Proteomic Analysis of Urine from Patients with Plasmodium vivax Malaria Unravels a Unique Plasmodium vivax Protein That Is Absent from Plasmodium falciparum
Abstract
1. Introduction
2. Material and Methods
2.1. Clinical Specimens
2.2. Mass Spectroscopy Analysis
2.3. Recombinant Protein and ELISA
3. Results
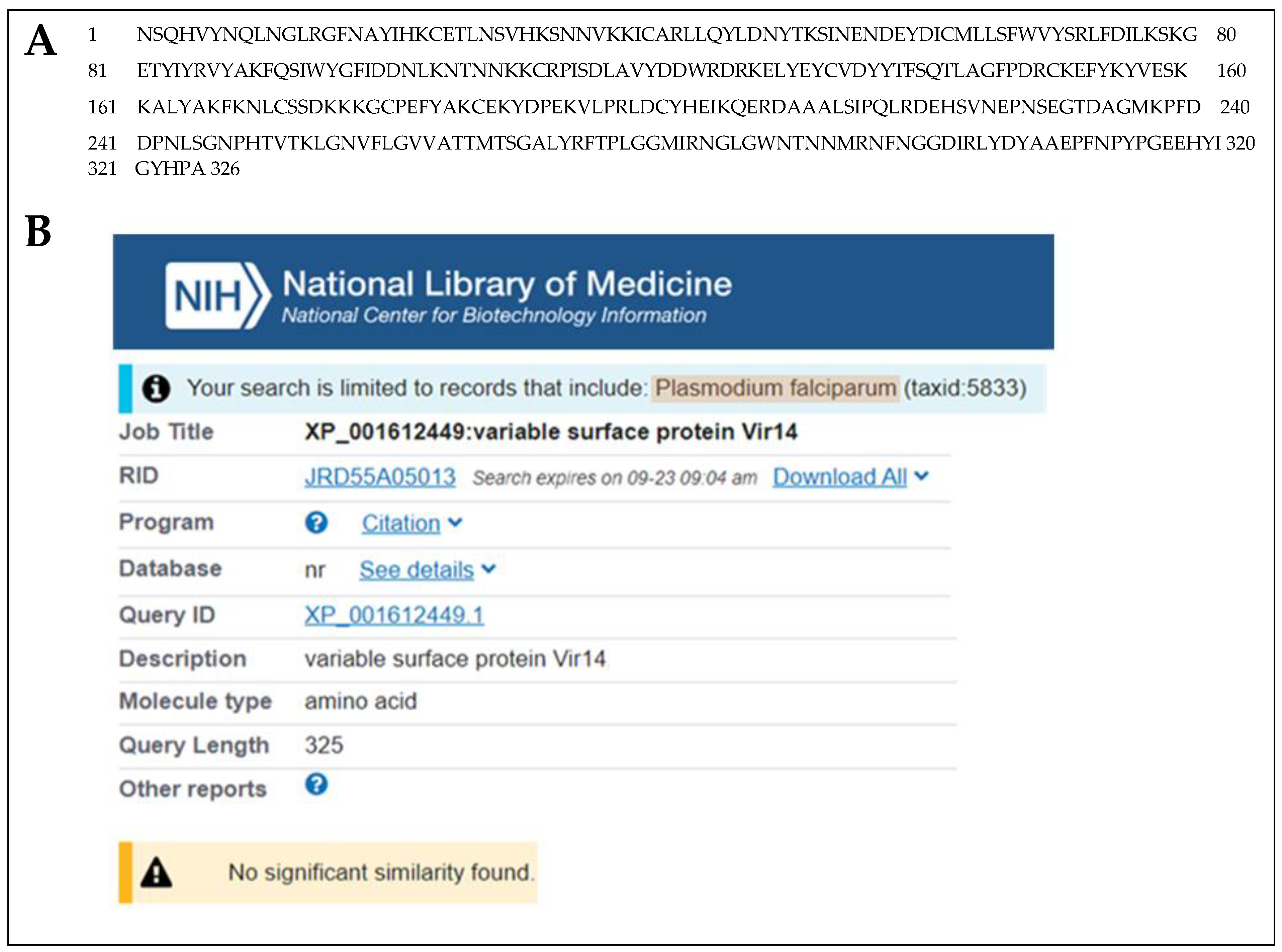
3.1. Discovery and Characterization of a Unique P. vivax Protein Present in the Urine of Patients with P. vivax Malaria from Brazil
3.2. Recognition of Vir14 by Sera from Patients with P. vivax Malaria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Prim. 2017, 3, 17050. [Google Scholar] [CrossRef] [PubMed]
- Payne, D. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull. World Health Organ. 1988, 66, 621–626. [Google Scholar] [PubMed]
- Johnston, S.P.; Pieniazek, N.J.; Xayavong, M.V.; Slemenda, S.B.; Wilkins, P.P.; da Silva, A.J. PCR as a confirmatory technique for laboratory diagnosis of malaria. J. Clin. Microbiol. 2006, 44, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, N.W.; Gaye, M.; Diallo, M.A.; Goldman, I.F.; Ljolje, D.; Deme, A.B.; Badiane, A.; Ndiaye, Y.D.; Barnwell, J.W.; Udhayakumar, V.; et al. Evaluation of the Illumigene Malaria LAMP: A Robust Molecular Diagnostic Tool for Malaria Parasites. Sci. Rep. 2016, 6, 36808. [Google Scholar] [CrossRef] [PubMed]
- Voller, A.; Draper, C.C. Immunodiagnosis and sero-epidemiology of malaria. Br. Med. Bull. 1982, 38, 173–177. [Google Scholar] [CrossRef]
- Chiodini, P.L. Malaria diagnostics: Now and the future. Parasitology 2014, 141, 1873–1879. [Google Scholar] [CrossRef]
- Jang, I.K.; Tyler, A.; Lyman, C.; Kahn, M.; Kalnoky, M.; Rek, J.C.; Arinaitwe, E.; Adrama, H.; Murphy, M.; Imwong, M.; et al. Simultaneous Quantification of Plasmodium Antigens and Host Factor C-Reactive Protein in Asymptomatic Individuals with Confirmed Malaria by Use of a Novel Multiplex Immunoassay. J. Clin. Microbiol. 2019, 57, e00948-18. [Google Scholar] [CrossRef]
- Mathison, B.A.; Pritt, B.S. Update on Malaria Diagnostics and Test Utilization. J. Clin. Microbiol. 2017, 55, 2009–2017. [Google Scholar] [CrossRef]
- Barber, B.E.; William, T.; Grigg, M.J.; Piera, K.; Yeo, T.W.; Anstey, N.M. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. J. Clin. Microbiol. 2013, 51, 1118–1123. [Google Scholar] [CrossRef]
