Abstract
Strongyloidiasis is a disease caused by Strongyloides stercoralis and remains a neglected tropical infection despite significant public health concerns. Challenges in the management of strongyloidiasis arise from wide ranging clinical presentations, lack of practical high sensitivity diagnostic tests, and a fatal outcome in immunocompromised hosts. Migration, globalization, and increased administration of immunomodulators, particularly during the COVID-19 era, have amplified the global impact of strongyloidiasis. Here, we comprehensively review the diagnostic tests, clinical manifestations, and treatment of strongyloidiasis. The review additionally focuses on complicated strongyloidiasis in immunocompromised patients and critical screening strategies. Diagnosis of strongyloidiasis is challenging because of non-specific presentations and low parasite load. In contrast, treatment is simple: administration of single dosage ivermectin or moxidectin, a recent anthelmintic drug. Undiagnosed infections result in hyperinfection syndrome and disseminated disease when patients become immunocompromised. Thus, disease manifestation awareness among clinicians is crucial. Furthermore, active surveillance and advanced diagnostic tests are essential for fundamental management.
1. Introduction
Strongyloidiasis, a disease caused by Strongyloides stercoralis, continues to persist as a worldwide public health issue. However, the real burden of this disease is unknown, and studies have been performed in limited geographical areas and populations [1,2]. An estimated 370 million people with strongyloidiasis worldwide, with a prevalence between 10% and 40% of the population in tropical and subtropical countries, was dated back to 2013 [1,3]. Although the treatment of chronic strongyloidiasis is straightforward, infection diagnosis remains a challenge, resulting in prevalence being under-reported. A unique autoinfection stage during the S. stercoralis life cycle potentially causes lifelong parasitic infections [4], lingering analogous to a ticking time bomb, which eventually bursts into escalated and life-threatening hyperinfection or disseminated strongyloidiasis when patients experience immune response impairment [5].
2. Materials and Methods
A web-based search was performed via PubMed and Google Scholar. We included original articles, reviews, case reports, and short communications in English published from 1987 to 2021. The keywords included ‘Strongyloides stercoralis’, ‘strongyloidiasis’, ‘strongyloidiasis management’, ‘strongyloidiasis epidemiology’, ‘strongyloidiasis diagnosis’, ‘Strongyloides stercoralis serology’, ‘Strongyloides hyperinfection syndrome’, ‘disseminated strongyloidiasis’, and ‘strongyloidiasis and COVID-19′. Standard textbooks, guidelines, and article bibliographies were used for additional references.
3. Epidemiology
Strongyloides is a soil-transmitted nematode that is endemic, yet not confined to tropical and subtropical countries. The global prevalence of S. stercoralis has been estimated at 10–40% of the population in tropical and subtropical regions, equivalent to an estimated 30–100 million cases [6]. However, it is under-reported in areas in which low-sensitivity diagnostic tests are used. The epidemiology of S. stercoralis infection differs from that of other helminth infections because of its unique ability for reinfection in humans, also defined as autoinfection [7]. The estimated prevalence has varied among community-based, hospital-based, and refugee and immigrant studies [6]. Asudai et al. reported a 2019-meta-analysis of studies involving migrants worldwide and demonstrated a pooled strongyloidiasis seroprevalence of 12.2%: 17.3% from East Asia and the Pacific, 14.6% from sub-Saharan Africa, and 11.4% from Latin America and the Caribbean [8]. S. stercoralis accounts for most human infections, whereas Strongyloides fuelleborni fuelleborni or S. fuelleborni kelleyi account for rare infections in certain geographical regions, including Papua New Guinea, Thailand, and the Philippines [1,6,9]. Brazil and Thailand are major hotspots for strongyloidiasis, with a prevalence of 10.8–17% and 23.7–34.7%, respectively. Most studies in European countries have focused on refugees, immigrants, and travelers from endemic countries because S. stercoralis has a lower prevalence in developed countries and in urban areas of developing countries where fecal contamination in soil is scarce [1,6]. The estimated prevalence in developed countries is heterogeneous, depending on study type and population ranging from < 0.1% in high-income countries in temperate zones [10] to 12.4–14.8% among farm workers on the Mediterranean coast [11].
The Committee to Advise on Tropical Medicine and Travel (CATMAT) categorized the epidemiological risk for Strongyloides infection and exposure as follows [12]: high risk (>10%) for birth, residence, or long-term travel (defined as cumulative six-month exposure, or contact of skin with sand or soil in a high risk area during shorter-term travel) in Southeast Asia, Oceania, Sub-Saharan Africa, South America, and the Caribbean; moderate risk (3–10%) in Mediterranean countries, Middle East, North Africa, Indian sub-continent, and Asia; and low risk (<3%) in Australia, North America, or Western Europe [10,12,13]. The risk of developing complicated strongyloidiasis is more pronounced in patients with compromised cell-mediated immunity, especially in patients from endemic locations who later receive immuno-suppressive treatment [1].
4. Life Cycle
Comprehension of the unique life cycle associated with S. stercoralis is important for clinical evaluation and management. The life cycle can be divided into parasitic (direct) and free-living (indirect) stages, as shown in Figure 1. The indirect life cycle of S. stercoralis (Figure 1) initiates when rhabditiform larvae (Figure 2A) pass from stools to soil. Subsequently, the larvae can either directly transform into invasive filariform larvae, shown in Figure 2B, which is the infective stage, or develop and molt into the free-living phase and thrive as adults in soil. In the free-living stage, female and male adults exhibiting rhabditiform type characteristic esophagus mate and enable female adults to deposit eggs in soil. The eggs hatch into rhabditiform larvae that develop into filariform larvae, which is the infective stage.
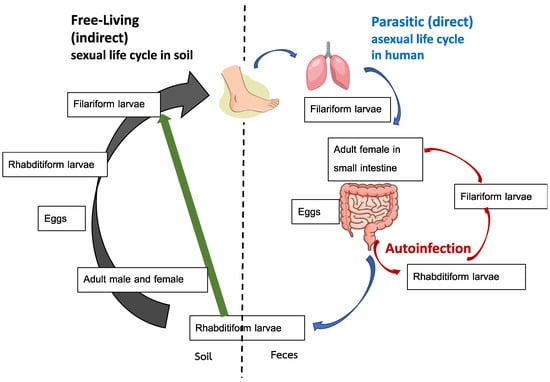
Figure 1.
Life cycle of Strongyloides stercoralis. Adapted from reference no. [17].
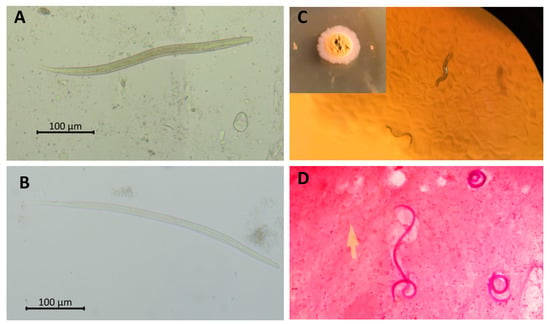
Figure 2.
Parasitological detection of Strongyloides stercoralis. Rhabditiform (A) and filariform; (B) larvae of S. stercoralis fresh smear. Migrating rhabditiform larvae in agar plate culture; (C). Gram staining of filariform larvae in sputum of a patient with S. stercoralis hyperinfection syndrome (100×) (D) Picture; (C) courtesy of Poom Adisakwattana.
S. stercoralis are soil-transmitted helminths. Humans are mainly infected via skin penetration of filariform larvae, especially through barefoot contact for the direct life cycle, or more rarely, through ingestion of contaminated food and drink. A serpiginous lesion (pruritus track) can be seen at the site of entry. Upon entry, larvae pass through the venous circulation and migrate to the heart and lungs. The filariform larvae, which later ascend the tracheobronchial tree, are expectorated into sputum and swallowed into the gastrointestinal (GI) tract, at which point molting and development into the adult stage are initiated. Subsequently, adults are embedded in the small intestinal mucosa, mainly in the duodenum. Female adults that asexually reproduce can be detected [4]. Finally, eggs hatch in the intestine and the rhabditiform larvae are excreted in stools.
