Molecular Characterization and Antifungal Susceptibility of Aspergillus spp. among Patients with Underlying Lung Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Area
2.2. Data Collections and Sample Size
2.3. Specimens’ Collection and Processing
2.4. Macroscopically and Microscopically Examinations
2.4.1. Direct Potassium Hydroxide (KOH)
2.4.2. Direct Gram Staining
2.5. Cultures of Specimens
2.6. Identification of Isolates
Needle Mount with Lactophenol Cotton Blue (LPCB) Stain
2.7. Antifungal Susceptibility Testing
2.7.1. Preparation of Inoculum
2.7.2. Seeding
2.7.3. Reading and Interpretation of Results
2.8. Molecular Identification of Aspergillus Isolates
2.8.1. Culture Preparation and DNA Extraction Method
2.8.2. PCR Method
2.8.3. DNA Sequencing
2.9. Bioinformatics Analysis
2.9.1. Sequences Similarity and Alignment
2.9.2. Phylogenetic Tree
2.10. Data Analysis
3. Results
3.1. Socio-Demographic and Clinical Data
3.2. Microscopy and Isolation of Fungal Pathogens
3.3. Antifungal Susceptibility Test
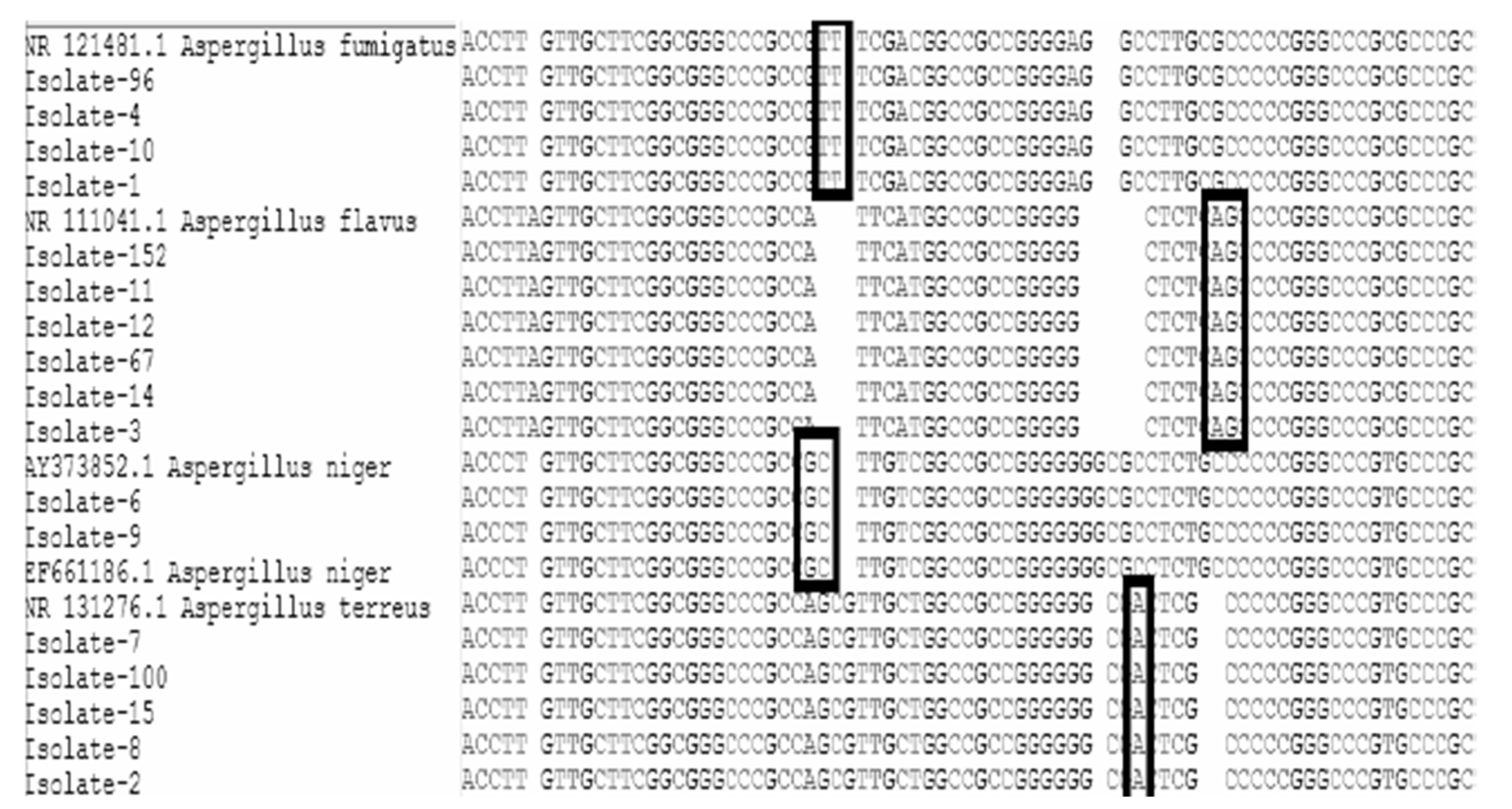
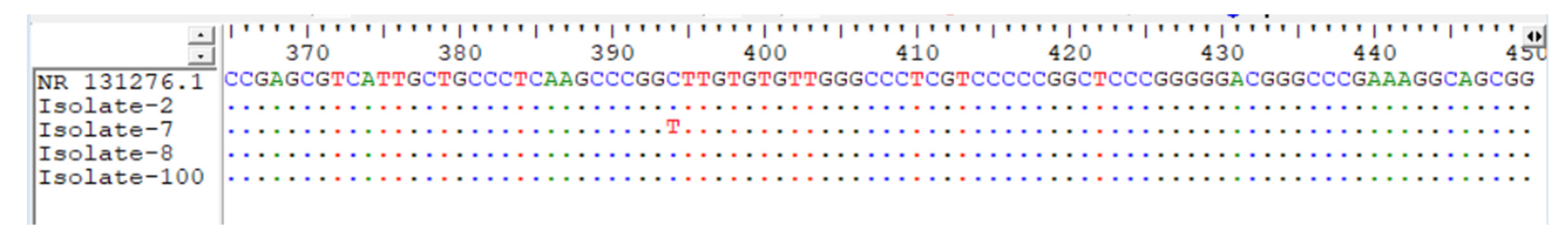
3.4. PCR Result for all Aspergillus Isolates
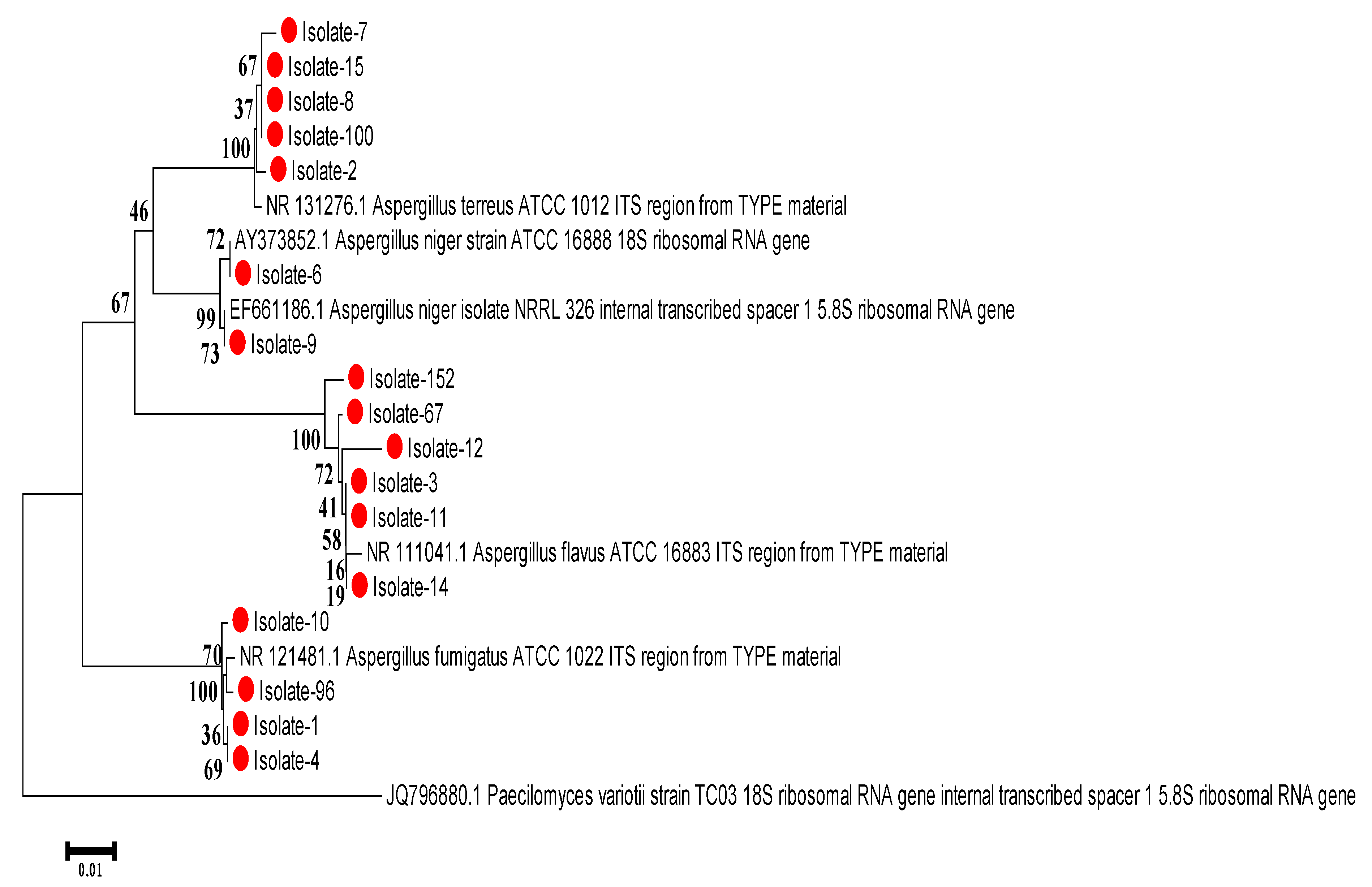
3.5. Bioinformatics Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Gianella, P.; Gasche-Soccal, P.; van Delden, C.; Hachulla, A.L.; Rochat, T. Invasive pulmonary aspergillosis and chronic pulmonary aspergillosis. Rev. Med. Suisse 2014, 10, 2202–2207. [Google Scholar] [PubMed]
- Patterson, K.C.; Strek, M.E. Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest 2014, 146, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Walicka-Serzysko, K.; Sands, D. The clinical presentations of pulmonary aspergillosis in children with cystic fibrosis—Preliminary report. Dev. Period Med. 2015, 19, 66–79. [Google Scholar] [PubMed]
- Natarajan, S.; Subramanian, P. Allergic bronchopulmonary aspergillosis: A clinical review of 24 patients: Are we right in frequent serologic monitoring? Ann. Thorac. Med. 2014, 9, 216–220. [Google Scholar] [CrossRef]
- Gupta, R.K.; Chandr, A.; Gautam, P.B. Allergic bronchopulmonary aspergillosis--a clinical review. J. Assoc. Physicians India 2012, 60, 46–51. [Google Scholar]