- Abeijon, C.; Alves, F.; Monnerat, S.; Mbui, J.; Viana, A.G.; Almeida, R.M.; Bueno, L.L.; Fujiwara, R.T.; Campos-Neto, A. Urine-based antigen detection assay for diagnosis of visceral leishmaniasis using monoclonal antibodies specific for six protein biomarkers of Leishmania infantum/Leishmania donovani. PLOS Negl. Trop. Dis. 2020, 14, e0008246. [Google Scholar] [CrossRef]
- Abeijon, C.; Alves, F.; Monnerat, S.; Wasunna, M.; Mbui, J.; Viana, A.G.; Bueno, L.L.; Siqueira, W.F.; Carvalho, S.G.; Agrawal, N.; et al. Development of a multiplexed assay for the detection of Leishmania donovani/Leishmania infantum protein biomarkers in the urine of patients with visceral leishmaniasis. J. Clin. Microbiol. 2019, 57, e02076-18. [Google Scholar] [CrossRef] [PubMed]
- Abeijon, C.; Kashino, S.S.; Silva, F.O.; Costa, D.L.; Fujiwara, R.T.; Costa, C.H.; Campos-Neto, A. Identification and diagnostic utility of Leishmania infantum proteins found in urine samples from patients with visceral leishmaniasis. Clin. Vaccine Immunol. 2012, 19, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Abeijon, C.; Campos-Neto, A. Potential non-invasive urine-based antigen (protein) detection assay to diagnose active visceral leishmaniasis. PLOS Negl. Trop. Dis. 2013, 7, e2161. [Google Scholar] [CrossRef]
- Abeijon, C.; Singh, O.P.; Chakravarty, J.; Sundar, S.; Campos-Neto, A. Novel Antigen Detection Assay to Monitor Therapeutic Efficacy of Visceral Leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 95, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Snounou, G. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol. Biol. 1996, 50, 263–291. [Google Scholar]
- Snounou, G.; Viriyakosol, S.; Jarra, W.; Thaithong, S.; Brown, K.N. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 1993, 58, 283–292. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- Anderson, D.C.; Li, W.; Payan, D.G.; Noble, W.S. A new algorithm for the evaluation of shotgun peptide sequencing in proteomics: Support vector machine classification of peptide MS/MS spectra and SEQUEST scores. J. Proteome Res. 2003, 2, 137–146. [Google Scholar] [CrossRef]
- Xu, T.; Park, S.K.; Venable, J.D.; Wohlschlegel, J.A.; Diedrich, J.K.; Cociorva, D.; Lu, B.; Liao, L.; Hewel, J.; Han, X.; et al. ProLuCID: An improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J. Proteom. 2015, 129, 16–24. [Google Scholar] [CrossRef]
