Evaluation of Two Different Strategies for Schistosomiasis Screening in High-Risk Groups in a Non-Endemic Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions
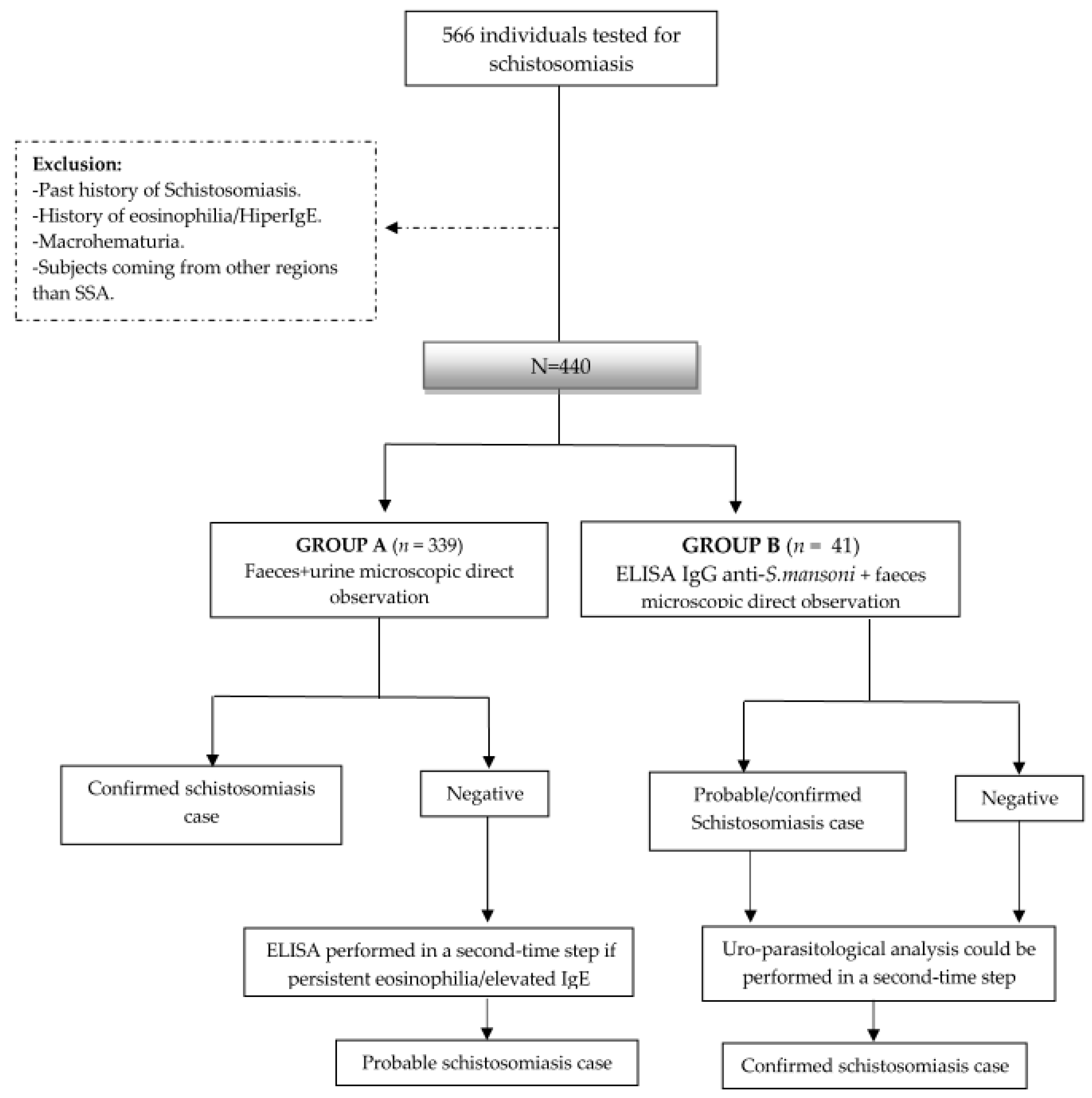
2.2. Study Design
2.3. Settings
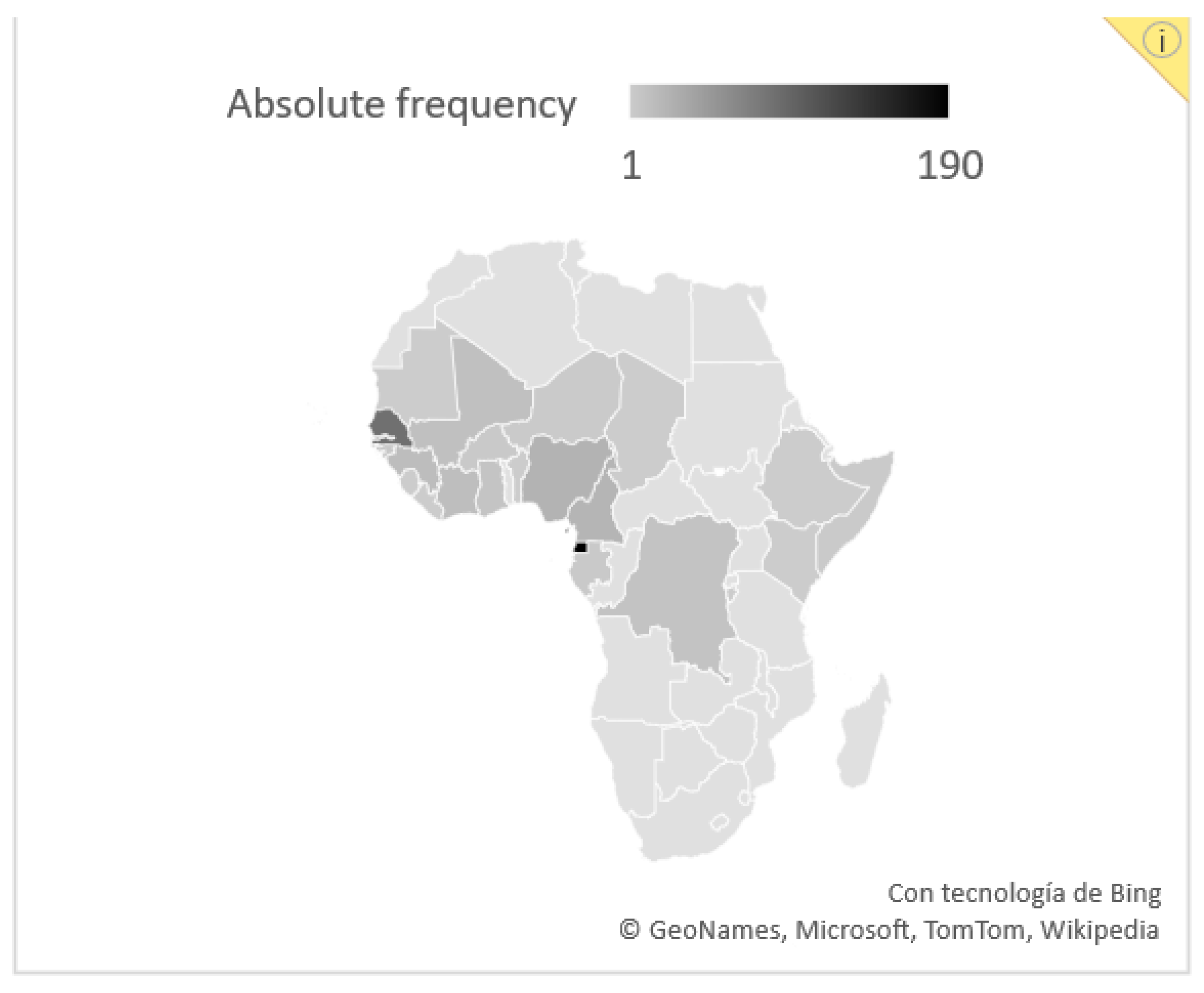
2.4. Study Population and Data Collection
2.5. Case Definition
2.6. Statistical Analysis
2.7. Ethics Statement
3. Results
3.1. Baseline Features
3.2. Schistosomiasis Screening Outcomes
3.2.1. Group A
3.2.2. Group B
3.3. Schistosomiasis Treatment, Complementary Examinations and Follow-Up
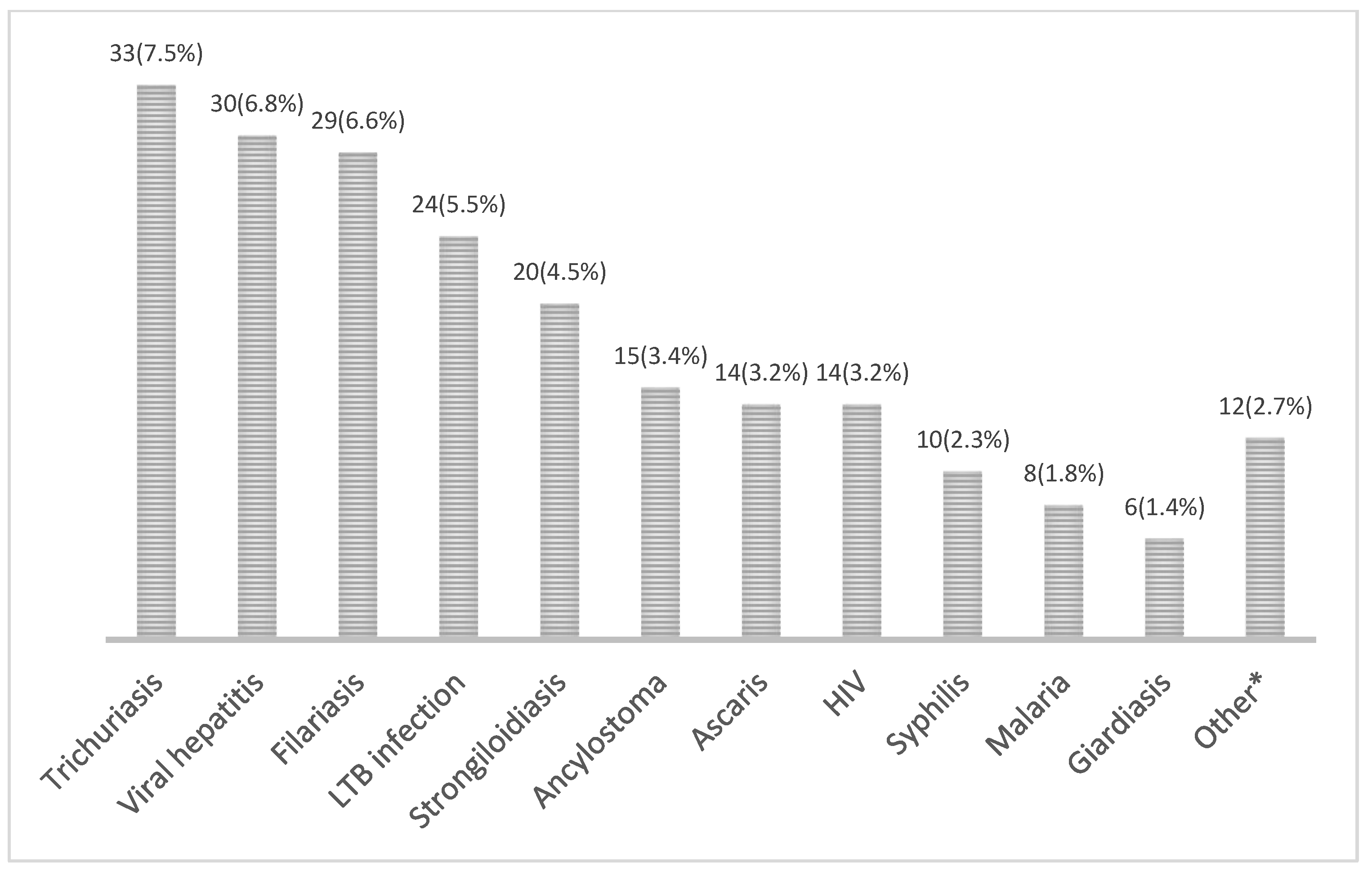
3.4. General Screening Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deol, A.K.; Fleming, F.M.; Calvo-Urbano, B.; Walker, M.; Bucumi, V.; Gnandou, I.; Tukahebwa, E.M.; Jemu, S.; Mwingira, U.J.; Alkohlani, A.; et al. Schistosomiasis—Assessing Progress toward the 2020 and 2025 Global Goals. N. Engl. J. Med. 2019, 381, 2519–2528. [Google Scholar] [CrossRef]