- Maturu, V.N.; Agarwal, R. Itraconazole in chronic pulmonary aspergillosis: In whom, for how long, and at what dose? Lung India 2015, 32, 309–312. [Google Scholar]
- Iqbal, N.; Irfan, M.; Zubairi, A.B.S.; Jabeen, K.; Awan, S.; Khan, J.A. Clinical manifestations and outcomes of pulmonary aspergillosis: Experience from Pakistan. BMJ Open Respir. Res. 2016, 3, e000155. [Google Scholar] [CrossRef]
- Denning, D.W.; Pleuvry, A.; Cole, D.C. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull. World Health Organ. 2011, 89, 864–872. [Google Scholar] [CrossRef]
- Agarwal, R.; Vishwanath, G.; Aggarwal, A.N.; Garg, M.; Gupta, D.; Chakrabarti, A. Itraconazole in chronic cavitary pulmonary aspergillosis: A randomised controlled trial and systematic review of literature. Mycoses 2013, 56, 559–570. [Google Scholar] [CrossRef]
- De Baets, F.; De Keyzer, L.; Van daele, S.; Schelstraete, P.; Van Biervliet, S.; Van Braeckel, E.; Thomas, M.; Wanyama, S.S. Risk Factors and impact of Allergic Bronchopulmonary Aspergillosis in P. aeruginosa negative CF Patients. Pediatr. Allergy Immunol. 2018, 29, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Perisson, C.; Destruys, L.; Grenet, D.; Bassinet, L.; Derelle, J.; Sermet-Gaudelus, I.; Thumerelle, C.; Prevotat, A.; Rosner, V.; Clement, A. Omalizumab treatment for allergic bronchopulmonary aspergillosis in young patients with cystic fibrosis. Respir. Med. 2017, 133, 12–15. [Google Scholar] [CrossRef]
- Knutsen, A.P. Allergic bronchopulmonary aspergillosis. Clin. Exp. Allergy 2015, 45, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Overton, N.L.; Brakhage, A.A.; Thywißen, A.; Denning, D.W.; Bowyer, P. Mutations in EEA1 are associated with allergic bronchopulmonary aspergillosis and affect phagocytosis of Aspergillus fumigatus by human macrophages. PLoS ONE 2018, 13, e0185706. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Saez-Lacy, D.; Wynkoop, W.; Walsh, T.J. Successful treatment of allergic bronchopulmonary aspergillosis with isavuconazole: Case report and review of the literature. Open Forum Infectious Diseases 2017, 4, ofx040. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, A.C. Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med. Mycol. 2009, 47, S261–S270. [Google Scholar] [CrossRef] [PubMed]
