Diagnosis of Schistosomiasis without a Microscope: Evaluating Circulating Antigen (CCA, CAA) and DNA Detection Methods on Banked Samples of a Community-Based Survey from DR Congo
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Laboratory Analysis
2.2.1. Real-Time PCR
2.2.2. POC-CCA
2.2.3. UCP-LF CAA
2.3. Statistical Analysis
2.4. Ethics Approval and Consent to Participate
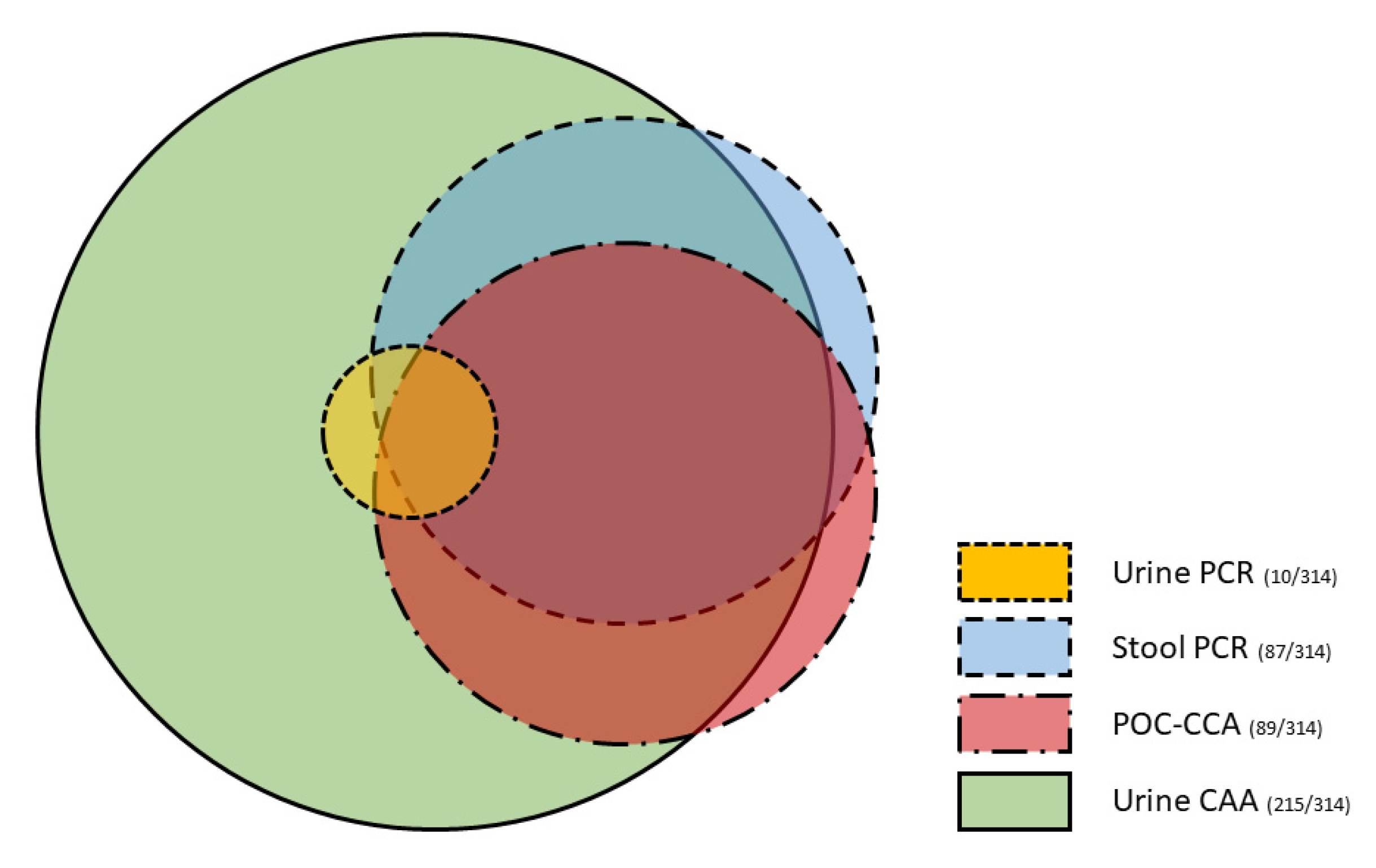
3. Results
3.1. Intensity of Infection
3.2. Diagnostic Accuracy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colley, D.G.; Andros, T.S.; Campbell, C.H., Jr. Schistosomiasis is more prevalent than previously thought: What does it mean for public health goals, policies, strategies, guidelines and intervention programs? Infect. Dis. Poverty 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, P.; Keiser, J.; Bos, R.; Tanner, M.; Utzinger, J. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006, 6, 411–425. [Google Scholar] [CrossRef]
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar] [PubMed]
- WHO. Helminth Control in School Age Children: A Guide for Managers of Control Programmes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Meurs, L.; Brienen, E.; Mbow, M.; Ochola, E.A.; Mboup, S.; Karanja, D.M.; Secor, W.E.; Polman, K.; van Lieshout, L. Is PCR the next reference standard for the diagnosis of Schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0003959. [Google Scholar] [CrossRef]
- Obeng, B.B.; Aryeetey, Y.A.; de Dood, C.J.; Amoah, A.S.; Larbi, I.A.; Deelder, A.M.; Yazdanbakhsh, M.; Hartgers, F.C.; Boakye, D.A.; Verweij, J.J.; et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobium in urine samples from Ghana. Ann. Trop. Med. Parasitol. 2008, 102, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, P.G.; Enyong, P.; Krijger, F.W.; De Jonge, N.; Zotter, G.M.; Thalhammer, F.; Muhlschlegel, F.; Bienzle, U.; Feldmeier, H.; Deelder, A.M. Circulating anodic and cathodic antigen in serum and urine from Schistosoma haematobium-infected Cameroonian children receiving praziquantel: A longitudinal study. Clin. Infect. Dis. 1994, 18, 408–413. [Google Scholar] [CrossRef]
- Van Dam, G.J.; Bogitsh, B.J.; van Zeyl, R.J.; Rotmans, J.P.; Deelder, A.M. Schistosoma mansoni: In vitro and in vivo excretion of CAA and CCA by developing schistosomula and adult worms. J. Parasitol. 1996, 82, 557–564. [Google Scholar] [CrossRef]
