Screening for Parasitic Infection and Tuberculosis in Immunosuppressed and Pre-Immunosuppressed Patients: An Observational Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design, Setting and Participants
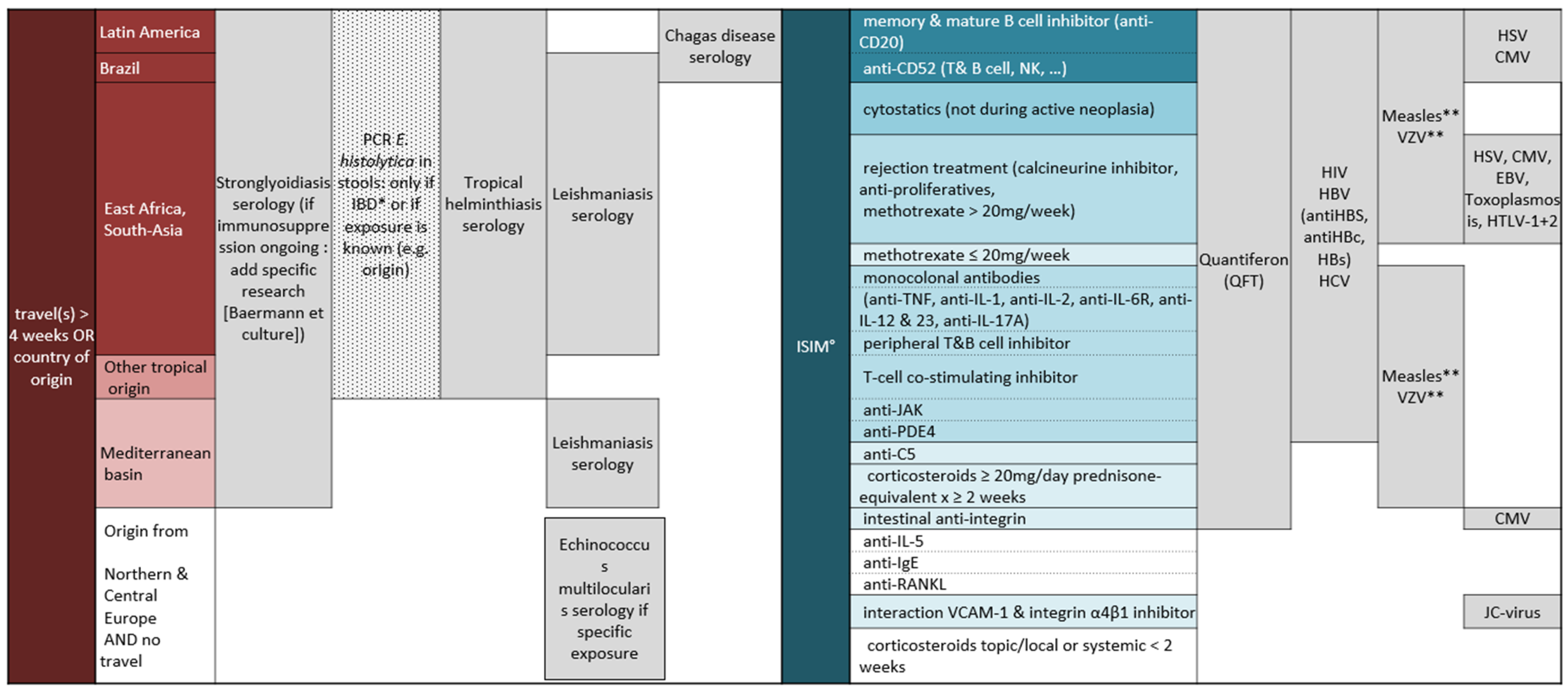
2.2. Screening Test Indications
2.3. Laboratory Tests
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Prevalence of Parasitic and Latent Tuberculosis Infection
3.2. Risk Factor for LTBI and Strongyloidiasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, B.I.; Kenney, B.; Malani, P.N.; Clauw, D.J.; Nallamothu, B.K.; Waljee, A.K. Prevalence of Immunosuppressive Drug Use Among Commercially Insured US Adults, 2018–2019. JAMA Netw. Open 2021, 4, e214920. [Google Scholar] [CrossRef]
- Patel, M.; Chen, J.; Kim, S.; Garg, S.; Flannery, B.; Haddadin, Z.; Rankin, D.; Halasa, N.; Talbot, H.K.; Reed, C. Analysis of MarketScan Data for Immunosuppressive Conditions and Hospitalizations for Acute Respiratory Illness, United States. Emerg. Infect. Dis. 2020, 26, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Montalvá, A.; Camps, I.R.; Barba, P.; Valcarcel, D.; Sulleiro, E.; Sanz-García, E.; Molina, I.; Salvador, F. Imported Disease Screening Prior to Chemotherapy and Bone Marrow Transplantation for Oncohematological Malignancies. Am. J. Trop. Med. Hyg. 2016, 95, 1463–1468. [Google Scholar] [CrossRef] [Green Version]
- Statistiques Cantonal Etat de Geneve. Available online: https://www.ge.ch/statistique/domaines/apercu.asp (accessed on 27 July 2021).