- Kousha, M.; Tadi, R.; Soubani, A.O. Pulmonary aspergillosis: A clinical review. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2011, 20, 156–174. [Google Scholar] [CrossRef]
- Al-Nakeeb, Z.; Sudan, A.; Jeans, A.R.; Gregson, L.; Goodwin, J.; Warn, P.A.; Felton, T.W.; Howard, S.J.; Hope, W.W. Pharmacodynamics of itraconazole against Aspergillus fumigatus in an in vitro model of the human alveolus: Perspectives on the treatment of triazole resistant infection and utility of airway administration. Antimicrob. Agents Chemother. 2012, 56, 4146–4153. [Google Scholar] [CrossRef]
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef]
- Henry, T.; Iwen, P.C.; Hinrichs, S.H. Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J. Clin. Microbiol. 2000, 38, 1510–1515. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Balajee, S.A.; Borman, A.M.; Brandt, M.E.; Cano, J.; Cuenca-Estrella, M.; Dannaoui, E.; Guarro, J.; Haase, G.; Kibbler, C.C.; Meyer, W.; et al. Sequence-based identification of Aspergillus, fusarium, and mucorales species in the clinical mycology laboratory: Where are we and where should we go from here? J. Clin. Microbiol. 2009, 47, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Iwen, P.C.; Hinrichs, S.H.; Rupp, M.E. Utilization of the internal transcribed spacer regions as molecular targets to detect and identify human fungal pathogens. Med. Mycol. 2002, 40, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Cheesbourgh, M. Medical Laboratory Manual for Tropical Countries, 2nd ed.; Cambridge University Press: New York, NY, USA, 2006. [Google Scholar]
- Afzal, H.; Shahzad, S.; Qamar, S.; Nisa, U. Morphological identification of Aspergillus species from the soil of larkana district (Sindh, Pakistan). Asian J. Agric. Biol. 2013, 1, 105–117. [Google Scholar]