- Van Lieshout, L.; Polderman, A.M.; Deelder, A.M. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 2000, 77, 69–80. [Google Scholar] [CrossRef]
- Knopp, S.; Corstjens, P.L.; Koukounari, A.; Cercamondi, C.I.; Ame, S.M.; Ali, S.M.; de Dood, C.J.; Mohammed, K.A.; Utzinger, J.; Rollinson, D.; et al. Sensitivity and Specificity of a Urine Circulating Anodic Antigen Test for the Diagnosis of Schistosoma haematobium in Low Endemic Settings. PLoS Negl. Trop. Dis. 2015, 9, e0003752. [Google Scholar] [CrossRef]
- Van Etten, L.; Engels, D.; Krijger, F.W.; Nkulikyinka, L.; Gryseels, B.; Deelder, A.M. Fluctuation of schistosome circulating antigen levels in urine of individuals with Schistosoma mansoni infection in Burundi. Am. J. Trop. Med. Hyg. 1996, 54, 348–351. [Google Scholar] [CrossRef]
- Polman, K.; Engels, D.; Fathers, L.; Deelder, A.M.; Gryseels, B. Day-to-day fluctuation of schistosome circulating antigen levels in serum and urine of humans infected with Schistosoma mansoni in Burundi. Am. J. Trop. Med. Hyg. 1998, 59, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Stothard, J.R.; Stanton, M.C.; Bustinduy, A.L.; Sousa-Figueiredo, J.C.; Van Dam, G.J.; Betson, M.; Waterhouse, D.; Ward, S.; Allan, F.; Hassan, A.A.; et al. Diagnostics for schistosomiasis in Africa and Arabia: A review of present options in control and future needs for elimination. Parasitology 2014, 141, 1947–1961. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, J.T.; Knopp, S.; N’Guessan, N.A.; Silue, K.D.; Furst, T.; Lohourignon, L.K.; Brou, J.K.; N’Gbesso, Y.K.; Vounatsou, P.; N’Goran, E.K.; et al. Accuracy of urine circulating cathodic antigen (CCA) test for Schistosoma mansoni diagnosis in different settings of Cote d’Ivoire. PLoS Negl. Trop. Dis. 2011, 5, e1384. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, J.T.; N’Gbesso, Y.K.; Knopp, S.; N’Guessan, N.A.; Silué, K.D.; van Dam, G.J.; N’Goran, E.K.; Utzinger, J. Accuracy of urine circulating cathodic antigen test for the diagnosis of Schistosoma mansoni in preschool-aged children before and after treatment. PLoS Negl. Trop. Dis. 2013, 7, e2109. [Google Scholar] [CrossRef] [PubMed]
- Shane, H.L.; Verani, J.R.; Abudho, B.; Montgomery, S.P.; Blackstock, A.J.; Mwinzi, P.N.; Butler, S.E.; Karanja, D.M.; Secor, W.E. Evaluation of urine CCA assays for detection of Schistosoma mansoni infection in Western Kenya. PLoS Negl. Trop. Dis. 2011, 5, e951. [Google Scholar] [CrossRef]