- Baird, J.K.; Valecha, N.; Duparc, S.; White, N.J.; Price, R.N. Diagnosis and Treatment of Plasmodium vivax Malaria. Am. J. Trop. Med. Hyg. 2016, 95, 35–51. [Google Scholar] [CrossRef]
- Bassat, Q. The importance of being vivax. J. Trop. Pediatr. 2014, 60, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Price, R.N.; Commons, R.J.; Battle, K.E.; Thriemer, K.; Mendis, K. Plasmodium vivax in the Era of the Shrinking P. falciparum Map. Trends Parasitol. 2020, 36, 560–570. [Google Scholar] [CrossRef] [PubMed]
- von Seidlein, L.; White, N.J. Taking on Plasmodium vivax malaria: A timely and important challenge. PLOS Med. 2021, 18, e1003593. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.C.; Ade, M.P.; Baird, J.K.; Cheng, Q.; Cunningham, J.; Dhorda, M.; Drakeley, C.; Felger, I.; Gamboa, D.; Harbers, M.; et al. Defining the next generation of Plasmodium vivax diagnostic tests for control and elimination: Target product profiles. PLOS Negl. Trop. Dis. 2017, 11, e0005516. [Google Scholar] [CrossRef] [PubMed]
- Kashino, S.S.; Pollock, N.; Napolitano, D.R.; Rodrigues, V., Jr.; Campos-Neto, A. Identification and characterization of Mycobacterium tuberculosis antigens in urine of patients with active pulmonary tuberculosis: An innovative and alternative approach of antigen discovery of useful microbial molecules. Clin. Exp. Immunol. 2008, 153, 56–62. [Google Scholar] [CrossRef]
- Napolitano, D.R.; Pollock, N.; Kashino, S.S.; Rodrigues, V., Jr.; Campos-Neto, A. Identification of Mycobacterium tuberculosis ornithine carboamyltransferase in urine as a possible molecular marker of active pulmonary tuberculosis. Clin. Vaccine Immunol. 2008, 15, 638–643. [Google Scholar] [CrossRef]
- Pollock, N.R.; Macovei, L.; Kanunfre, K.; Dhiman, R.; Restrepo, B.I.; Zarate, I.; Pino, P.A.; Mora-Guzman, F.; Fujiwara, R.T.; Michel, G.; et al. Validation of Mycobacterium tuberculosis Rv1681 protein as a diagnostic marker of active pulmonary tuberculosis. J. Clin. Microbiol. 2013, 51, 1367–1373. [Google Scholar] [CrossRef]
- Bernabeu, M.; Lopez, F.J.; Ferrer, M.; Martin-Jaular, L.; Razaname, A.; Corradin, G.; Maier, A.G.; Del Portillo, H.A.; Fernandez-Becerra, C. Functional analysis of Plasmodium vivax VIR proteins reveals different subcellular localizations and cytoadherence to the ICAM-1 endothelial receptor. Cell. Microbiol. 2012, 14, 386–400. [Google Scholar] [CrossRef]
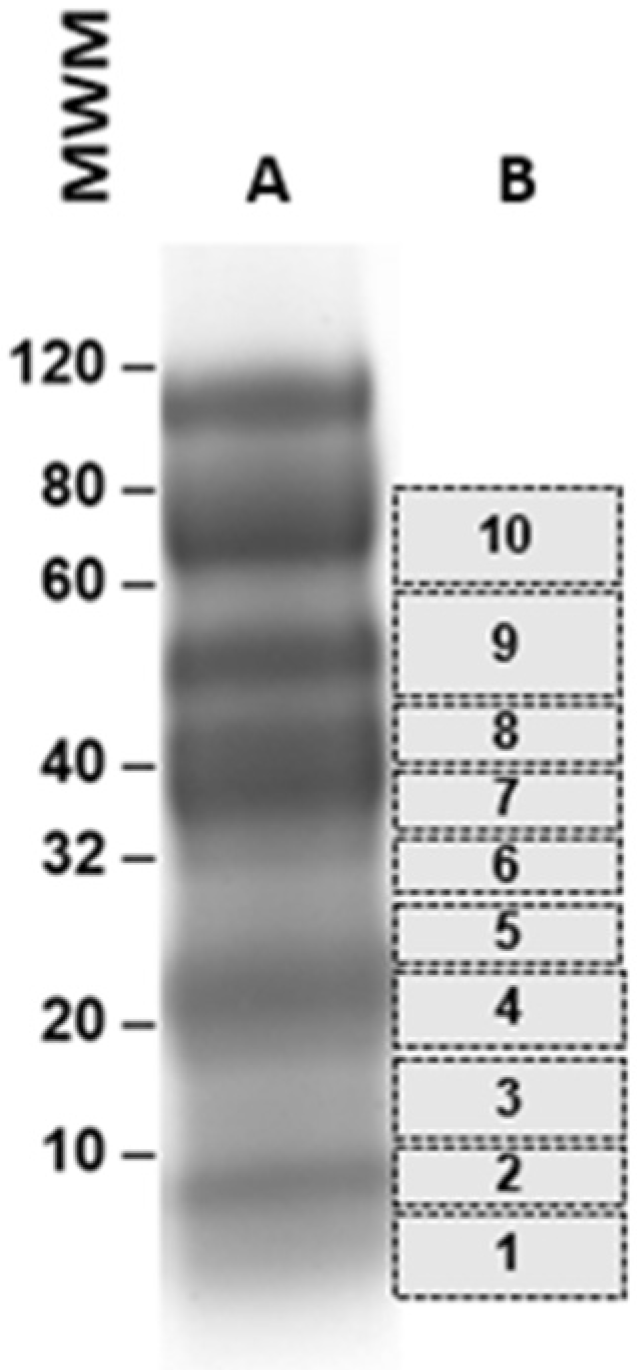