The autoinfection life cycle occurs when the rhabditiform larvae develop into invasive filariform larvae prior to expulsion in stools. Subsequently, reinfection occurs during filariform larvae penetration into the intestinal mucosa (internal autoinfection) or perianal skin (external autoinfection) [14]. This unique autoinfection cycle enables S. stercoralis to cause persistent or even lifelong infection. The simultaneous detection of rhabditiform and filariform larvae in stools can reflect the autoinfection that usually occurs in hyperinfection syndrome or in immunosuppressed hosts [15]. Theoretically, the autoinfection life cycle spans 2–3 weeks. Thus, antiparasitic treatment should be administered in repeating 2–3-week intervals to ensure cure of strongyloidiasis [16].
5. Laboratory Diagnosis
At present, there is no gold standard diagnostic technique for S. stercoralis infection [18], and the available parasitological and serological diagnostic methods still possess limitations. The advantages and disadvantages of each diagnostic method are compared in Table 1. A combination of diagnostic techniques is recommended.

Table 1.
Summary of diagnostic tests for strongyloidiasis.
For parasitological detection, a single stool concentration examination has limited sensitivity to diagnose chronic strongyloidiasis because of low parasite load and irregular fecal shedding of larvae. Thus, repeated stool examination consisting of 3–7 specimens is recommended [17]. In contrast to chronic strongyloidiasis, an abundance of larvae can be obtained in stools through a simple smear from patients with hyperinfection or disseminated infection because of the high parasite load.
The Baermann funnel method is more sensitive than the single stool concentration technique. Although the Baermann funnel is less sensitive than the stool culture techniques for strongyloidiasis diagnosis, its quantification of parasite burden makes it suitable for utilization in study settings.
The culture techniques, such as Harada Mori culture, polyethylene tube culture, and agar plate culture have higher sensitivity than stool concentration techniques and are recommended as the laboratory investigations of choice. However, the disadvantages of the culture techniques are time consumption, high cost, availability limitations, and requirement for fresh stool samples [17,19]. For instance, agar plate culture, which is the most efficient stool culture technique, requires fresh stools and 2–3 days to detect migrating rhabditiform larvae on agar (Figure 2C). Stool culture techniques only enable detection of Strongyloides spp., Trichostrongylus spp., and hookworm larvae, thus requiring other stool examination techniques for the identification of concurrent parasite infections.
The presence of larvae in sputum and/or bronchoalveolar lavage indicates hyperinfection syndrome, and simple fresh smear and special staining can be performed (Figure 2D). A high index of suspicion is required, and bedside sputum examination is essential for the diagnosis of strongyloidiasis hyperinfection syndrome. Tissue biopsy is required to diagnose ectopic foci (organs not involved in the life cycle) of S. stercoralis in disseminated infection. Furthermore, rhabditiform larvae can be found in duodenal biopsy when patients (especially transplant hosts) undergo upper GI endoscopy for other reasons [20].
Eosinophilia is common in strongyloidiasis, but it is usually mild (5–15%) and nonspecific [17]. A study in an Aboriginal community in the endemic area of North Australia reported that eosinophilia had 60.9% sensitivity, 71.1% specificity, 54.6% positive predictive value, and 76.1% negative predictive value for S. stercoralis infection [21]. The presence of eosinophilia may suggest parasitic infections including S. stercoralis but the absence of eosinophilia cannot rule out strongyloidiasis. Moreover, eosinophilia tended to present less in S. stercoralis infection in immunocompromised individuals [22].
In terms of serological methods, techniques used to detect antibody response to S. stercoralis include enzyme-linked immunosorbent assay (ELISA), indirect agglutination, indirect immunofluorescence, and western blotting (immunoblotting). Their respective sensitivities and specificities depend on the types of antigens used and the detected immunoglobulins. In principle, serology is more sensitive than fecal-based methods. Serology is effective for diagnosis of stronyloidiasis in people residing in endemic areas, and immigrants who require a screening test prior to immunosuppressive treatment, and for use in seroepidemiological studies. Furthermore, serology is useful in post-treatment follow-up and in monitoring outcomes of public health control intervention programs [17,23,24,25]. The drawbacks of serology include low sensitivity in patients with impaired immunity [26,27] and cross-reactivity with other helminthiases, such as filariasis, ascariasis, and schistosomiasis [17,19]. However, cross-reactivity is dependent upon prepared antigen type and additional diseases present in respective laboratory studies. Our center, the Faculty of Tropical Medicine, Mahidol University is a center of excellence in parasite diagnostic serology. Our indirect IgG-ELISA diagnosis of strongyloidiasis, prepared from a modified molecular weight cut-off antigen, yielded a sensitivity and specificity of 96% and 94%, respectively, in immunocompetent hosts, and 42.9% and 96.3%, in immunocompromised hosts [27,28]. Recently, several sensitive and specific point-of-care serological tests for strongyloidiasis were developed and showed sensitivities and specificities of 91.3–93.3% and 83.8–100%, respectively [29,30,31]. The rapid point-of-care test also worked well in a field study, with 82% sensitivity and 96% specificity [32].
For a decade, the molecular techniques of polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) have been developed for diagnosis of strongyloidiasis with varying results [33,34]. Apart from stool examination, the molecular techniques retain compatibility for other clinical specimens including sputum, blood, urine, bronchoalveolar lavage, and intestinal aspiration [33,35]. A meta-analysis reported that high specificity with limited sensitivity validates PCR as more suitable for diagnostic confirmation, as opposed to an initial screening test for strongyloidiasis [36]. Although LAMP offers a rapid and economic testing alternative, it appears less effective for S. stercoralis detection in clinical specimens when compared with PCR [33].
6. Clinical Syndromes
S. stercoralis can cause a wide spectrum of disease presentations depending on the host’s immunity and parasite load. The clinical syndromes include acute strongyloidiasis, chronic strongyloidiasis, hyperinfection syndrome, and disseminated infection. Strongylodiasis can be simply classified as “uncomplicated” (acute and chronic strongyloidiasis) or “severe or complicated” (hyperinfection syndrome and disseminated infection) [17].
6.1. Acute Strongyloidiasis
This syndrome is rarely diagnosed. It is mainly reported in travelers who returned from endemic areas [5,37]. The symptoms at this stage are from reactions at the site of larval entry and migration, which normally occur immediately to several weeks post-infection. Skin manifestations include a serpiginous lesion (urticarial track with severe pruritus) at the entry site; the lung migration of parasites can result in dry cough and wheezing or even the classic Loeffler-like syndrome [38]. Lastly, GI symptoms begin when the parasites reach the intestine ~2 weeks after infection [4]. At this stage, serological testing is usually negative, and diagnosis is based on rhabditiform larvae detection in stools which is normally found 2–4 weeks after infection [39].
6.2. Chronic Strongyloidiasis
Most people with chronic strongyloidiasis are asymptomatic or have mild symptoms. The common GI symptoms include abdominal bloating, epigastric pain that worsens by eating, intermittent vomiting, diarrhea, constipation, and borborygmus. Larva currens, a rapid intradermal migration of larvae (5–15 cm/h), is the pathognomonic skin lesion (Figure 3A) [38]. Other skin manifestations include urticarial and recurrent maculopapular rashes. A meta-analysis reported that the significant symptoms associated with chronic strongyloidiasis were abdominal pain, diarrhea, and urticaria [2].