- Tchuem Tchuente, L.A.; Kuete Fouodo, C.J.; Kamwa Ngassam, R.I.; Sumo, L.; Dongmo Noumedem, C.; Kenfack, C.M.; Gipwe, N.F.; Nana, E.D.; Stothard, J.R.; Rollinson, D. Evaluation of circulating cathodic antigen (CCA) urine-tests for diagnosis of Schistosoma mansoni infection in Cameroon. PLoS Negl. Trop. Dis. 2012, 6, e1758. [Google Scholar] [CrossRef]
- Colley, D.G.; Binder, S.; Campbell, C.; King, C.H.; Tchuem Tchuenté, L.A.; N’Goran, E.K.; Erko, B.; Karanja, D.M.; Kabatereine, N.B.; van Lieshout, L.; et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 2013, 88, 426–432. [Google Scholar] [CrossRef]
- Erko, B.; Medhin, G.; Teklehaymanot, T.; Degarege, A.; Legesse, M. Evaluation of urine-circulating cathodic antigen (Urine-CCA) cassette test for the detection of Schistosoma mansoni infection in areas of moderate prevalence in Ethiopia. Trop. Med. Int. Health 2013, 18, 1029–1035. [Google Scholar] [CrossRef]
- Adriko, M.; Standley, C.J.; Tinkitina, B.; Tukahebwa, E.M.; Fenwick, A.; Fleming, F.M.; Sousa-Figueiredo, J.C.; Stothard, J.R.; Kabatereine, N.B. Evaluation of circulating cathodic antigen (CCA) urine-cassette assay as a survey tool for Schistosoma mansoni in different transmission settings within Bugiri District, Uganda. Acta Trop. 2014, 136, 50–57. [Google Scholar] [CrossRef]
- Poole, H.; Terlouw, D.J.; Naunje, A.; Mzembe, K.; Stanton, M.; Betson, M.; Lalloo, D.G.; Stothard, J.R. Schistosomiasis in pre-school-age children and their mothers in Chikhwawa district, Malawi with notes on characterization of schistosomes and snails. Parasites Vectors 2014, 7, 153. [Google Scholar] [CrossRef]
- Midzi, N.; Butterworth, A.E.; Mduluza, T.; Munyati, S.; Deelder, A.M.; van Dam, G.J. Use of circulating cathodic antigen strips for the diagnosis of urinary schistosomiasis. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bärenbold, O.; Garba, A.; Colley, D.G.; Fleming, F.M.; Haggag, A.A.; Ramzy, R.M.R.; Assare, R.K.; Tukahebwa, E.M.; Mbonigaba, J.B.; Bucumi, V.; et al. Translating preventive chemotherapy prevalence thresholds for Schistosoma mansoni from the Kato-Katz technique into the point-of-care circulating cathodic antigen diagnostic test. PLoS Negl. Trop. Dis. 2018, 12, e0006941. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guideline on Control and Elimination of Human Schistosomiasis; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. Schistosomiasis. Available online: https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis (accessed on 1 January 2022).