- Hassan, R.M.; Kadry Ismail, D.; Samy Elkholy, Y. Comparison of E Test and Disc Diffusion Methods for Susceptibility Testing of Filamentous Fungi; Experience of a Routine Lab. Arch. Clin. Infect. Dis. 2018, 13, e57889. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Melhem, M.S.C.; Bonfietti, L.X.; Rodriguez-Tudela, J.L. Susceptibility Test for Fungi: Clinical and Laboratorial Correlations in Medical Mycology. Rev. Inst. Med. Trop. Sao Paulo 2015, 57 (Suppl. 19), 57–64. [Google Scholar] [CrossRef]
- Colosi, I.A.; Faure, O.; Dessaigne, B.; Bourdon, C.; Lebeau, B.; Colosi, H.A.; Pelloux, H. Susceptibility of 100 filamentous fungi: Comparison of two diffusion methods, Neo-Sensitabs and E-test, for amphotericin B, caspofungin, itraconazole, voriconazole and posaconazole. Med. Mycol. 2012, 50, 378–385. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.J.; Zhang, S.; Liu, X.Z.; Wen, H.A.; Wang, M. A simple method of genomic DNA extraction suitable for analysis of bulk fungal strains. Lett. Appl. Microbiol. 2010, 51, 114–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. In PCR Protocols; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1999; Volume 41, pp. 95–98. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Chawla, K.; Kosaraju, K.; Rayasam, S.; Mukhopadhyay, C. Clinico-microbiological profile of chronic pulmonary aspergillosis from a tertiary care centre in southern India. J. Clin. Diagn. Res. 2013, 7, 2712–2715. [Google Scholar] [CrossRef]
- Chabi, M.L.; Goracci, A.; Roche, N.; Paugam, A.; Lupo, A.; Revel, M.P. Pulmonary aspergillosis. Diagn. Interv. Imaging 2015, 96, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, A.; Ryan, C.F.; Flint, J.D.; Müller, N.L. Aspergillus-related lung disease. Can. Respir. J. 2005, 12, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Paugam, A.; Baixench, M.T.; Lebuisson, A.; Dupouy-Camet, J. Diagnosis of invasive pulmonary aspergillosis: Value of bronchoalveolar lavage galactomannan for immunocompromised patients. Pathol. Biol. 2010, 58, 100–103. [Google Scholar] [CrossRef]
- Anna, N.; Ewang, A.; Kamga, H.L.; Nsagha, D.S.; Assob, J.; Ndah, D.; Tebit, K.E. Respiratory Tract Aspergillosis in the Sputum of Patients Suspected of Tuberculosis in Fako Division-Cameroon. J. Microbiol. Res. 2012, 2, 68–72. [Google Scholar]