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Mc Manus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuem Tchuenté, L.A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef] [PubMed]
- de Laval, F.; Savini, H.; Biance- Valero, E.; Simon, F. Human Schistosomiasis: An Emerging Threat for Europe. Lancet 2014, 384, 1094–1095. Available online: http://linkinghub.elsevier.com/retrieve/pii/S014067361461669X (accessed on 12 December 2021). [CrossRef] [PubMed]
- Beltrame, A.; Buonfrate, D.; Gobbi, F.; Angheben, A.; Marchese, V.; Monteiro, G.B.; Bisoffi, Z. The hidden epidemic of schistosomiasis in recent African immigrants and asylum seekers to Italy. Eur. J. Epidemiol. 2017, 32, 733–735. [Google Scholar] [CrossRef]
- Boissier, J.; Grech-Angelini, S.; Webster, B.L.; Allienne, J.-F.; Huyse, T.; Mas-Coma, S.; Toulza, E.; Barré-Cardi, H.; Rollinson, D.; Kincaid-Smith, J.; et al. Outbreak of urogenital schistosomiasis in Corsica (France): An epidemiological case study. Lancet Infect. Dis. 2016, 16, 971–979. [Google Scholar] [CrossRef]
- Agbata, E.N.; Morton, R.L.; Bisoffi, Z.; Bottieau, E.; Greenaway, C.; Biggs, B.-A.; Montero, N.; Tran, A.; Rowbotham, N.; Arevalo-Rodriguez, I.; et al. Effectiveness of screening and treatment approaches for schistosomiasis and strongyloidiasis in newly-arrived migrants from endemic countries in the EU/EEA: A systematic review. Int. J. Environ. Res. Public Health 2019, 16, 11. [Google Scholar] [CrossRef]
- Katz, N.; Chaves, A.; Pellegrino, J.P. A simple device for quantitative stool thick-smear in Schistosoma mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar] [PubMed]
- Bärenbold, O.; Raso, G.; Coulibaly, J.T.; N’ Goran, E.K.; Utzinger, J.; Vounatsou, P. Estimating sensitivity of the Kato-Katz technique for the diagnosis of Schistosoma mansoni and hookworm in relation to infection intensity. PLoS Negl. Trop. Dis. 2017, 11, e0005953. [Google Scholar] [CrossRef]
- Kittur, N.; Castleman, J.D.; Campbell, C.H.; King, C.H.; Colley, D.G. Comparison of schistosoma mansoni prevalence and intensity of infection, as determined by the circulating cathodic antigen urine assay or by the kato-katz fecal assay: A systematic review. Am. J. Trop. Med. Hyg. 2016, 94, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Hinz, R.; Schwarz, N.G.; Hahn, A.; Frickmann, H. Serological approaches for the diagnosis of schistosomiasis—A review. Mol. Cell. Probes 2017, 31, 2–21. [Google Scholar] [CrossRef] [PubMed]
- United Nations Educational Scientific and Cultural Organization UNESCO. Learning to Live Together [Internet]. Available online: https://wayback.archive-it.org/10611/20171126022441/http://www.unesco.org/new/en/social-and-human-sciences/themes/international-migration/glossary/migrant/ (accessed on 20 December 2021).