- WHO. Report of the First Meeting of the WHO Diagnostic Technical Advisory Group for Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Corstjens, P.; de Dood, C.J.; Knopp, S.; Clements, M.N.; Ortu, G.; Umulisa, I.; Ruberanziza, E.; Wittmann, U.; Kariuki, T.; LoVerde, P.; et al. Circulating Anodic Antigen (CAA): A Highly Sensitive Diagnostic Biomarker to Detect Active Schistosoma Infections-Improvement and Use during SCORE. Am. J. Trop. Med. Hyg. 2020, 103 (Suppl. S1), 50–57. [Google Scholar] [CrossRef] [PubMed]
- van Dam, G.J.; Odermatt, P.; Acosta, L.; Bergquist, R.; de Dood, C.J.; Kornelis, D.; Muth, S.; Utzinger, J.; Corstjens, P.L. Evaluation of banked urine samples for the detection of circulating anodic and cathodic antigens in Schistosoma mekongi and S. japonicum infections: A proof-of-concept study. Acta Trop. 2015, 141 Pt B, 198–203. [Google Scholar] [CrossRef]
- van Dam, G.J.; Xu, J.; Bergquist, R.; de Dood, C.J.; Utzinger, J.; Qin, Z.Q.; Guan, W.; Feng, T.; Yu, X.L.; Zhou, J.; et al. An ultra-sensitive assay targeting the circulating anodic antigen for the diagnosis of Schistosoma japonicum in a low-endemic area, People’s Republic of China. Acta Trop. 2015, 141 Pt B, 190–197. [Google Scholar] [CrossRef]
- Vonghachack, Y.; Sayasone, S.; Khieu, V.; Bergquist, R.; van Dam, G.J.; Hoekstra, P.T.; Corstjens, P.; Nickel, B.; Marti, H.; Utzinger, J.; et al. Comparison of novel and standard diagnostic tools for the detection of Schistosoma mekongi infection in Lao People’s Democratic Republic and Cambodia. Infect. Dis. Poverty 2017, 6, 127. [Google Scholar] [CrossRef]
- Kanobana, K.; Praet, N.; Kabwe, C.; Dorny, P.; Lukanu, P.; Madinga, J.; Mitashi, P.; Verwijs, M.; Lutumba, P.; Polman, K. High prevalence of Taenia solium cysticerosis in a village community of Bas-Congo, Democratic Republic of Congo. Int. J. Parasitol. 2011, 41, 1015–1018. [Google Scholar] [CrossRef]
- Madinga, J.; Polman, K.; Kanobana, K.; van Lieshout, L.; Brienen, E.; Praet, N.; Kabwe, C.; Gabriel, S.; Dorny, P.; Lutumba, P.; et al. Epidemiology of polyparasitism with Taenia solium, schistosomes and soil-transmitted helminths in the co-endemic village of Malanga, Democratic Republic of Congo. Acta Trop. 2017, 171, 186–193. [Google Scholar] [CrossRef]
- Cools, P.; van Lieshout, L.; Koelewijn, R.; Addiss, D.; Ajjampur, S.S.R.; Ayana, M.; Bradbury, R.S.; Cantera, J.L.; Dana, D.; Fischer, K.; et al. First international external quality assessment scheme of nucleic acid amplification tests for the detection of Schistosoma and soil-transmitted helminths, including Strongyloides: A pilot study. PLoS Negl. Trop. Dis. 2020, 14, e0008231. [Google Scholar] [CrossRef]
- Pillay, P.; Taylor, M.; Zulu, S.G.; Gundersen, S.G.; Verweij, J.J.; Hoekstra, P.; Brienen, E.A.; Kleppa, E.; Kjetland, E.F.; van Lieshout, L. Real-time polymerase chain reaction for detection of Schistosoma DNA in small-volume urine samples reflects focal distribution of urogenital Schistosomiasis in primary school girls in KwaZulu Natal, South Africa. Am. J. Trop. Med. Hyg. 2014, 90, 546–552. [Google Scholar] [CrossRef]