- Khodavaisy, S.; Hedayati, M.T.; Alialy, M.; Habibi, M.R.; Badali, H. Detection of galactomannan in bronchoalveolar lavage of the intensive care unit patients at risk for invasive aspergillosis. Curr. Med. Mycol. 2015, 1, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef]
- Ader, F.; Nseir, S.; Guery, B.; Tillie-Leblond, I. Acute invasive pulmonary aspergillosis in chronic lung disease--A review. Rev. Des Mal. Respir. 2006, 23, 6s11–16s20. [Google Scholar]
- Al-Malaky, K.A.; Al-Charrakh, A.H.; Imran, Z.K. Diagnostic Aspergillosis in the Sputum of Patients with Pulmonary Complains and Antifungals Susceptibility of Triazoles and Caspofungin Using E-Test. Int. J. Med. Sci. Clin. Invent. 2015, 2, 1448–1455. [Google Scholar] [CrossRef]
- Tashiro, T.; Izumikawa, K.; Tashiro, M.; Takazono, T.; Morinaga, Y.; Yamamoto, K.; Imamura, Y.; Miyazaki, T.; Seki, M.; Kakeya, H.; et al. Diagnostic significance of Aspergillus species isolated from respiratory samples in an adult pneumology ward. Med. Mycol. 2011, 49, 581–587. [Google Scholar]
- Badiee, P.; Alborzi, A.; Moeini, M.; Haddadi, P.; Farshad, S.; Japoni, A.; Ziyaeyan, M. Antifungal susceptibility of the aspergillus species by Etest and CLSI reference methods. Arch. Iran. Med. 2012, 15, 429–432. [Google Scholar]
- Diekema, D.J.; Messer, S.A.; Hollis, R.J.; Jones, R.N.; Pfaller, M.A. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 2003, 41, 3623–3626. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Shapiro, B.L.; Srinivasan, M.; Prajna, N.V.; Acharya, N.R.; Fothergill, A.W.; Ruiz, J.; Chidambaram, J.D.; Maxey, K.J.; Hong, K.C.; et al. Antimicrobial susceptibility of Fusarium, Aspergillus, and other filamentous fungi isolated from keratitis. Arch. Ophthalmol. 2007, 125, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.A.; Kasuya, M.C.M.; Barros EGd Borges, A.C.; Araújo, E.F. Polymorphism in the internal transcribed spacer (ITS) of the ribosomal DNA of 26 isolates of ectomycorrhizal fungi. Genet. Mol. Biol. 2002, 25, 477–483. [Google Scholar] [CrossRef]