- Centers for Disease Control and Prevention. CDC Yellow Book 2020: Health Information for International Travel [Internet]; Oxford University Press: Oxford, UK, 2020; Chapter 9. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-for-work-other-reasons/visiting-friends-and-relatives-vfr-travel (accessed on 23 December 2021).
- Salas-Coronas, J.; Ramírez-Olivencia, G.; Pérez-Arellano, J.L.; Belhassen-García, M.; Carranza-Rodríguez, C.; García-Rodríguez, M.; Villar-Garcia, J.; Treviño-Maruri, B.; Serre-Delcor, N.; López-Vélez, R.; et al. Diagnosis and treatment of imported eosinophilia in travellers and immigrants: Recommendations of the Spanish Society of Tropical Medicine and International Health (SEMTSI). Rev. Esp. Quimioter Public Soc. Esp. Quimioter. 2017, 30, 62–78. [Google Scholar]
- World Health Organisation. Schistosomiasis. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (accessed on 19 November 2021).
- Salas-Coronas, J.; Cabezas-Fernandez, M.T.; Lozano-Serrano, A.B.; Soriano-Perez, M.J.; Vazquez-Villegas, J.; Cuenca-Gomez, J. Newly arrived african migrants to Spain: Epidemiology and burden of disease. Am. J. Trop. Med. Hyg. 2018, 98, 319–325. [Google Scholar] [CrossRef]
- Delcor, N.S.; Ascaso, C.; Essadik, H.O.; Maruri, B.T.; Romero, I.M.; Guiu, I.C.; Soley, M.E.; Arandes, A.S. Infectious diseases in sub-Saharan immigrants to Spain. Am. J. Trop. Med. Hyg. 2016, 94, 750–756. [Google Scholar] [CrossRef]
- Kärki, T.; Napoli, C.; Riccardo, F.; Fabiani, M.; Dente, M.G.; Carballo, M.; Noori, T.; Declich, S. Screening for Infectious Diseases among Newly Arrived Migrants in EU/EEA Countries—Varying Practices but Consensus on the Utility of Screening. Int. J. Environ. Res. Public Health 2014, 11, 11004–11014. [Google Scholar] [CrossRef]
- Bocanegra, C.; Salvador, F.; Sulleiro, E.; Sánchez-Montalvá, A.; Pahissa, A.; Molina, I. Screening for imported diseases in an immigrant population: Experience from a teaching hospital in Barcelona, Spain. Am. J. Trop. Med. Hyg. 2014, 91, 1277–1281. [Google Scholar] [CrossRef]
- Asundi, A.; Beliavsky, A.; Liu, X.J.; Akaberi, A.; Schwarzer, G.; Bisoffi, Z.; Requena-Méndez, A.; Shrier, I.; Greenaway, C. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e236–e248. [Google Scholar] [CrossRef]
- Salvador, F.; Ribera, E.; Crespo, M.; Falcó, V.; Curran, A.; Ocaña, I.; Eynde, E.V.D.; Molina, I.; Pahissa, A.; Navarro, J.; et al. Tropical diseases screening in immigrant patients with human immunodeficiency virus infection in Spain. Am. J. Trop. Med. Hyg. 2013, 88, 1196–1202. [Google Scholar] [CrossRef]
- Sánchez-Montalvá, A.; Camps, I.R.; Barba, P.; Valcarcel, D.; Sulleiro, E.; Sanz-García, E.; Molina, I.; Salvador, F. Imported Disease Screening Prior to Chemotherapy and Bone Marrow Transplantation for Oncohematological Malignancies. Am. J. Trop. Med. Hyg. 2016, 95, 1463–1468. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27928093 (accessed on 20 December 2021). [CrossRef]
- Utzinger, J.; Booth, M.; N’ Goran, E.K.; Müller, I.; Tanner, M.; Lengeler, C. Relative contribution of day-to-day and intra-specimen variation in faecal egg counts of Schistosoma mansoni before and after treatment with praziquantel. Parasitology 2001, 122, 537–544. [Google Scholar] [CrossRef] [PubMed]