- Jackson, Y.; Paignon, A.; Wolff, H.; Delicado, N. Health of undocumented migrants in primary care in Switzerland. PLoS ONE 2018, 13, e0201313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longchamp, C.; Aebersold, M.; Rousselot, B.; Ratelband-Pally, S. Sans Papier in der Schweiz: Arbeitsmarkt, nicht Asylpolitik ist entscheidend; Gfs: Bern, Switzerland, 2005. [Google Scholar]
- Davis, J.S.; Ferreira, D.; Paige, E.; Gedye, C.; Boyle, M. Infectious Complications of Biological and Small Molecule Targeted Immunomodulatory Therapies. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Goyal, K.; Merola, J.F. Screening and Vaccinations in Patients Requiring Systemic Immunosuppression: An Update for Dermatologists. Am. J. Clin. Dermatol. 2015, 16, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.E.; Kumar, D.; Green, M.; Ison, M.G.; Kaul, D.; Michaels, M.G. Considerations for Screening Live Kidney Donors for Endemic Infections: A Viewpoint on the UNOS Policy: Live Kidney Donor Screening for Endemic Infections. Am. J. Transplant. 2014, 14, 1003–1011. [Google Scholar] [CrossRef] [Green Version]
- Requena-Méndez, A.; Muñoz, J.; Gomez-Junyent, J.; Bisoffi, Z.; Buonfrate, D.; Zammarchi, L. Evidence-Based Guidelines for Screening and Management of Strongyloidiasis in Non-Endemic Countries. Am. J. Trop. Med. Hyg. 2017, 97, 645–652. [Google Scholar] [CrossRef]
- Eperon, G.; Bühler, S.; Enriquez, N.; Vaudaux, B. The immunosuppressed traveler: Vaccination guidelines. Rev. Med. Suisse 2018, 14, 922–933. [Google Scholar] [PubMed]
- Kern, P.; Ammon, A.; Kron, M.; Sinn, G.; Sander, S.; Petersen, L.R.; Gaus, W.; Kern, P. Risk factors for alveolar echinococcosis in humans. Emerg. Infect. Dis. 2004, 10, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.M.; Masiá, M.; Rodriguez, J.C.; Lopez, C.; Padilla, S.; Robledano, C.; Navarro-Blasco, F.J.; Matarredona, J.; Garcia-Sepulcre, M.F.; Gutiérrez, F. Negative effect of immunosuppressive therapy in the performance of the QuantiFERON Gold In-Tube test in patients with immune-mediated inflammatory diseases. Clin. Exp. Med. 2012, 13, 177–186. [Google Scholar] [CrossRef]
- Cohen, A.; Mathiasen, V.D.; Schön, T.; Wejse, C. The global prevalence of latent tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2019, 54, 1900655. [Google Scholar] [CrossRef] [PubMed]
- Houben, R.M.G.J.; Dodd, P.J. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, T.; Au, E.; Chen, S.; Tong, A.; Wong, G. Screening and prevention for latent tuberculosis in immunosuppressed patients at risk for tuberculosis: A systematic review of clinical practice guidelines. BMJ Open 2018, 8, e022445. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, D.R.; Sayeed, L.; Rose, M.; Budd, E.; Mohammed, M.; Harrison, S.; Azad, J.; Maddox, J. Adherence to guidelines across different specialties to prevent infections in patients undergoing immunosuppressive therapies. BMC Infect. Dis. 2020, 20, 359. [Google Scholar] [CrossRef]
- Sullivan, R.; Gaskell, C.; Lewis, C.R.; Vollmer-Conna, U.; Post, J.J. Infectious disease screening in patients prior to undergoing immunosuppressive therapy. Int. J. Clin. Pract. 2019, 73, e13406. [Google Scholar] [CrossRef]
- Winnicki, W.; Eder, M.; Mazal, P.; Mayer, F.J.; Sengölge, G.; Wagner, L. Prevalence of Strongyloides stercoralis infection and hyperinfection syndrome among renal allograft recipients in Central Europe. Sci. Rep. 2018, 8, 15406. [Google Scholar] [CrossRef]
- Luvira, V.; Chantawat, N.; Naaglor, T.; Dekumyoy, P.; Mungthin, M.; Trakulhun, K.; Phiboonbanakit, D.; Pakdee, W. Comparative Diagnosis of Strongyloidiasis in Immunocompromised Patients. Am. J. Trop. Med. Hyg. 2016, 95, 401–404. [Google Scholar] [CrossRef] [Green Version]
- Presumptive Treatment and Screening for Strongyloidiasis, Infections Caused by Other Soil-Transmitted Helminths, and Schistosomiasis Among Newly Arrived Refugees. [Internet]. CDC Website; 2021. Available online: https://www.cdc.gov/immigrantrefugeehealth/guidelines/domestic/intestinal-parasites-domestic.html (accessed on 3 July 2021).