- Haugland, R.A.; Varma, M.; Wymer, L.J.; Vesper, S.J. Quantitative PCR Analysis of Selected Aspergillus, Penicillium and Paecilomyces Species. Syst. Appl. Microbiol. 2004, 27, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Kopchinskiy, A.G.; Komoń, M.; Bissett, J.; Szakacs, G.; Kubicek, C.P. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet. Biol. 2005, 42, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Leema, G.; Kaliamurthy, J.; Geraldine, P.; Thomas, P.A. Keratitis due to Aspergillus flavus: Clinical profile, molecular identification of fungal strains and detection of aflatoxin production. Mol. Vis. 2010, 16, 843–854. [Google Scholar]
| Variables | Total n (%) | Aspergillus spp. | |
|---|---|---|---|
| Negative | Positive | ||
| (n = 356) | (n = 28) | ||
| Sex: | |||
| Male | 233 (61.0) | 23 | 210 |
| Female | 151 (39.0) | 5 | 146 |
| Specimens: | |||
| Sputum | 341 (89) | 27 | 314 |
| Bronchoalveolar Lavage | 43 (11) | 1 | 42 |
| Underlying lung diseases: | |||
| Chronic pulmonary infection | 219 (57.0) | 15 | 204 |
| Asthma | 77 (20.1) | 7 | 70 |
| Cystic fibrosis | 34 (8.9) | 3 | 31 |
| Pulmonary tuberculosis | 29 (7.6) | 3 | 26 |
| Pleural effusions | 12 (3.1) | 0 | 12 |
| Malignancy | 9 (2.3) | 0 | 9 |
| Emphysema, hemoptysis and lung abscess | 4 (1.0) | 0 | 4 |
| Age group: | |||
| <10 | 2 (0.5) | 0 | 2 |
| 11–49 | 258 (67.2) | 22 | 236 |
| ≥50 | 124 (32.2) | 6 | 118 |
| Underlying Lung Diseases | Aspergillosis | Candidiasis | Total |
|---|---|---|---|
| CPI (n= 219) | 15 (46.9%) | 2 (6.2%) | 17 (53.1%) |
| Asthma (n= 77) | 7 (21.9%) | 0 (0.0%) | 7 (21.9%) |
| CF (n= 34) | 3 (9.4%) | 0 (0.0%) | 3 (9.4%) |
| PTB (n= 29) | 3 (9.4%) | 0 (0.0%) | 3 (9.4%) |
| Malignancy + pleural effusions (n= 21) | 0 (0.0%) | 2 (6.2%) | 2 (6.2%) |
| Total | 28 (87.5%) | 4 (12.5%) | 32 (100.0%) |
| Disease | Isolates | |||||
|---|---|---|---|---|---|---|
| A. fumigatus | A. flavus | A. terreus | A. niger | Candida spp. | Total | |
| CPI (n= 219) | 6 (18.8%) | 6 (18.8%) | 3 (9.4%) | 0 (0.0%) | 2 (6.2%) | 17 (53.1%) |
| Asthma (n= 77) | 3 (9.4%) | 2 (6.2%) | 2 (6.2%) | 0 (0.0%) | 0 (0.0%) | 7 (21.9%) |
| CF (n= 34) | 0 (0.0%) | 0 (0.0%) | 2 (6.2%) | 1 (3.1%) | 0 (0.0%) | 3 (9.4%) |
| PTB (n= 29) | 0 (0.0%) | 3 (9.4) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (9.4%) |