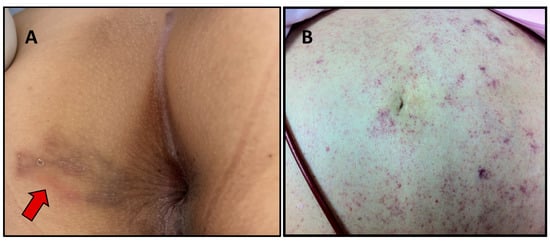
Figure 3.
Skin lesions that can be found in strongyloidiasis. Larva currens in the perianal area (arrow) (A) and periumbilical parasitic thumbprint purpura in a patient with disseminated strongyloidiasis (B). Picture (A) courtesy of Than Narkwiboonwong.
6.3. Hyperinfection Syndrome
Hyperinfection syndrome is defined by an increase in parasite load from the autoinfection life cycle, usually (but not always) caused by impairment of immune status. Thus, the larvae in non-disseminated hyperinfection syndrome are confined to organs directly involved in the S. stercoralis life cycle, which includes the GI tract, peritoneum, and lungs. Skin lesions in hyperinfection syndrome consist of larva currens, petechial, and purpuric rashes that are commonly found in the lower trunk, thighs, and buttocks [4].
A retrospective study reported the common clinical manifestations of Strongyloides hyperinfection syndrome which included fever (80.8%), respiratory (88.6%), and GI (71.2%) symptoms [40]. The pulmonary symptoms of hyperinfection syndrome included cough, shortness of breath and asthma-like presentations; acute respiratory distress can also occur [41]. However, diagnostic difficulties may arise as immunocompromised patients often possess underlying pulmonary diseases, or the symptoms might be masked by secondary infections. Chest radiography is often variable. The classic bilateral or focal interstitial infiltrates can be detected. However, the consolidations and abscesses can occur especially with concurrent bacterial pneumonia. Sputum examination demonstrates filariform or rhabditiform larvae and even occasionally eggs [4].
6.4. Disseminated Infection
Disseminated infection describes the migration of larvae to organs beyond the range of the pulmonary autoinfective cycle. The larvae are found in ectopic sites including the skin, liver, brain, heart, and urinary tract. Periumbilical parasitic thumbprint purpura is a rare skin presentation classically found in disseminated strongyloidiasis (Figure 3B) [42]. This particular skin lesion results from the penetration of larva into the skin.
Concurrent bacterial and fungal infections, mostly from enteric pathogens, often occur in hyperinfection and disseminated infection, and the clinical presentations include bacteremia, peritonitis, and pneumonia [17]. Blood and cerebrospinal fluid cultures were positive for bacteria in 29.1% and 15.2%, respectively, of 151 patients with severe strongyloidiasis [43]. Shock and respiratory failure were reported in up to 57.3% and 67.9%, respectively, of patients with hyperinfection syndrome [41].
7. Strongyloidiasis in Immunocompromised Patients
The immunocompromised conditions associated with severe strongyloidiasis are mainly defects in the cell mediated immune response. The most common condition is corticosteroid treatment. One study reported that the mean corticosteroid dose in patients with severe strongyloidiasis was 52 ± 42 mg prednisolone equivalent per day, and the duration ranged from four days to 20 years [43]. Other treatments associated with severe strongyloidiasis are chemotherapy, cyclosporine, azathioprine, total body irradiation, and transplantation. Diseases with a high risk of severe strongyloidiasis are hematological malignancy, human T-cell lymphotropic virus type 1 (HTLV-1) infection, malnutrition and hypogammaglobulinemia [4,26]. The risk of severe strongyloidiasis in people living with HIV has been debated; disseminated strongyloidiasis was removed from the list of opportunistic infections because corticosteroid use in severe Pneumocystis pneumonia was suspected to be a confounding factor [1,4]. However, the prevalence of strongyloidiasis is high in patients with HIV infection, especially among residents and travelers from endemic areas. A previous study reported up to 25% seroprevalence among antiretroviral-naive HIV patients with CD4 count ≤ 100 cells/µL [44].
The real impact of strongyloidiasis among immunocompromised individuals is unknown and it is believed to be under-reported because of inadequate screening and difficulties in diagnosis. The reported prevalence varies from 3% to 23.0% depending on the population and method of testing [27,45,46,47]. The clinical presentation of strongyloidiasis varies from asymptomatic in chronic strongyloidiasis to a fatal disseminated syndrome, depending on host immunity. A cross-sectional study in immunocompromised patients in Thailand revealed a strongyloidiasis prevalence of 6.7%; of which 62.5% of the cases were asymptomatic or chronic strongyloidiasis [27].
Transplantation patients can develop a severe form of strongyloidiasis from their previous infections or new infections during transplantation. Donor derived S. stercoralis infection was documented in solid organ transplant (SOT), especially in renal transplantation [20,48,49], leading to recommendations for S. stercoralis screening in both donors and recipients. Complicated strongyloidiasis developed earlier and had an increased fatality rate in hematopoietic stem cell transplantation (HSCT) when compared with SOT as a result of supplementary intensive immunosuppression [20]. In SOT, complicated strongyloidiasis usually occurred within three months after transplantation [20]. Cyclosporin metabolite produces anti-parasitic effects against Strongyloides spp. Thus, reduced use of cyclosporin-based regimens may result in increased prevalence of severe complicated strongyloidiasis [50,51].
The critical challenge in the diagnosis of severe strongyloidiasis in immunocompromised patients is the potential absence of symptoms and presentations (caused by poor immune response) until the patients reach the full-blown stage. A seroprevalence study among renal transplant recipients reported a significant decrease in IgG titer in repeatedly transplanted patients, suggesting decreased antibody response in more immunosuppressed hosts [47]. Thus, serological testing for strongyloidiasis appears to decrease in sensitivity for immunocompromised patients [26]. Our previous comparative diagnostic study of strongyloidiasis among hospitalized immunocompromised patients in Bangkok, Thailand, reported a decrease in sensitivity of IgG obtained by indirect-ELISA testing from 96% to 42.9% while specificity remained high [27]. The stool agar plate culture remains the investigation method of choice for this group of patients [27].
Immunocompromised hosts with secondary bacterial and fungal infections have poor outcomes in complicated strongyloidiasis. A mortality rate of 69% was reported among immunocompromised patients with severe strongyloidiasis in the USA [52]. An example of S. stercoralis hyperinfection syndrome in an immunocompromised patient is shown in Figure 4.
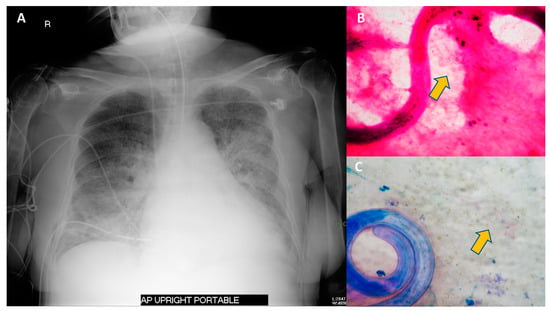
Figure 4.
Chest radiography and sputum examination from a case of S. stercoralis hyperinfection syndrome in an immunocompromised patient. A 75-year-old Thai woman with temporal arteritis had been treated with prednisolone 20 mg/day for 4 months. She developed fever and diarrhea for 2 days prior to respiratory failure. The chest radiography revealed bilateral patchy infiltration (A). Sputum examination with Gram staining (B) and Modified Acid-Fast staining (C) revealed filariform larvae of S. stercoralis and a positive branching filamentous organism (arrow), indicating Nocardia species. Blood cultures grew Escherichia coli. She was diagnosed with S. stercoralis hyperinfection syndrome with concurrent Gram-negative bacteremia and pulmonary nocardiasis. She was treated with broad-spectrum cephalosporin, cotrimoxazole and ivermectin.