- Vinkeles Melchers, N.V.; van Dam, G.J.; Shaproski, D.; Kahama, A.I.; Brienen, E.A.; Vennervald, B.J.; van Lieshout, L. Diagnostic performance of Schistosoma real-time PCR in urine samples from Kenyan children infected with Schistosoma haematobium: Day-to-day variation and follow-up after praziquantel treatment. PLoS Negl. Trop. Dis. 2014, 8, e2807. [Google Scholar] [CrossRef]
- Casacuberta-Partal, M.; Beenakker, M.; de Dood, C.; Hoekstra, P.; Kroon, L.; Kornelis, D.; Corstjens, P.; Hokke, C.H.; van Dam, G.; Roestenberg, M.; et al. Specificity of the Point-of-Care Urine Strip Test for Schistosoma Circulating Cathodic Antigen (POC-CCA) Tested in Non-Endemic Pregnant Women and Young Children. Am. J. Trop. Med. Hyg. 2021, 104, 1412–1417. [Google Scholar] [CrossRef]
- Corstjens, P.L.; De Dood, C.J.; Kornelis, D.; Fat, E.M.; Wilson, R.A.; Kariuki, T.M.; Nyakundi, R.K.; Loverde, P.T.; Abrams, W.R.; Tanke, H.J.; et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology 2014, 141, 1841–1855. [Google Scholar] [CrossRef]
- Corstjens, P.L.; Nyakundi, R.K.; de Dood, C.J.; Kariuki, T.M.; Ochola, E.A.; Karanja, D.M.; Mwinzi, P.N.; van Dam, G.J. Improved sensitivity of the urine CAA lateral-flow assay for diagnosing active Schistosoma infections by using larger sample volumes. Parasites Vectors 2015, 8, 241. [Google Scholar] [CrossRef]
- Assaré, R.K.; Tra-Bi, M.I.; Coulibaly, J.T.; Corstjens, P.; Ouattara, M.; Hürlimann, E.; van Dam, G.J.; Utzinger, J.; N’Goran, E.K. Accuracy of Two Circulating Antigen Tests for the Diagnosis and Surveillance of Schistosoma mansoni Infection in Low-Endemicity Settings of Côte d’Ivoire. Am. J. Trop. Med. Hyg. 2021, 105, 677–683. [Google Scholar] [CrossRef]
- Hoekstra, P.T.; Chernet, A.; de Dood, C.J.; Brienen, E.A.T.; Corstjens, P.; Labhardt, N.D.; Nickel, B.; Wammes, L.; van Dam, G.J.; Neumayr, A.; et al. Sensitive diagnosis and post-treatment follow-up of Schistosoma mansoni infections in asymptomatic Eritrean refugees by circulating anodic antigen detection and polymerase chain reaction. Am. J. Trop. Med. Hyg. 2022, 106, 1240–1246. [Google Scholar] [CrossRef]
- Hoekstra, P.T.; Casacuberta-Partal, M.; van Lieshout, L.; Corstjens, P.; Tsonaka, R.; Assaré, R.K.; Silué, K.D.; N’Goran, E.K.; N’Gbesso, Y.K.; Brienen, E.A.T.; et al. Limited efficacy of repeated praziquantel treatment in Schistosoma mansoni infections as revealed by highly accurate diagnostics, PCR and UCP-LF CAA (RePST trial). PLoS Negl. Trop. Dis. 2022. under review. [Google Scholar]
- Meurs, L.; Mbow, M.; Vereecken, K.; Menten, J.; Mboup, S.; Polman, K. Epidemiology of mixed Schistosoma mansoni and Schistosoma haematobium infections in northern Senegal. Int. J. Parasitol. 2012, 42, 305–311. [Google Scholar] [CrossRef]
- Cunin, P.; Tchuem Tchuenté, L.A.; Poste, B.; Djibrilla, K.; Martin, P.M. Interactions between Schistosoma haematobium and Schistosoma mansoni in humans in north Cameroon. Trop. Med. Int. Health 2003, 8, 1110–1117. [Google Scholar] [CrossRef]