- de Vlas, S.J.; Gryseels, B. Underestimation of Schistosoma mansoni prevalences. Parasitol. Today 1992, 8, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.J.; Magalhães, F.D.C.; Elias, A.M.S.; De Castro, V.N.; Favero, V.; Lindholz, C.G.; Oliveira, A.; Barbosa, F.S.; Gil, F.; Gomes, M.A.; et al. Evaluation of diagnostic methods for the detection of intestinal schistosomiasis in endemic areas with low parasite loads: Saline gradient, Helmintex, Kato-Katz and rapid urine test. PLoS Negl. Trop. Dis. 2018, 12, e0006232. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Mgeni, A.F.; Khamis, I.S.; Steinmann, P.; Stothard, R.; Rollinson, D.; Marti, H.; Utzinger, J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: Effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl. Trop. Dis. 2008, 2, e331. [Google Scholar] [CrossRef]
- Bierman, W.F.W.; Wetsteyn, J.C.F.M.; Van Gool, T. Presentation and diagnosis of imported schistosomiasis: Relevance of eosinophilia, microscopy for ova, and serology. J. Travel Med. 2005, 12, 9–13. [Google Scholar] [CrossRef]
- Kinkel, H.F.; Dittrich, S.; Baümer, B.; Weitzel, T. Evaluation of eight serological tests for diagnosis of imported schistosomiasis. Clin. Vaccine Immunol. 2012, 19, 948–953. [Google Scholar] [CrossRef]
- Elfaki, T.E.M.; Arndts, K.; Wiszniewsky, A.; Ritter, M.; Goreish, I.A.; Mekki, M.E.Y.A.A.E.; Arriens, S.; Pfarr, K.; Fimmers, R.; Doenhoff, M.; et al. Multivariable Regression Analysis in Schistosoma mansoni-Infected Individuals in the Sudan Reveals Unique Immunoepidemiological Profiles in Uninfected, egg+ and Non-egg+ Infected Individuals. PLoS Negl. Trop. Dis. 2016, 10, e0004629. [Google Scholar] [CrossRef]
- Marchese, V.; Beltrame, A.; Angheben, A.; Monteiro, G.B.; Giorli, G.; Perandin, F.; Buonfrate, D.; Bisoffi, Z. Schistosomiasis in immigrants, refugees and travellers in an Italian referral centre for tropical diseases. Infect. Dis. Poverty 2018, 7, 56–65. [Google Scholar] [CrossRef]
- Belhassen-García, M.; Pardo-Lledías, J.; Pérez Del Villar, L.; Muro, A.; Velasco-Tirado, V.; Blázquez De Castro, A.; Vicente, B.; García García, M.I.; Bellido, J.L.M.; Cordero-Sánchez, M. Relevance of eosinophilia and hyper-IgE in immigrant children. Medicine 2014, 93, e43. [Google Scholar] [CrossRef]
- Chaves, N.J.; Paxton, G.A.; Biggs, B.-A.; Thambiran, A.; Gardiner, J.; Williams, J.; Smith, M.M.; Davis, J.S. The Australasian Society for Infectious Diseases and Refugee Health Network of Australia recommendations for health assessment for people from refugee-like backgrounds: An abridged outline. Med. J. Aust. 2017, 206, 310–315. [Google Scholar] [CrossRef]
- Tilli, M.; Gobbi, F.; Rinaldi, F.; Testa, J.; Caligaris, S.; Magro, P.; Buonfrate, D.; Degani, M.; Minervini, A.; Carini, M.; et al. The diagnosis and treatment of urogenital schistosomiasis in Italy in a retrospective cohort of immigrants from Sub-Saharan Africa. Infection 2019, 47, 447–459. [Google Scholar] [CrossRef]
