
- Wang, X.Y.; Zhang, J.F.; Xing, Y.T.; Abdalla, F.M. The Novel Strategy of China-Zanzibar Cooperation Project of Schistosomiasis Control: The Integrated Strategy. In Sino-African Cooperation for Schistosomiasis Control in Zanzibar; Springer: Berlin/Heidelberg, Germany, 2021; pp. 213–234. [Google Scholar]


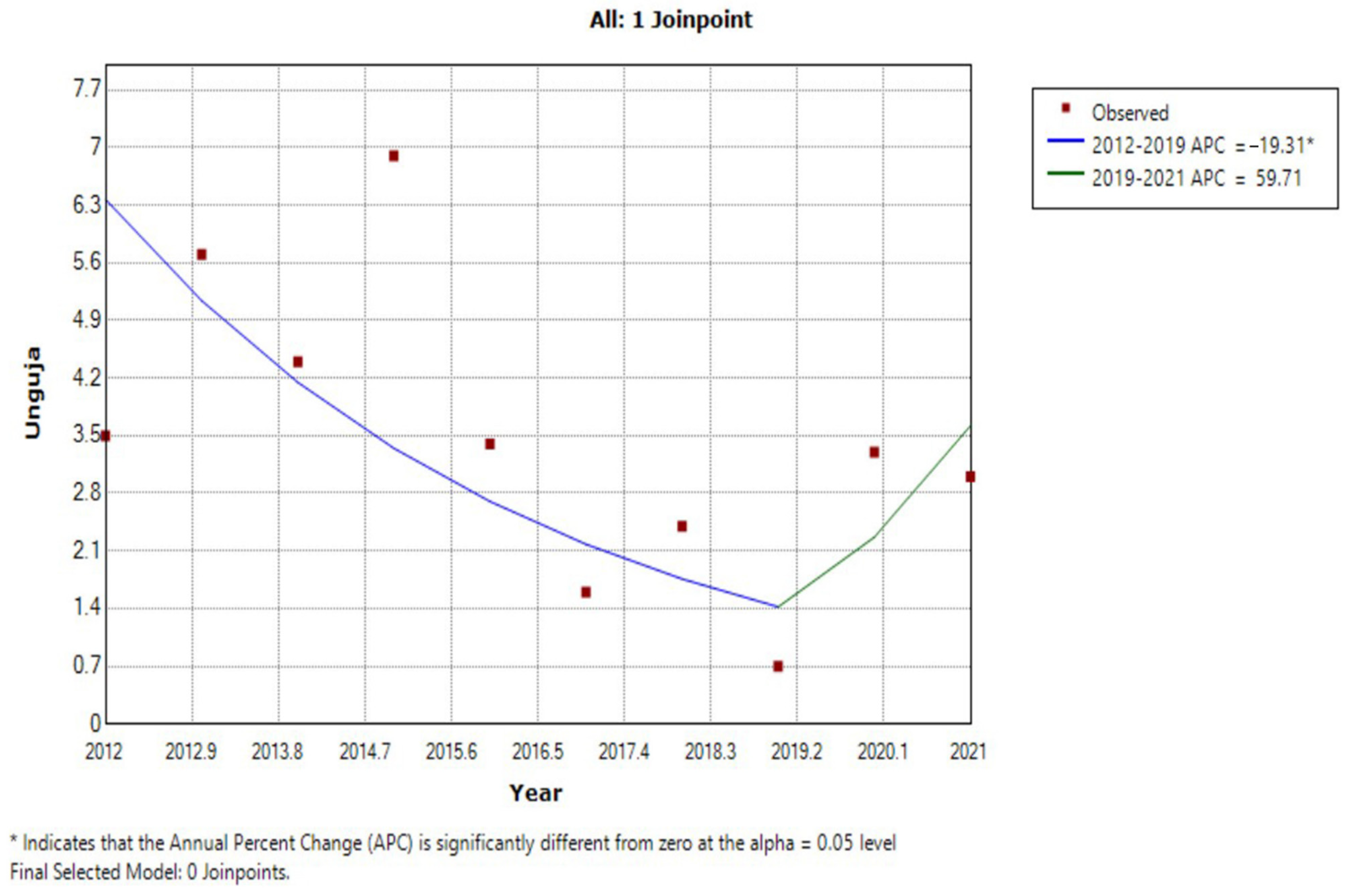
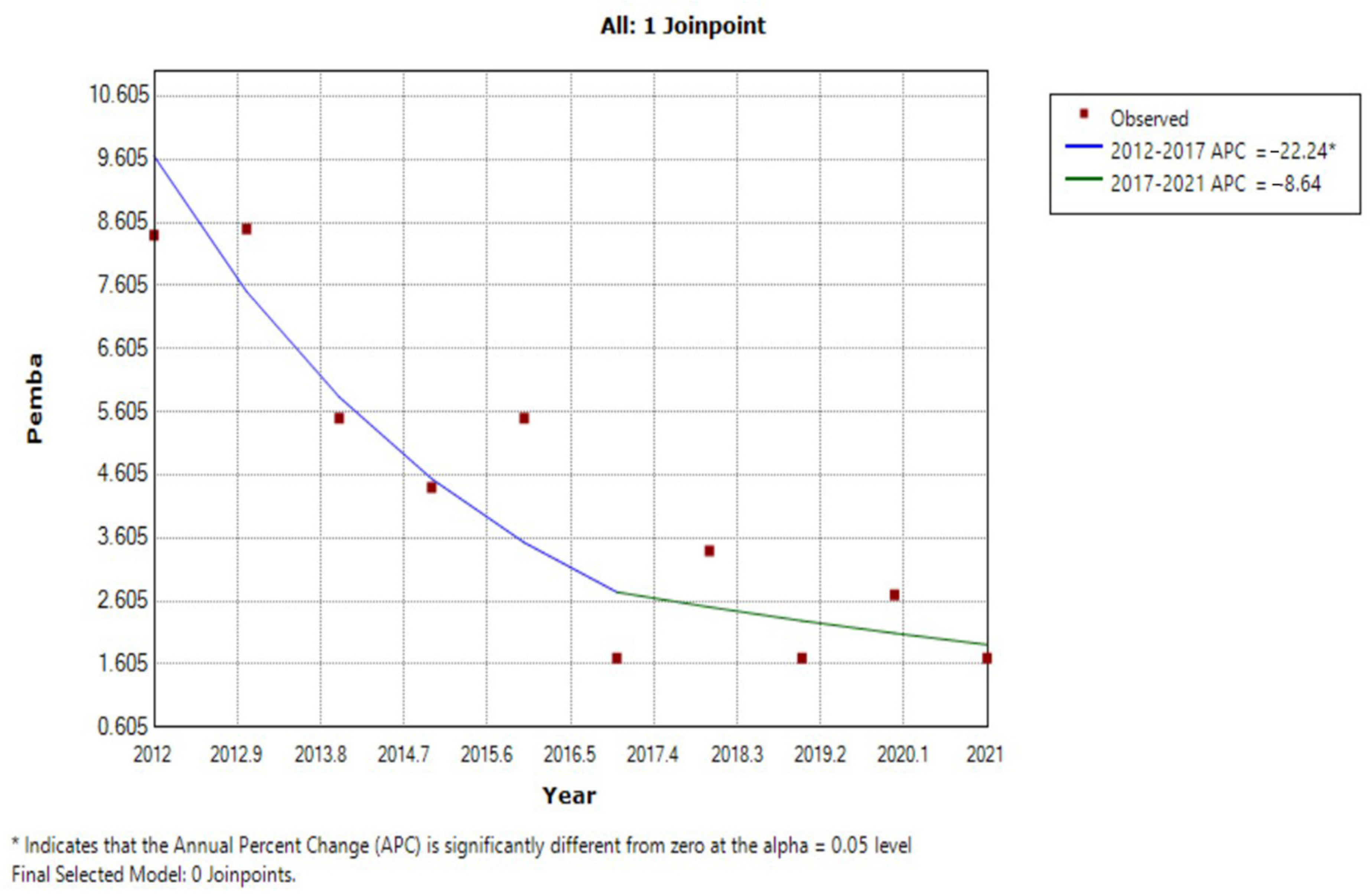
| Year | Unguja Island Regions | Pemba Island Regions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Central (%) | North A (%) | North B (%) | Urban (%) | West A (%) | West B (%) | Average (%) | Chake (%) | Micheweni (%) | Mkoani (%) | Wete (%) | Average (%) | |
| 2012 | 3.7 | 7.2 | 5.1 | 1.5 | 2.8 | 0.4 | 3.5 | 7.8 | 10.2 | 6.5 | 8.9 | 8.4 |
| 2013 | 8.2 | 14.2 | 5.8 | 1.2 | 3 | 1.9 | 5.7 | 7.1 | 10.3 | 5.1 | 11.3 | 8.5 |
| 2014 | 4.9 | 12.9 | 2.5 | 3.1 | 2 | 0.7 | 4.4 | 5.3 | 6.9 | 3.3 | 6.4 | 5.5 |
| 2015 | 8.6 | 15.6 | 6.2 | 0.7 | 5.9 | 4.3 | 6.9 | 4.2 | 4.9 | 4.8 | 3.7 | 4.4 |
| 2016 | 3.9 | 11.5 | 3.3 | 0.2 | 1.5 | 0 | 3.4 | 7.6 | 5.2 | 4 | 5.1 | 5.5 |