- Meulah, B.; Oyibo, P.; Bengtson, M.; Agbana, T.; Lontchi, R.A.L.; Adegnika, A.A.; Oyibo, W.; Hokke, C.H.; Diehl, J.C.; van Lieshout, L. Performance evaluation of the Schistoscope 5.0 for (semi-) automated digital detection and quantification of Schistosoma haematobium eggs in urine: A field-based study in Nigeria. Am. J. Trop. Med. Hyg. 2022. accepted. [Google Scholar] [CrossRef]
- Polman, K.; Stelma, F.F.; Gryseels, B.; Van Dam, G.J.; Talla, I.; Niang, M.; Van Lieshout, L.; Deelder, A.M. Epidemiologic application of circulating antigen detection in a recent Schistosoma mansoni focus in northern Senegal. Am. J. Trop. Med. Hyg. 1995, 53, 152–157. [Google Scholar] [CrossRef]
- Polman, K.; Stelma, F.F.; Le Cessie, S.; De Vlas, S.J.; Falcao Ferreira, S.T.; Talla, I.; Deelder, A.M.; Gryseels, B. Evaluation of the patterns of Schistosoma mansoni infection and re-infection in Senegal, from faecal egg counts and serum concentrations of circulating anodic antigen. Ann. Trop. Med. Parasitol. 2002, 96, 679–689. [Google Scholar] [CrossRef]
- Faust, C.L.; Osakunor, D.N.M.; Downs, J.A.; Kayuni, S.; Stothard, J.R.; Lamberton, P.H.L.; Reinhard-Rupp, J.; Rollinson, D. Schistosomiasis Control: Leave No Age Group Behind. Trends Parasitol. 2020, 36, 582–591. [Google Scholar] [CrossRef]
- Utzinger, J.; Becker, S.L.; van Lieshout, L.; van Dam, G.J.; Knopp, S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 2015, 21, 529–542. [Google Scholar] [CrossRef]
- Hoekstra, P.T.; van Dam, G.J.; van Lieshout, L. Context-specific procedures for the diagnosis of human schistosomiasis—A mini review. Front. Trop. Dis. 2021, 2, 722438. [Google Scholar] [CrossRef]
- Worrell, C.M.; Bartoces, M.; Karanja, D.M.; Ochola, E.A.; Matete, D.O.; Mwinzi, P.N.; Montgomery, S.P.; Secor, W.E. Cost analysis of tests for the detection of Schistosoma mansoni infection in children in western Kenya. Am. J. Trop. Med. Hyg. 2015, 92, 1233–1239. [Google Scholar] [CrossRef]



| Diagnostic Method | N (%) |
|---|---|
| PCR (urine) | |
| 35 ≤ Ct < 50 (low) | 2 (0.6%) |
| 30 ≤ Ct < 35 (medium) | 4 (1.3%) |
| 25 ≤ Ct < 30 (high) | 3 (1.0%) |
| Ct < 25 (very high) | 1 (0.3%) |
| PCR (stool) | |
| 35 ≤ Ct < 50 (low) | 15 (4.8%) |
| 30 ≤ Ct < 35 (medium) | 4 (1.3%) |
| 25 ≤ Ct < 30 (high) | 18 (5.7%) |
| Ct < 25 (very high) | 50 (15.9%) |
| POC-CCA | |
| Trace | 44 (14.0%) |
| 1+ (low) | 48 (15.3%) |
| 2+ (moderate) | 28 (8.9%) |
| 3+ (high) | 10 (3.2%) |
| UCP-LF CAA (urine) | |
| 0.1–1 pg/mL (very low) | 64 (20.4%) |
| 1–10 pg/mL (low) | 66 (21.0%) |
| 10–100 pg/mL (moderate) | 58 (18.5%) |
| >100 pg/mL (high) | 27 (8.6%) |
| Diagnostic Test | Reference Test | K Value | Interpretation 1 | p Value | McNemar’s p Value | |
|---|---|---|---|---|---|---|
| PCR (urine) | ||||||
| PCR (stool) | Positive | Negative | ||||
| Positive | 8 | 79 | 0.114 | Slight | <0.001 | <0.001 |