8. Management
8.1. Management of Uncomplicated Strongyloidiasis
The antiparasitic drugs effective against strongyloidiasis include ivermectin, benzimidazole compounds (thiabendazole, albendazole and mebendazole), and pyrvinium pamoate. However, most anti-parasitic agents cannot kill migrating larvae and eggs in autoinfection. Thus, repeating the regimen at 2–3-week intervals is usually recommended to eradicate autoinfection. The clinical studies of treatment for chronic uncomplicated strongyloidiasis are summarized in Table 2.

Table 2.
Clinical studies in treatments of chronic uncomplicated strongyloidiasis.
Albendazole shows less efficacy than thiabendazole in the treatment of chronic strongyloidiasis, although it is better tolerated. Albendazole also has activity against a broad range of intestinal helminths.
Nowadays, ivermectin is the drug of choice for uncomplicated strongyloidiasis. A recent phase III randomized control trial revealed that a single dose of ivermectin (200 µg/kg) was sufficient for treatment of uncomplicated strongyloidiasis [65]. In addition to regimen simplicity, this drug is also generally well tolerated in patients. The common adverse events of ivermectin are abnormal liver enzymes (13.6%), itching (1.8–11.8%), headache (8.8–10.6%), loose stools (1.2–9.8%), cough (6.7%), fever (6.3%), and fatigue (0.8–5.9%) [16].
Moxidectin is a veterinary antiparasitic drug that has FDA approval for the treatment of human onchocerciasis and is in line for clinical trials in strongyloidiasis. A single dose of 8 mg veterinary form moxidectin was safe and demonstrated non-inferior efficacy to ivermectin for treatment of uncomplicated strongyloidiasis in a phase II randomized controlled trial [64]. A randomized controlled trial with ascending doses supported a single 8-mg dose of human moxidectin tablets (similar dose for onchocerciasis) for treatment of chronic strongyloidiasis [66]. The advantages of moxidectin over ivermectin include more convenience as a single oral dose independent of patient weight, less neurotoxicity, and a larger volume of distribution with a longer half-life, which might be beneficial towards eradication of auto- and re-infection [66,67]. The drug was also proposed as an alternative in cases with ivermectin failure [67].
8.2. Management of Complicated Strongyloidiasis
The treatment of complicated strongyloidiasis is based on case reports and series. The systematic analysis of case reports showed better outcomes for ivermectin treatment when compared with other single regimen treatments [68]. Although there are no standard guidelines for treatment of severe strongyloidiasis, ivermectin at 200 µg/kg/day until resolution of clinical syndromes and absence of parasite detection in three consecutive specimens has been recommended [4,16,20]. Additionally, continuing treatment until negative fecal culture of S. stercoralis for two weeks has been suggested to eradicate autoinfection [4,69].
Due to the serious condition of cases of complicated strongylodiasis occurring in nature, multiple antiparasitic drugs or multiple routes of ivermectin have been applied in combination with oral ivermectin [70]. Subcutaneous injection of a veterinary parenteral form of ivermectin has been used as a salvage regimen or in patients with absorption problems [71]. The common dosage of parenteral ivermectin was 200 µg/kg/day (range: 75–200 µg/kg/day) and the duration of treatment was in the range of 3–22 doses [16,71]. Parenteral ivermectin is not authorized for human usage and severe neurotoxicity has been reported in patients with complicated strongyloidiasis [72,73]. Thus, patients’ consent before prescription is suggested. To date, there is no report of moxidectin treatment for complicated strongyloidiasis in immunocompromised individuals.
Apart from antiparasitic treatment, the key management of complicated strongyloidiasis is restoring host immunity. The use of immunosuppressive agents needs to be minimized. Furthermore, the concurrent bacterial and fungal infection must be evaluated and empirically treated.
8.3. Follow-Up after Treatment
Both clinical and laboratory evaluation should be performed after treatment. Laboratory evaluations include stool examination, complete blood count and serology. In severe forms of strongyloidiasis, stool follow-up examination should be performed for at least two weeks to ensure eradication [69]. Eosinophilia usually declines to normal levels within one month while serology requires 6–12 months. Therefore, sequential testing every 3–6 months for two years is recommended [16,23]. Screening for HTLV-1 infection is advised in cases of treatment failure [38].
9. Screening and Prevention
To prevent severe forms of strongyloidiasis in immunocompromised patients, vigilant screening before and during immunosuppressive treatment is essential. A combination of tests, including stool concentration examination, stool culture, and serological methods, is recommended [4,27,74]. Some experts have suggested that combination screening should include stool PCR [75]. In cases where sensitive screening tests are not available, pre-emptive treatment with ivermectin is suggested [68,76]. Parasites can be transmitted via SOT, and there is a high prevalence of strongyloidiasis among donors from endemic areas [77]. Therefore, screening tests are recommended in both recipients and donors before transplantation [51,78]. The infected living donors should be cured prior to transplantation while recipients of untreated infected donors require empiric therapy after transplantation and close monitoring [51].
In addition to screening, routine empiric treatment of strongyloidiasis with ivermectin in immunocompromised patients or prior to immunosuppressive treatment has been the recommendation, especially in endemic areas, although no standard regimen or significant evidence-based study has been reported [79,80]. Re-infection is common in endemic areas. Thus, routine periodic deworming has been suggested [78].
In immunocompetent individuals, screening for strongyloidiasis with a preference for serological techniques is recommended in immigrants or long-term travelers (>1 year) from an endemic area [76].
Contact isolation should be applied in all strongyloidiasis cases in addition to screening of all patients’ family members. Patient education that focuses on personal hygiene (using latrines and wearing shoes in endemic areas) should be provided to people at risk (immunocompromised individuals) and the general population.
In endemic areas, community control was successful through proactive case screening and pharmacological treatment regardless of environmental sanitation improvements [81,82]. Mass administration of ivermectin for strongyloidiasis or other parasitic infections has produced beneficial effects toward sustained reduction in prevalence [83,84].
10. Strongyloidiasis and COVID-19
Dexamethasone has been shown to reduce mortality in hospitalized patients with moderate-to-severe COVID-19 [85]. Although most immunocompetent patients have chronic asymptomatic strongyloidiasis, immunocompromised patients, especially those undergoing corticosteroid therapy, can progress to advanced disease, such as disseminated strongyloidiasis and hyperinfection syndrome [86,87]. The prevalence of COVID-19 and strongyloidiasis coinfection is unclear. Pereira and colleagues summarized the reported cases, in which the majority were male, with an average age of 61.3 years, were discharged from hospital, and then returned with skin presentations and symptoms followed by respiratory and GI symptoms [86,88,89,90,91,92]. Symptoms of severe COVID-19 are mainly cough (68.9%), fever (71.6%), dyspnea (71.2%), and diarrhea (20%), while those of Strongyloides hyperinfection syndrome are cough, fever (80.8%), dyspnea or wheeze (88.6%), GI symptoms (71.2%), and disseminated larva currens [93]. Chronic strongyloidiasis and COVID-19 could become commonplace, especially in endemic low- and middle-income countries. Thus, clinicians should evaluate underlying chronic strongyloidiasis infection independent of signs and symptoms, epidemiology, and other behavioral risk factors prior to corticosteroid initiation [87]. According to the 2016 CATMAT recommendation of risk stratification, a test-and-treat strategy is suggested for mild COVID-19 where serological testing is available. Presumptive ivermectin treatment is considered reasonable for moderate- to high-risk patients who are candidates for corticosteroids (equivalent to 20 mg/day prednisolone for ≥ 2 weeks) and have not previously received testing or treatment [12,86,87]. Although Strongyloides serology and stool tests are ideally recommended prior to initiation of immunosuppression, if immediate circumstances limit feasibility, the tests should subsequently be performed as soon as possible. Regarding a limited supply of ivermectin, presumptive ivermectin treatment for COVID-19 patients should be reserved for: (i) empiric therapy for patients with very high epidemiological risk; (ii) patients with asymptomatic strongyloidiasis with positive serology; (iii) Strongyloides hyperinfection or disseminated disease; and (iv) patients with symptomatic Strongyloides infection or high risk of progression to Strongyloides hyperinfection or disseminated disease [13]. Potential, but not high-risk, patients should be monitored for clinical deterioration upon immunosuppression. Prompt investigation with stool microscopy, respiratory samples examination, or culture should be undertaken if Strongyloides hyperinfection is suspected [93].