| Peptide Identified in Patient Urines | XCorr | ΔCorr | P. vivax Donor Protein | MW (Da) | Identity with P. falciparum Protein | Accession Numbers P. vivax (Pv) P. falciparum (Pf) |
|---|---|---|---|---|---|---|
| KMNLDEFNELVEQRNR | 2.48 | 0.125 | Proteasome regulatory subunit p27 | 33,710.41 | >75% | XP_001614534.1 Pv XP_001351248.1 Pf |
| TINEGQTLLTVFK YQFINIER | 3.46 1.88 | 0.623 0.160 | Profilin | 19,287.32 | >95% | XP_001608363.1 Pv XP_001352188.1 Pf |
| GVDMHNEEIKAVIK | 2.46 | 0.205 | Uncharacterized protein | 44,559.84 | 50% | XP_001614264.1 Pv XP_001350199.1 Pf |
| DAAALSIPQLR | 2.51 | 0.248 | Variable surface protein Vir14 | 37,719.38 | None | XP_001612449.1 Pv |
| EELNKINYNPR | 2.63 | 0.351 | Heat shock protein, class I | 26,949.43 | >58% | XP_001616584.1 Pv XP_001350519.1 Pf |
| Normal Healthy Control Subjects (6) | P. vivax Malaria Patients (n = 121) | |
|---|---|---|
| Positive sera (% of patients) * | 0 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantin, R.F.; Abeijon, C.; Pereira, D.B.; Fujiwara, R.T.; Bueno, L.L.; Campos-Neto, A. Proteomic Analysis of Urine from Patients with Plasmodium vivax Malaria Unravels a Unique Plasmodium vivax Protein That Is Absent from Plasmodium falciparum. Trop. Med. Infect. Dis. 2022, 7, 314. https://doi.org/10.3390/tropicalmed7100314
Fantin RF, Abeijon C, Pereira DB, Fujiwara RT, Bueno LL, Campos-Neto A. Proteomic Analysis of Urine from Patients with Plasmodium vivax Malaria Unravels a Unique Plasmodium vivax Protein That Is Absent from Plasmodium falciparum. Tropical Medicine and Infectious Disease. 2022; 7(10):314. https://doi.org/10.3390/tropicalmed7100314
Chicago/Turabian StyleFantin, Raianna F., Claudia Abeijon, Dhelio B. Pereira, Ricardo T. Fujiwara, Lilian L. Bueno, and Antonio Campos-Neto. 2022. "Proteomic Analysis of Urine from Patients with Plasmodium vivax Malaria Unravels a Unique Plasmodium vivax Protein That Is Absent from Plasmodium falciparum" Tropical Medicine and Infectious Disease 7, no. 10: 314. https://doi.org/10.3390/tropicalmed7100314
APA StyleFantin, R. F., Abeijon, C., Pereira, D. B., Fujiwara, R. T., Bueno, L. L., & Campos-Neto, A. (2022). Proteomic Analysis of Urine from Patients with Plasmodium vivax Malaria Unravels a Unique Plasmodium vivax Protein That Is Absent from Plasmodium falciparum. Tropical Medicine and Infectious Disease, 7(10), 314. https://doi.org/10.3390/tropicalmed7100314