- Salas-Coronas, J.; Vázquez-Villegas, J.; Lozano-Serrano, A.B.; Soriano-Pérez, M.J.; Cabeza-Barrera, I.; Cabezas-Fernández, M.T.; Villarejo-Ordóñez, A.; Sánchez-Sánchez, J.C.; Vivas-Pérez, J.I.A.; Vázquez-Blanc, S.; et al. Severe complications of imported schistosomiasis, Spain: A retrospective observational study. Travel Med. Infect. Dis. 2020, 35, 101508. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.K.; Beckett, C.L.; Leder, K.; Biggs, B.A.; Torresi, J.; O’ Brien, D.P. Long-term follow-up of schistosomiasis serology post-treatment in Australian Travelers and Immigrants. J. Travel Med. 2010, 17, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Pavli, A.; Maltezou, H. Health problems of newly arrived migrants and refugees in Europe. J. Travel Med. 2017, 24, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Netto, G.; Bhopal, R.; Lederle, N.; Khatoon, J.; Jackson, A. How can health promotion interventions be adapted for minority ethnic communities? Five principles for guiding the development of behavioural interventions. Health Promot Int. 2010, 25, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Rechel, B.; Mladovsky, P.; Ingleby, D.; Mackenbach, J.P.; Mc Kee, M. Migration and health in an increasingly diverse Europe. Lancet 2013, 381, 1235–1245. [Google Scholar] [CrossRef]
- Beltrame, A.; Guerriero, M.; Angheben, A.; Gobbi, F.; Requena-Mendez, A.; Zammarchi, L.; Formenti, F.; Perandin, F.; Buonfrate, D.; Bisoffi, Z. Accuracy of parasitological and immunological tests for the screening of human schistosomiasis in immigrants and refugees from African countries: An approach with Latent Class Analysis. PLoS Negl. Trop. Dis. 2017, 11, e0005593. [Google Scholar] [CrossRef] [PubMed]
- Guegan, H.; Fillaux, J.; Charpentier, E.; Robert-Gangneux, F.; Chauvin, P.; Guemas, E.; Boissier, J.; Valentin, A.; Cassaing, S.; Gangneux, J.-P.; et al. Real-time PCR for diagnosis of imported schistosomiasis. PLoS Negl. Trop. Dis. 2019, 13, e0007711. [Google Scholar] [CrossRef]
- van Grootveld, R.; van Dam, G.J.; de Dood, C.; de Vries, J.J.C.; Visser, L.G.; Corstjens, P.L.A.M.; van Lieshout, L. Improved diagnosis of active Schistosoma infection in travellers and migrants using the ultra-sensitive in-house lateral flow test for detection of circulating anodic antigen (CAA) in serum. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1709–1716. [Google Scholar] [CrossRef]
- van Lieshout, L.; Polderman, A.M.; Deelder, A.M. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop. 2000, 77, 69–80. [Google Scholar] [CrossRef]
- Casacuberta-Partal, M.; Janse, J.J.; van Schuijlenburg, R.; de Vries, J.J.C.; Erkens, M.A.A.; Suijk, K.; van Aalst, M.; Maas, J.J.; Grobusch, M.P.; van Genderen, P.J.J.; et al. Antigen-based diagnosis of Schistosoma infection in travellers: A prospective study. J. Travel Med. 2020, 27, taaa055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cucchetto, G.; Buonfrate, D.; Marchese, V.; Rodari, P.; Ferrari, A.; Zanotti, P.; Bottieau, E.; Silva, R.; Bisoffi, Z.; Gobbi, F. High-dose or multi-day praziquantel for imported schistosomiasis? A systematic review. J. Travel Med. 2019, 26, taz050. [Google Scholar] [CrossRef]