| 2017 | 1.2 | 5.4 | 1.5 | 0.4 | 1.2 | 0 | 1.6 | 2.1 | 1.5 | 1.2 | 2.1 | 1.7 |
| 2018 | 1.1 | 10.6 | 1.4 | 0 | 1.3 | 0 | 2.4 | 2.6 | 3.3 | 5.3 | 2.2 | 3.4 |
| 2019 | 0.3 | 2.9 | 0.9 | 0 | 0.2 | 0 | 0.7 | 1.7 | 0.9 | 1.7 | 2.4 | 1.7 |
| 2020 | 0.5 | 13.1 | 5.6 | 0 | 0.5 | 0 | 3.3 | 4.9 | 4 | 0.7 | 1.3 | 2.7 |
| 2021 | 4.6 | 7.6 | 3.2 | 0.3 | 1.5 | 0.7 | 3.0 | 2.1 | 0.6 | 1.6 | 2.4 | 1.7 |
| Polynomial Regression | Island | Equation Describing the Graph | R2 |
|---|---|---|---|
| First order polynomial | Unguja | Y = −0.334x + 676.879 | 0.305 |
| Pemba | Y = −0.768x + 1552.778 | 0.798 | |
| Second order polynomial | Unguja | Y = 0.0152x2 − 61.44x + 62,287 | 0.309 |
| Pemba | Y = 0.0894x2 − 361.29x + 365,052 | 0.867 | |
| Third order polynomial | Unguja | Y = 0.0589x3 − 356.28x2 + 718,407x − 5 × 108 | 0.664 |
| Pemba | Y = 0.0018x3 − 10.957x2 + 21,913x − 1 × 107 | 0.867 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhsin, M.A.; Wang, X.; Kabole, F.M.; Zilabumba, J.; Yang, K. The Indispensability of Snail Control for Accelerating Schistosomiasis Elimination: Evidence from Zanzibar. Trop. Med. Infect. Dis. 2022, 7, 347. https://doi.org/10.3390/tropicalmed7110347
Muhsin MA, Wang X, Kabole FM, Zilabumba J, Yang K. The Indispensability of Snail Control for Accelerating Schistosomiasis Elimination: Evidence from Zanzibar. Tropical Medicine and Infectious Disease. 2022; 7(11):347. https://doi.org/10.3390/tropicalmed7110347
Chicago/Turabian StyleMuhsin, Mtumweni Ali, Xinyao Wang, Fatma Mohammed Kabole, January Zilabumba, and Kun Yang. 2022. "The Indispensability of Snail Control for Accelerating Schistosomiasis Elimination: Evidence from Zanzibar" Tropical Medicine and Infectious Disease 7, no. 11: 347. https://doi.org/10.3390/tropicalmed7110347
APA StyleMuhsin, M. A., Wang, X., Kabole, F. M., Zilabumba, J., & Yang, K. (2022). The Indispensability of Snail Control for Accelerating Schistosomiasis Elimination: Evidence from Zanzibar. Tropical Medicine and Infectious Disease, 7(11), 347. https://doi.org/10.3390/tropicalmed7110347