11. Conclusions
The unique nature of S. stercoralis results in clinical practice challenges, including diagnostic complexities, a broad spectrum of disease presentations depending on the host’s immunity, and corresponding difficulties caused by treatment complications. The diagnosis of acute and chronic strongyloidiasis is difficult because of the non-specific presentations and low parasite load. All available investigations have limited sensitivity. Thus, the combination of parasitological and serological methods is recommended. The gold standard regimen is a single dose of ivermectin. Moxidectin has the potential to become the drug of choice in the future. In contrast, poor host immunity, high parasite burden from autoinfection, and concurrent bacterial and fungal infection lead to treatment complexity and high mortality in hyperinfection and disseminated strongyloidiasis. Increases in traveling and migration, as well as advances in immunosuppressive treatment, particularly during the COVID-19 pandemic, have raised the impact and awareness of strongyloidiasis. Active surveillance and further research regarding diagnostic techniques are required to reveal the real burden of this under-reported disease. The standard recommendations need to be strengthened for screening and prophylactic strategies in immunocompromised patients, individuals undergoing immunosuppressive treatment, as well as immigrants and long-term travelers from endemic areas.
Author Contributions
V.L. conceptualized the work. V.L. and T.S. performed literature review. D.P. and P.D. provided resources. V.L. and T.S. drafted the manuscript. D.P., K.S., D.W. and P.D. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Faculty of Tropical Medicine, Mahidol University. The funders had no role in study design, decision to publish or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Than Narkwiboonwong and Poom Adisakwattana for providing images.
Conflicts of Interest
The authors declare no competing interest.
References
- Schär, F.; Trostdorf, U.; Giardina, F.; Khieu, V.; Muth, S.; Marti, H.; Vounatsou, P.; Odermatt, P. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl. Trop. Dis. 2013, 7, e2288. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Martello, E.; Giorli, G.; Fittipaldo, A.; Staffolani, S.; Montresor, A.; Bisoffi, Z.; Buonfrate, D. Morbidity Associated with Chronic Strongyloides stercoralis Infection: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2019, 100, 1305–1311. [Google Scholar] [CrossRef]
- Bisoffi, Z.; Buonfrate, D.; Montresor, A.; Requena-Méndez, A.; Muñoz, J.; Krolewiecki, A.J.; Gotuzzo, E.; Mena, M.A.; Chiodini, P.L.; Anselmi, M.; et al. Strongyloides stercoralis: A plea for action. PLoS Negl. Trop. Dis. 2013, 7, e2214. [Google Scholar] [CrossRef]
- Keiser, P.B.; Nutman, T.B. Strongyloides stercoralis in the Immunocompromised Population. Clin. Microbiol. Rev. 2004, 17, 208–217. [Google Scholar] [CrossRef]
- Caumes, E.; Keystone, J.S. Acute strongyloidiasis: A rarity. Chronic strongyloidiasis: A time bomb! J. Travel Med. 2011, 18, 71–72. [Google Scholar] [CrossRef]
- Krolewiecki, A.; Nutman, T.B. Strongyloidiasis: A Neglected Tropical Disease. Infect. Dis. Clin. North Am. 2019, 33, 135–151. [Google Scholar] [CrossRef] [PubMed]
- Montes, M.; Sawhney, C.; Barros, N. Strongyloides stercoralis: There but not seen. Curr. Opin. Infect. Dis. 2010, 23, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Asundi, A.; Beliavsky, A.; Liu, X.J.; Akaberi, A.; Schwarzer, G.; Bisoffi, Z.; Requena-Méndez, A.; Shrier, I.; Greenaway, C. Prevalence of strongyloidiasis and schistosomiasis among migrants: A systematic review and meta-analysis. Lancet Glob. Health 2019, 7, e236–e248. [Google Scholar] [CrossRef]
- Genta, R.M. Global prevalence of strongyloidiasis: Critical review with epidemiologic insights into the prevention of disseminated disease. Rev. Infect. Dis. 1989, 11, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Bisanzio, D.; Giorli, G.; Odermatt, P.; Fürst, T.; Greenaway, C.; French, M.; Reithinger, R.; Gobbi, F.; Montresor, A.; et al. The Global Prevalence of Strongyloides stercoralis Infection. Pathogens 2020, 9, 468. [Google Scholar] [CrossRef] [PubMed]
- Román-Sánchez, P.; Pastor-Guzmán, A.; Moreno-Guillén, S.; Igual-Adell, R.; Suñer-Generoso, S.; Tornero-Estébanez, C. High prevalence of Strongyloides stercoralis among farm workers on the Mediterranean coast of Spain: Analysis of the predictive factors of infection in developed countries. Am. J. Trop. Med. Hyg. 2003, 69, 336–340. [Google Scholar] [CrossRef]
- Boggild, A.K.; Libman, M.; Greenaway, C.; McCarthy, A.E. CATMAT statement on disseminated strongyloidiasis: Prevention, assessment and management guidelines. Can. Commun. Dis. Rep. 2016, 42, 12–19. [Google Scholar] [CrossRef]
- Leung, E.; McIntyre, M.; Andany, N.; Ciccotelli, W.A.; Graham, C.; Jüni, P.; Langford, B.; McCarthy, A.E.; Nott, C.; Gold, W.L.; et al. Ivermectin to Prevent Disseminated Strongyloides Infection in Patients with COVID-19. Sci. Briefs Ont. COVID-19 Sci. Advis. Table 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Ganesh, S.; Cruz, R.J., Jr. Strongyloidiasis: A multifaceted disease. Gastroenterol. Hepatol. 2011, 7, 194–196. [Google Scholar]
- Sing, A.; Leitritz, L.; Bogner, J.R.; Heesemann, J. First-glance diagnosis of Strongyloides stercoralis autoinfection by stool microscopy. J. Clin. Microbiol. 1999, 37, 1610–1611. [Google Scholar] [CrossRef]
- Luvira, V.; Watthanakulpanich, D.; Pittisuttithum, P. Management of Strongyloides stercoralis: A puzzling parasite. Int. Health 2014, 6, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Berk, S.L. Diagnosis of Strongyloides stercoralis infection. Clin. Infect. Dis. 2001, 33, 1040–1047. [Google Scholar] [CrossRef]
- Mendes, T.; Minori, K.; Ueta, M.; Miguel, D.C.; Allegretti, S.M. Strongyloidiasis Current Status with Emphasis in Diagnosis and Drug Research. J. Parasitol. Res. 2017, 2017, 5056314. [Google Scholar] [CrossRef] [PubMed]
- Requena-Méndez, A.; Chiodini, P.; Bisoffi, Z.; Buonfrate, D.; Gotuzzo, E.; Muñoz, J. The Laboratory Diagnosis and Follow Up of Strongyloidiasis: A Systematic Review. PLoS Negl. Trop. Dis. 2013, 7, e2002. [Google Scholar] [CrossRef] [PubMed]
- Roxby, A.C.; Gottlieb, G.S.; Limaye, A.P. Strongyloidiasis in transplant patients. Clin. Infect. Dis. 2009, 49, 1411–1423. [Google Scholar] [CrossRef]