- Ciccacci, F.; Orlando, S.; Majid, N.; Marazzi, C. Epidemiological transition and double burden of diseases in low-income countries: The case of Mozambique. Pan. Afr. Med. J. 2020, 37, 49. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 440) | Group A (n = 399) | Group B (n = 41) | p-Value | |
|---|---|---|---|---|
| Male * | 250 (56.8%) | 225 (56.4%) | 25 (61.0%) | 0.32 |
| Age (years) ** | 36.0 [20] | 36.0 [20] | 32.0 [18] | 0.06 |
Country of origin *
| ||||
| 190 (43.2%) | 178 (44.6%) | 12 (37.1%) | 0.06 | |
Past medical history *
| 15 (3.4%) 16 (3.6%) 43 (9.8%) | 11 (2.8%) 14 (3.5%) 41 (10.3%) | 4 (9.8%) 2 (4.9%) 2 (4.9%) | 0.02 0.66 0.27 |
Type of migrant *
| 13 (3.0%) 427 (97.0%) 145 (33.0%) 157 (35.7%) | 7 (1.8%) 392 (98.2%) 136 (34.1%) 139 (34.8%) | 6 (14.6%) 35 (85.4%) 9 (22.0%) 18 (43.9%) | <0.001 - 0.03 0.26 |
| Peripheric eosinophilia * | 82 (18.6%) | 72 (18.1%) | 10 (24.4%) | 0.32 |
| Elevated IgE * | 242 (55.0%) | 239 (61.3%) | 3 (100%) | <0.001 |
| Schistosomiasis Cases * | Group A (n = 399) | Group B (n = 41) |
|---|---|---|
Confirmed cases
| 37 (9.3%) 26 (6.5%) 4 (1.0%) 6 (1.5%) | 3 (7.3%) 3 (7.3%) - - |
| Probable cases | 9 (2.0%) | 1 (2.4%) |
| Total | 46 (11%) | 4 (9.8%) |
| Copro/Uroparasitological Sample Examination | ||||||
|---|---|---|---|---|---|---|
| S. mansoni | S. intercalatum | S. haematobium | No eggs | Total | ||
| S. mansoni. serology (ELISA IgG) | Positive | 0 (0%) | 0 (0%) | 3 (17.6%) | 14 (82.4%) | 17 |
| Negative | 1 (1.4%) | 1 (1.4%) | 0 (0%) | 69 (97.2%) | 71 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roade, L.; Sulleiro, E.; Bocanegra, C.; Salvador, F.; Treviño, B.; Zarzuela, F.; Goterris, L.; Serre-Delcor, N.; Oliveira-Souto, I.; Aznar, M.L.; et al. Evaluation of Two Different Strategies for Schistosomiasis Screening in High-Risk Groups in a Non-Endemic Setting. Trop. Med. Infect. Dis. 2023, 8, 44. https://doi.org/10.3390/tropicalmed8010044
Roade L, Sulleiro E, Bocanegra C, Salvador F, Treviño B, Zarzuela F, Goterris L, Serre-Delcor N, Oliveira-Souto I, Aznar ML, et al. Evaluation of Two Different Strategies for Schistosomiasis Screening in High-Risk Groups in a Non-Endemic Setting. Tropical Medicine and Infectious Disease. 2023; 8(1):44. https://doi.org/10.3390/tropicalmed8010044
Chicago/Turabian StyleRoade, Luisa, Elena Sulleiro, Cristina Bocanegra, Fernando Salvador, Begoña Treviño, Francesc Zarzuela, Lidia Goterris, Nuria Serre-Delcor, Inés Oliveira-Souto, Maria Luisa Aznar, and et al. 2023. "Evaluation of Two Different Strategies for Schistosomiasis Screening in High-Risk Groups in a Non-Endemic Setting" Tropical Medicine and Infectious Disease 8, no. 1: 44. https://doi.org/10.3390/tropicalmed8010044
APA StyleRoade, L., Sulleiro, E., Bocanegra, C., Salvador, F., Treviño, B., Zarzuela, F., Goterris, L., Serre-Delcor, N., Oliveira-Souto, I., Aznar, M. L., Pou, D., Sánchez-Montalvà, A., Bosch-Nicolau, P., Espinosa-Pereiro, J., & Molina, I. (2023). Evaluation of Two Different Strategies for Schistosomiasis Screening in High-Risk Groups in a Non-Endemic Setting. Tropical Medicine and Infectious Disease, 8(1), 44. https://doi.org/10.3390/tropicalmed8010044