| Negative | 2 | 225 | ||||
| POC-CCA | ||||||
| PCR (stool) | Positive | Negative | ||||
| Positive | 60 | 27 | 0.577 | Moderate | <0.001 | 1 |
| Negative | 26 | 201 | ||||
| UCP-LF CAA | ||||||
| PCR (stool) | Positive | Negative | ||||
| Positive | 80 | 7 | 0.223 | Fair | <0.001 | <0.001 |
| Negative | 135 | 92 | ||||
| UCP-LF CAA | ||||||
| POC-CCA | Positive | Negative | ||||
| Positive | 79 | 7 | 0.220 | Fair | <0.001 | <0.001 |
| Negative | 136 | 92 | ||||
| PCR (urine) | ||||||
| POC-CCA | Positive | Negative | ||||
| Positive | 8 | 78 | 0.116 | Slight | <0.001 | <0.001 |
| Negative | 2 | 226 | ||||
| PCR (urine) | ||||||
| UCP-LF CAA | Positive | Negative | ||||
| Positive | 10 | 205 | 0.030 | Slight | 0.029 | <0.001 |
| Negative | 0 | 99 |
| CRS (PCR & UCP-LF CAA) 1 | Diagnostic Accuracy | ||||
|---|---|---|---|---|---|
| Positive | Negative | Sensitivity | Specificity | ||
| PCR (urine) | Positive | 10 | 0 | 4.5% | 100% 2 |
| Negative | 212 | 92 | |||
| PCR (stool) | Positive | 87 | 0 | 39.2% | 100% 2 |
| Negative | 135 | 92 | |||
| POC-CCA | Positive | 80 | 6 | 36.0% | 93.5% |
| Negative | 142 | 86 | |||
| UCP-LF CAA | Positive | 215 | 0 | 96.8% | 100% 2 |
| Negative | 7 | 92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoekstra, P.T.; Madinga, J.; Lutumba, P.; van Grootveld, R.; Brienen, E.A.T.; Corstjens, P.L.A.M.; van Dam, G.J.; Polman, K.; van Lieshout, L. Diagnosis of Schistosomiasis without a Microscope: Evaluating Circulating Antigen (CCA, CAA) and DNA Detection Methods on Banked Samples of a Community-Based Survey from DR Congo. Trop. Med. Infect. Dis. 2022, 7, 315. https://doi.org/10.3390/tropicalmed7100315
Hoekstra PT, Madinga J, Lutumba P, van Grootveld R, Brienen EAT, Corstjens PLAM, van Dam GJ, Polman K, van Lieshout L. Diagnosis of Schistosomiasis without a Microscope: Evaluating Circulating Antigen (CCA, CAA) and DNA Detection Methods on Banked Samples of a Community-Based Survey from DR Congo. Tropical Medicine and Infectious Disease. 2022; 7(10):315. https://doi.org/10.3390/tropicalmed7100315
Chicago/Turabian StyleHoekstra, Pytsje T., Joule Madinga, Pascal Lutumba, Rebecca van Grootveld, Eric A. T. Brienen, Paul L. A. M. Corstjens, Govert J. van Dam, Katja Polman, and Lisette van Lieshout. 2022. "Diagnosis of Schistosomiasis without a Microscope: Evaluating Circulating Antigen (CCA, CAA) and DNA Detection Methods on Banked Samples of a Community-Based Survey from DR Congo" Tropical Medicine and Infectious Disease 7, no. 10: 315. https://doi.org/10.3390/tropicalmed7100315
APA StyleHoekstra, P. T., Madinga, J., Lutumba, P., van Grootveld, R., Brienen, E. A. T., Corstjens, P. L. A. M., van Dam, G. J., Polman, K., & van Lieshout, L. (2022). Diagnosis of Schistosomiasis without a Microscope: Evaluating Circulating Antigen (CCA, CAA) and DNA Detection Methods on Banked Samples of a Community-Based Survey from DR Congo. Tropical Medicine and Infectious Disease, 7(10), 315. https://doi.org/10.3390/tropicalmed7100315