- Hays, R.; Thompson, F.; Esterman, A.; McDermott, R. Strongyloides stercoralis, Eosinophilia, and Type 2 Diabetes Mellitus: The Predictive Value of Eosinophilia in the Diagnosis of S. stercoralis Infection in an Endemic Community. Open Forum Infect. Dis. 2016, 3, ofw029. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Pérez, A.; Soriano-Pérez, M.J.; Salvador, F.; Gomez-Junyent, J.; Villar-Garcia, J.; Santin, M.; Muñoz, C.; González-Cordón, A.; Salas-Coronas, J.; Sulleiro, E.; et al. Clinical Features Associated with Strongyloidiasis in Migrants and the Potential Impact of Immunosuppression: A Case Control Study. Pathogens 2020, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, M.R.; Wilson, M.; Keystone, J.S.; Kain, K.C. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am. J. Trop. Med. Hyg. 2002, 66, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Vargas, P.; Krolewiecki, A.J.; Echazú, A.; Juarez, M.; Cajal, P.; Gil, J.F.; Caro, N.; Nasser, J.; Lammie, P.; Cimino, R.O. Serologic Monitoring of Public Health Interventions against Strongyloides stercoralis. Am. J. Trop. Med. Hyg. 2017, 97, 166–172. [Google Scholar] [CrossRef]
- Buonfrate, D.; Sequi, M.; Mejia, R.; Cimino, R.O.; Krolewiecki, A.J.; Albonico, M.; Degani, M.; Tais, S.; Angheben, A.; Requena-Mendez, A.; et al. Accuracy of Five Serologic Tests for the Follow up of Strongyloides stercoralis Infection. PLoS Negl. Trop. Dis. 2015, 9, e0003491. [Google Scholar] [CrossRef]
- Ramanathan, R.; Nutman, T. Strongyloides stercoralis infection in the immunocompromised host. Curr. Infect. Dis. Rep. 2008, 10, 105–110. [Google Scholar] [CrossRef]
- Luvira, V.; Trakulhun, K.; Mungthin, M.; Naaglor, T.; Chantawat, N.; Pakdee, W.; Phiboonbanakit, D.; Dekumyoy, P. Comparative Diagnosis of Strongyloidiasis in Immunocompromised Patients. Am. J. Trop. Med. Hyg. 2016, 95, 401–404. [Google Scholar] [CrossRef]
- Dekumyoy, P.; Somtana, K.; Jantanawiwat, P.; Nuamtanong, S.; Sa-nguankiat, S.; Nuchfaong, S.; Janyapoon, K.; Chindanond, D. Improved antigens for IgG-ELISA diagnosis of strongyloidiasis. Southeast Asian J. Trop. Med. Public Health 2002, 33 (Suppl. 3), 53–59. [Google Scholar]
- Yunus, M.H.; Arifin, N.; Balachandra, D.; Anuar, N.S.; Noordin, R. Lateral Flow Dipstick Test for Serodiagnosis of Strongyloidiasis. Am. J. Trop. Med. Hyg. 2019, 101, 432–435. [Google Scholar] [CrossRef]
- Sadaow, L.; Sanpool, O.; Rodpai, R.; Boonroumkaew, P.; Maleewong, W.; Intapan, P.M. Development of immunochromatographic device as a point-of-care tool for serodiagnosis of human strongyloidiasis cases. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 465–470. [Google Scholar] [CrossRef]
- Boonroumkaew, P.; Sadaow, L.; Sanpool, O.; Rodpai, R.; Thanchomnang, T.; Phupiewkham, W.; Intapan, P.M.; Maleewong, W. Effectiveness of Strongyloides Recombinant IgG Immunoreactive Antigen in Detecting IgG and IgG4 Subclass Antibodies for Diagnosis of Human Strongyloidiasis Using Rapid Immunochromatographic Tests. Diagnostics 2020, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Noordin, R.; Anuar, N.S.; Juri, N.M.; Wongphutorn, P.; Ruantip, S.; Kopolrat, K.Y.; Worasith, C.; Sithithaworn, J.; Sithithaworn, P. Evaluation of a Rapid IgG4 Lateral Flow Dipstick Test to Detect Strongyloides stercoralis Infection in Northeast Thailand. Am. J. Trop. Med. Hyg. 2021, 105, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.R.; Kim, R.; Ahuja, V.; Robertson, G.J.; Sultana, Y.; Wehrhahn, M.C.; Bradbury, R.S.; Gilbert, G.L.; Lee, R.; Loeffelholz, M.J. Comparison of Loop-Mediated Isothermal Amplification and Real-Time PCR Assays for Detection of Strongyloides Larvae in Different Specimen Matrices. J. Clin. Microbiol. 2019, 57, e01173-18. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.J.; Koehler, A.V.; Gasser, R.B.; Watts, M.; Norton, R.; Bradbury, R.S. Application of PCR-Based Tools to Explore Strongyloides Infection in People in Parts of Northern Australia. Trop. Med. Infect. Dis. 2017, 2, 62. [Google Scholar] [CrossRef] [PubMed]
- Formenti, F.; La Marca, G.; Perandin, F.; Pajola, B.; Romano, M.; Santucci, B.; Silva, R.; Giorli, G.; Bisoffi, Z.; Buonfrate, D. A diagnostic study comparing conventional and real-time PCR for Strongyloides stercoralis on urine and on faecal samples. Acta Trop. 2019, 190, 284–287. [Google Scholar] [CrossRef]
- Buonfrate, D.; Requena-Mendez, A.; Angheben, A.; Cinquini, M.; Cruciani, M.; Fittipaldo, A.; Giorli, G.; Gobbi, F.; Piubelli, C.; Bisoffi, Z. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection-A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2018, 12, e0006229. [Google Scholar] [CrossRef]
- Pattison, D.A.; Speare, R. Strongyloidiasis in personnel of the Regional Assistance Mission to Solomon Islands (RAMSI). Med. J. Aust. 2008, 189, 203–206. [Google Scholar] [CrossRef]
- Greaves, D.; Coggle, S.; Pollard, C.; Aliyu, S.H.; Moore, E.M. Strongyloides stercoralis infection. BMJ 2013, 347, f4610. [Google Scholar] [CrossRef]
- Kling, K.; Kuenzli, E.; Blum, J.; Neumayr, A. Acute strongyloidiasis in a traveller returning from South East Asia. Travel Med. Infect. Dis. 2016, 14, 535–536. [Google Scholar] [CrossRef]
- Geri, G.; Rabbat, A.; Mayaux, J.; Zafrani, L.; Chalumeau-Lemoine, L.; Guidet, B.; Azoulay, E.; Pène, F. Strongyloides stercoralis hyperinfection syndrome: A case series and a review of the literature. Infection 2015, 43, 691–698. [Google Scholar] [CrossRef]
- Vasquez-Rios, G.; Pineda-Reyes, R.; Pineda-Reyes, J.; Marin, R.; Ruiz, E.F.; Terashima, A. Strongyloides stercoralis hyperinfection syndrome: A deeper understanding of a neglected disease. J. Parasit. Dis. 2019, 43, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.A.; Scully, B.E.; Bulman, W.A.; Husain, S.; Grossman, M.E. Periumbilical parasitic thumbprint purpura: Strongyloides hyperinfection syndrome acquired from a cadaveric renal transplant. Transpl. Infect. Dis. 2011, 13, 58–62. [Google Scholar] [CrossRef]
- Fardet, L.; Genereau, T.; Poirot, J.L.; Guidet, B.; Kettaneh, A.; Cabane, J. Severe strongyloidiasis in corticosteroid-treated patients: Case series and literature review. J. Infect. 2007, 54, 18–27. [Google Scholar] [CrossRef]