| Malignancy + pleural effusions (n= 21) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (6.2%) | 2 (6.2%) |
| Total | 9 (28.1%) | 11 (34.4%) | 7 (21.9%) | 1 (3.1%) | 4 (12.5%) | 32 (100.0%) |
| Isolates (n) | Itraconazole | Voriconazole | p-Value |
|---|---|---|---|
| A. fumigatus (9) | R: 4 (44.4%) | R: 1 (11.1%) | 0.08 |
| I: 2 (22.3%) | I: 1 (11.1%) | ||
| S: 3 (33.3%) | S: 7 (77.8%) | ||
| MIC g/mL | 0.047–6 | 0.008–4 | |
| A. flavus (11) | R: 1 (9.1%) | R: 0 | 0.003 * |
| I: 4 (36.4%) | I: 0 | ||
| S: 6 (54.5%) | S: 11 (100%) | ||
| MIC g/mL | 0.125–4 | 0.064–1.5 | |
| A. terreus (7) | R: 1 (14.3%) | R: 1 (8.4%) | 0.06 |
| I: 1 (14.3%) | I: 1 (8.3%) | ||
| S: 5 (71.4%) | S: 5 (83.3%) | ||
| MIC g/mL | 0.125–2.5 | 0.125–3 | |
| A. niger (1) | R: 1 (100%) | I: 1 (100%) | |
| MIC g/mL | 8 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moglad, E.; Saeed, S.; Saeed, H.; Ahmed, H.; Salih, K.; Altayb, H.; Elhag, W. Molecular Characterization and Antifungal Susceptibility of Aspergillus spp. among Patients with Underlying Lung Diseases. Trop. Med. Infect. Dis. 2022, 7, 274. https://doi.org/10.3390/tropicalmed7100274
Moglad E, Saeed S, Saeed H, Ahmed H, Salih K, Altayb H, Elhag W. Molecular Characterization and Antifungal Susceptibility of Aspergillus spp. among Patients with Underlying Lung Diseases. Tropical Medicine and Infectious Disease. 2022; 7(10):274. https://doi.org/10.3390/tropicalmed7100274
Chicago/Turabian StyleMoglad, Ehssan, Samar Saeed, Humodi Saeed, Hind Ahmed, Kwathar Salih, Hisham Altayb, and Wafa Elhag. 2022. "Molecular Characterization and Antifungal Susceptibility of Aspergillus spp. among Patients with Underlying Lung Diseases" Tropical Medicine and Infectious Disease 7, no. 10: 274. https://doi.org/10.3390/tropicalmed7100274
APA StyleMoglad, E., Saeed, S., Saeed, H., Ahmed, H., Salih, K., Altayb, H., & Elhag, W. (2022). Molecular Characterization and Antifungal Susceptibility of Aspergillus spp. among Patients with Underlying Lung Diseases. Tropical Medicine and Infectious Disease, 7(10), 274. https://doi.org/10.3390/tropicalmed7100274