- Nabha, L.; Krishnan, S.; Ramanathan, R.; Mejia, R.; Roby, G.; Sheikh, V.; McAuliffe, I.; Nutman, T.; Sereti, I. Prevalence of Strongyloides stercoralis in an urban US AIDS cohort. Pathog. Glob. Health 2012, 106, 238–244. [Google Scholar] [CrossRef]
- Rafiei, R.; Rafiei, A.; Rahdar, M.; Keikhaie, B. Seroepidemiology of Strongyloides stercoralis amongst immunocompromised patients in Southwest Iran. Parasite Epidemiol. Control 2016, 1, 229–232. [Google Scholar] [CrossRef]
- Getaz, L.; Castro, R.; Zamora, P.; Kramer, M.; Gareca, N.; Torrico-Espinoza, M.D.C.; Macias, J.; Lisarazu-Velasquez, S.; Rodriguez, G.; Valencia-Rivero, C.; et al. Epidemiology of Strongyloides stercoralis infection in Bolivian patients at high risk of complications. PLoS Negl. Trop. Dis. 2019, 13, e0007028. [Google Scholar] [CrossRef]
- Winnicki, W.; Eder, M.; Mazal, P.; Mayer, F.J.; Sengölge, G.; Wagner, L. Prevalence of Strongyloides stercoralis infection and hyperinfection syndrome among renal allograft recipients in Central Europe. Sci. Rep. 2018, 8, 15406. [Google Scholar] [CrossRef]
- Abanyie, F.A.; Gray, E.B.; Delli Carpini, K.W.; Yanofsky, A.; McAuliffe, I.; Rana, M.; Chin-Hong, P.V.; Barone, C.N.; Davis, J.L.; Montgomery, S.P.; et al. Donor-derived Strongyloides stercoralis infection in solid organ transplant recipients in the United States, 2009-2013. Am. J. Transplant. 2015, 15, 1369–1375. [Google Scholar] [CrossRef]
- Hoy, W.E.; Roberts, N.J., Jr.; Bryson, M.F.; Bowles, C.; Lee, J.C.K.; Rivero, A.J.; Ritterson, A.L. Transmission of Strongyloidiasis by Kidney Transplant?: Disseminated Strongyloidiasis in Both Recipients of Kidney Allografts From a Single Cadaver Donor. JAMA 1981, 246, 1937–1939. [Google Scholar] [CrossRef]
- Schad, G.A. Cyclosporine may eliminate the threat of overwhelming strongyloidiasis in immunosuppressed patients. J. Infect. Dis. 1986, 153, 178. [Google Scholar] [CrossRef]
- Schwartz, B.S.; Mawhorter, S.D. Parasitic infections in solid organ transplantation. Am. J. Transplant. 2013, 13 (Suppl. 4), 280–303. [Google Scholar] [CrossRef]
- Marcos, L.A.; Terashima, A.; Canales, M.; Gotuzzo, E. Update on strongyloidiasis in the immunocompromised host. Curr. Infect. Dis. Rep. 2011, 13, 35–46. [Google Scholar] [CrossRef]
- Pungpak, S.; Bunnag, D.; Chindanond, D.; Radmoyos, B. Albendazole in the treatment of strongyloidiasis. Southeast Asian J. Trop. Med. Public Health 1987, 18, 207–210. [Google Scholar]
- Archibald, L.K.; Beeching, N.J.; Gill, G.V.; Bailey, J.W.; Bell, D.R. Albendazole is effective treatment for chronic strongyloidiasis. Q. J. Med. 1993, 86, 191–195. [Google Scholar]
- Pitisuttithum, P.; Supanaranond, W.; Chindanond, D. A randomized comparative study of albendazole and thiabendazole in chronic strongyloidiasis. Southeast Asian J. Trop. Med. Public Health 1995, 26, 735–738. [Google Scholar]
- Shikiya, K.; Zaha, O.; Niimura, S.; Uehara, T.; Ohshiro, J.; Kinjo, F.; Saito, A.; Asato, R. Clinical study on ivermectin against 125 strongyloidiasis patients. Kansenshogaku Zasshi 1994, 68, 13–20. [Google Scholar] [CrossRef]
- Datry, A.; Hilmarsdottir, I.; Mayorga-Sagastume, R.; Lyagoubi, M.; Gaxotte, P.; Biligui, S.; Chodakewitz, J.; Neu, D.; Danis, M.; Gentilini, M. Treatment of Strongyloides stercoralis infection with ivermectin compared with albendazole: Results of an open study of 60 cases. Trans. R Soc. Trop. Med. Hyg. 1994, 88, 344–345. [Google Scholar] [CrossRef]
- Gann, P.H.; Neva, F.A.; Gam, A.A. A randomized trial of single- and two-dose ivermectin versus thiabendazole for treatment of strongyloidiasis. J. Infect. Dis. 1994, 169, 1076–1079. [Google Scholar] [CrossRef]
- Toma, H.; Sato, Y.; Shiroma, Y.; Kobayashi, J.; Shimabukuro, I.; Takara, M. Comparative studies on the efficacy of three anthelminthics on treatment of human strongyloidiasis in Okinawa, Japan. Southeast Asian J. Trop. Med. Public Health 2000, 31, 147–151. [Google Scholar]
- Igual-Adell, R.; Oltra-Alcaraz, C.; Soler-Company, E.; Sanchez-Sanchez, P.; Matogo-Oyana, J.; Rodriguez-Calabuig, D. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin. Pharmacother. 2004, 5, 2615–2619. [Google Scholar] [CrossRef]
- Suputtamongkol, Y.; Kungpanichkul, N.; Silpasakorn, S.; Beeching, N.J. Efficacy and safety of a single-dose veterinary preparation of ivermectin versus 7-day high-dose albendazole for chronic strongyloidiasis. Int. J. Antimicrob. Agents 2008, 31, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Bisoffi, Z.; Buonfrate, D.; Angheben, A.; Boscolo, M.; Anselmi, M.; Marocco, S.; Monteiro, G.; Gobbo, M.; Bisoffi, G.; Gobbi, F. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Negl. Trop. Dis. 2011, 5, e1254. [Google Scholar] [CrossRef] [PubMed]
- Suputtamongkol, Y.; Premasathian, N.; Bhumimuang, K.; Waywa, D.; Nilganuwong, S.; Karuphong, E.; Anekthananon, T.; Wanachiwanawin, D.; Silpasakorn, S. Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLoS Negl. Trop. Dis. 2011, 5, e1044. [Google Scholar] [CrossRef]
- Barda, B.; Sayasone, S.; Phongluxa, K.; Xayavong, S.; Keoduangsy, K.; Odermatt, P.; Puchkov, M.; Huwyler, J.; Hattendorf, J.; Keiser, J. Efficacy of Moxidectin Versus Ivermectin Against Strongyloides stercoralis Infections: A Randomized, Controlled Noninferiority Trial. Clin. Infect. Dis. 2017, 65, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Buonfrate, D.; Salas-Coronas, J.; Munoz, J.; Maruri, B.T.; Rodari, P.; Castelli, F.; Zammarchi, L.; Bianchi, L.; Gobbi, F.; Cabezas-Fernandez, T.; et al. Multiple-dose versus single-dose ivermectin for Strongyloides stercoralis infection (Strong Treat 1 to 4): A multicentre, open-label, phase 3, randomised controlled superiority trial. Lancet Infect. Dis. 2019, 19, 1181–1190. [Google Scholar] [CrossRef]
- Hofmann, D.; Sayasone, S.; Sengngam, K.; Chongvilay, B.; Hattendorf, J.; Keiser, J. Efficacy and safety of ascending doses of moxidectin against Strongyloides stercoralis infections in adults: A randomised, parallel-group, single-blinded, placebo-controlled, dose-ranging, phase 2a trial. Lancet Infect. Dis. 2021, 21, 1151–1160. [Google Scholar] [CrossRef]
- Prichard, R.K.; Geary, T.G. Perspectives on the utility of moxidectin for the control of parasitic nematodes in the face of developing anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 69–83. [Google Scholar] [CrossRef]
- Buonfrate, D.; Requena-Mendez, A.; Angheben, A.; Munoz, J.; Gobbi, F.; Van Den Ende, J.; Bisoffi, Z. Severe strongyloidiasis: A systematic review of case reports. BMC Infect. Dis. 2013, 13, 78. [Google Scholar] [CrossRef]
- Mejia, R.; Nutman, T.B. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr. Opin. Infect. Dis. 2012, 25, 458–463. [Google Scholar] [CrossRef]
- Hamilton, K.W.; Abt, P.L.; Rosenbach, M.A.; Bleicher, M.B.; Levine, M.S.; Mehta, J.; Montgomery, S.P.; Hasz, R.D.; Bono, B.R.; Tetzlaff, M.T.; et al. Donor-derived Strongyloides stercoralis infections in renal transplant recipients. Transplantation 2011, 91, 1019–1024. [Google Scholar] [CrossRef]
- Barrett, J.; Broderick, C.; Soulsby, H.; Wade, P.; Newsholme, W. Subcutaneous ivermectin use in the treatment of severe Strongyloides stercoralis infection: Two case reports and a discussion of the literature. J. Antimicrob. Chemother. 2016, 71, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.A.; Maclean, J.D.; Fleckenstein, L.; Greenaway, C. Parenteral administration of ivermectin in a patient with disseminated strongyloidiasis. Am. J. Trop. Med. Hyg. 2005, 73, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Donadello, K.; Cristallini, S.; Taccone, F.S.; Lorent, S.; Vincent, J.L.; de Backer, D.; Jacobs, F. Strongyloides disseminated infection successfully treated with parenteral ivermectin: Case report with drug concentration measurements and review of the literature. Int. J. Antimicrob. Agents 2013, 42, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Sithithaworn, P.; Srisawangwong, T.; Tesana, S.; Daenseekaew, W.; Sithithaworn, J.; Fujimaki, Y.; Ando, K. Epidemiology of Strongyloides stercoralis in north-east Thailand: Application of the agar plate culture technique compared with the enzyme-linked immunosorbent assay. Trans. R Soc. Trop. Med. Hyg. 2003, 97, 398–402. [Google Scholar] [CrossRef]
- Carnino, L.; Schwob, J.-M.; Gétaz, L.; Nickel, B.; Neumayr, A.; Eperon, G. A Practical Approach to Screening for Strongyloides stercoralis. Trop. Med. Infect. Dis. 2021, 6, 203. [Google Scholar] [CrossRef]
- Requena-Méndez, A.; Buonfrate, D.; Gomez-Junyent, J.; Zammarchi, L.; Bisoffi, Z.; Muñoz, J. Evidence-Based Guidelines for Screening and Management of Strongyloidiasis in Non-Endemic Countries. Am. J. Trop. Med. Hyg. 2017, 97, 645–652. [Google Scholar] [CrossRef]
- Gómez-Junyent, J.; Paredes, D.; Hurtado, J.C.; Requena-Méndez, A.; Ruiz, A.; Valls, M.E.; Vila, J.; Muñoz, J. High seroprevalence of Strongyloides stercoralis among individuals from endemic areas considered for solid organ transplant donation: A retrospective serum-bank based study. PLoS Negl. Trop. Dis. 2018, 12, e0007010. [Google Scholar] [CrossRef]
- Pierrotti, L.C.; Kotton, C.N. Transplantation in the Tropics: Lessons on Prevention and Management of Tropical Infectious Diseases. Curr. Infect. Dis. Rep. 2015, 17, 34. [Google Scholar] [CrossRef]
- Santiago, M.; Leitao, B. Prevention of Strongyloides hyperinfection syndrome: A rheumatological point of view. Eur. J. Intern. Med. 2009, 20, 744–748. [Google Scholar] [CrossRef]
- Hayes, J.; Nellore, A. Management of Strongyloides in Solid Organ Transplant Recipients. Infect. Dis. Clin. North Am. 2018, 32, 749–763. [Google Scholar] [CrossRef]
- Forrer, A.; Khieu, V.; Schindler, C.; Schär, F.; Marti, H.; Char, M.C.; Muth, S.; Odermatt, P. Ivermectin Treatment and Sanitation Effectively Reduce Strongyloides stercoralis Infection Risk in Rural Communities in Cambodia. PLoS Negl. Trop. Dis. 2016, 10, e0004909. [Google Scholar] [CrossRef] [PubMed]
- Hays, R.; Esterman, A.; McDermott, R. Control of chronic Strongyloides stercoralis infection in an endemic community may be possible by pharmacological means alone: Results of a three-year cohort study. PLoS Negl. Trop. Dis. 2017, 11, e0005825. [Google Scholar] [CrossRef] [PubMed]
- Barda, B.; Albonico, M.; Buonfrate, D.; Ame, S.M.; Ali, S.; Speich, B.; Keiser, J. Side Benefits of Mass Drug Administration for Lymphatic Filariasis on Strongyloides stercoralis Prevalence on Pemba Island, Tanzania. Am. J. Trop. Med. Hyg. 2017, 97, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Kearns, T.M.; Currie, B.J.; Cheng, A.C.; McCarthy, J.; Carapetis, J.R.; Holt, D.C.; Page, W.; Shield, J.; Gundjirryirr, R.; Mulholland, E.; et al. Strongyloides seroprevalence before and after an ivermectin mass drug administration in a remote Australian Aboriginal community. PLoS Negl. Trop. Dis. 2017, 11, e0005607. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Pereira, C.V.M.; Mastandrea, G.R.A.; Medeiros, A.C.C.d.S.; Gryschek, R.C.B.; Paula, F.M.d.; Corral, M.A. COVID-19 and strongyloidiasis: What to expect from this coinfection? Clinics (Sao Paulo) 2021, 76, e3528. [Google Scholar] [CrossRef]
- Stauffer, W.M.; Alpern, J.D.; Walker, P.F. COVID-19 and Dexamethasone: A Potential Strategy to Avoid Steroid-Related Strongyloides Hyperinfection. JAMA 2020, 324, 623–624. [Google Scholar] [CrossRef]
- Feria, L.; Torrado, M.; Anton-Vazquez, V. Reactivation of Strongyloides stercoralis in patients with SARS-CoV-2 pneumonia receiving dexamethasone. Med. Clin. (Barc) 2022, 158, 242–243. [Google Scholar] [CrossRef]
- Gautam, D.; Gupta, A.; Meher, A.; Siddiqui, F.; Singhai, A. Corticosteroids in Covid-19 pandemic have the potential to unearth hidden burden of strongyloidiasis. IDCases 2021, 25, e01192. [Google Scholar] [CrossRef]
- Lier, A.J.; Tuan, J.J.; Davis, M.W.; Paulson, N.; McManus, D.; Campbell, S.; Peaper, D.R.; Topal, J.E. Case Report: Disseminated Strongyloidiasis in a Patient with COVID-19. Am. J. Trop. Med. Hyg. 2020, 103, 1590–1592. [Google Scholar] [CrossRef]
- Marchese, V.; Crosato, V.; Gulletta, M.; Castelnuovo, F.; Cristini, G.; Matteelli, A.; Castelli, F. Strongyloides infection manifested during immunosuppressive therapy for SARS-CoV-2 pneumonia. Infection 2021, 49, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Pintos-Pascual, I.; López-Dosil, M.; Castillo-Núñez, C.; Múñez-Rubio, E. Eosinophilia and abdominal pain after severe pneumonia due to COVID 19. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2021, 39, 478–480. [Google Scholar] [CrossRef] [PubMed]
- De Wilton, A.; Nabarro, L.E.; Godbole, G.S.; Chiodini, P.L.; Boyd, A.; Woods, K. Risk of Strongyloides Hyperinfection Syndrome when prescribing dexamethasone in severe COVID-19. Travel Med. Infect. Dis. 2021, 40, 101981. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).