- Stauffer, W.M.; Alpern, J.D.; Walker, P.F. COVID-19 and Dexamethasone: A Potential Strategy to Avoid Steroid-Related Strogyloides Hyperinfection. JAMA 2020, 324, 623. [Google Scholar] [CrossRef]
- Akuffo, H.; Costa, C.; Van Griensven, J.; Burza, S.; Moreno, J.; Herrero, M. New insights into leishmaniasis in the immunosuppressed. PLoS Negl. Trop. Dis. 2018, 12, e0006375. [Google Scholar] [CrossRef]
- Dujardin, J.-C.; Campino, L.; Cañavate, C.; Dedet, J.-P.; Gradoni, L.; Soteriadou, K.; Mazeris, A.; Özbel, Y.; Boelaert, M. Spread of Vector-borne Diseases and Neglect of Leishmaniasis, Europe. Emerg. Infect. Dis. 2008, 14, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Y.; Chappuis, F. Chagas disease in Switzerland: History and challenges. Eurosurveillance 2011, 16, 19963. [Google Scholar] [CrossRef] [Green Version]
- Moreira, M.d.C.V.; Cunha-Melo, R.J. Chagas Disease Infection Reactivation after Heart Transplant. Trop. Med. 2020, 5, 106. [Google Scholar] [CrossRef]
- Schweiger, A.; Ammann, R.W.; Candinas, D.; Clavien, P.-A.; Eckert, J.; Gottstein, B.; Halkic, N.; Muellhaupt, B.; Prinz, B.M.; Reichen, J.; et al. Human Alveolar Echinococcosis after Fox Population Increase, Switzerland. Emerg. Infect. Dis. 2007, 13, 878–882. [Google Scholar] [CrossRef] [PubMed]
- Chauchet, A.; Grenouillet, F.; Knapp, J.; Richou, C.; Delabrousse, E.; Dentan, C.; Millon, L.; Di Martino, V.; Contreras, R.; Deconinck, E.; et al. Increased Incidence and Characteristics of Alveolar Echinococcosis in Patients With Immunosuppression-Associated Conditions. Clin. Infect. Dis. 2014, 59, 1095–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, J.; Sako, Y.; Grenouillet, F.; Bresson-Hadni, S.; Richou, C.; Gbaguidi-Haore, H.; Ito, A.; Millon, L. Comparison of the serological tests ICT and ELISA for the diagnosis of alveolar echinococcosis in France. Parasite 2014, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Leray, E.; Moreau, T.; Fromont, A.; Edan, G. Epidemiology of multiple sclerosis. Rev. Neurol. Paris 2016, 172, 3–13. [Google Scholar] [CrossRef] [PubMed]
| Pathogen | Test | Cut Off Value |
|---|---|---|
| Mycobacterium tuberculosis | Quantiferon® | Positive ≥ 0.35 IU/mL Indeterminate: blood cells have not responded to a positive control stimulant. Negative < 0.35 IU/ml |
| Strongyloides spp. | In–house ELISA IgG (S. ratti antigen) SWISS TPH | Positive > 0.7 OD Doubtful ≥ 0.5 ≤ 0.69 OD Negative < 0.5 OD |
| Euroimmune® ELISA IgG (S. papillosus antigen) Swiss TPH | Negative < 0.8 Doubtful ≥ 0.8 to < 1.1 Positive ≥ 1.1 | |
| Baermann test and stool culture (HUG) | Positive/Negative | |
| Entomoeba histolytica | Stool PCR (HUG) | Positive/Negative |
| Trypanosoma cruzi | Chagas STAT-PAK® | Positive/Negative |
| ELISA IgG (HUG) | Positive > 1 | |
| Echinococcus multilocularis | ELISA IgG (UniBern) E. multilocularis Em2 E. multilocularis Em18 | Positive/Negative |
| Leishmania spp. | IFAT | Positive ≥ 80 Negative < 80 |
| Characteristic | N = 406 |
|---|---|
| Sex, n (%) | |
| Female | 259 (63.7%) |
| Male | 147 (36.3%) |
| Age, median (range) | 42.2 (18–84) |
| Nationality, n (%) | |
| Switzerland/Europe | 347 (85.5%) |
| Others | 59 (14.5%) |
| Region of origin, n (%) | |
| Africa | 19 (4.7%) |
| Asia | 12 (3.0%) |
| Mediterranean Basin and Middle East | 84 (20.7%) |
| Latin America | 20 (5.0%) |
| Northern Europe, USA, Australia | 271 (66.7%) |
| Travel, n (%) | |
| Africa | |
| for more than 4 consecutive weeks * | 30 (7.4%) |
| less than 4 consecutive weeks or never | 317 (78.0%) |
| unknown duration | 59 (14.6%) |
| Asia | |
| for more than 4 consecutive weeks * | 41 (10.0%) |
| less than 4 weeks or never | 284 (70.0%) |
| unknown duration | 81 (20.0%) |
| Mediterranean Basin and Middle East | |
| for more than 4 consecutive weeks * | 111 (27.4%) |
| less than 4 weeks or never | 158 (38.9%) |
| unknown duration | 137 (33.7%) |
| Latin America | |
| for more than 4 consecutive weeks * | 37 (9.1%) |
| less than 4 consecutive weeks or never | 310 (76.3%) |
| unknown duration | 59 (14.5%) |
| Disease for which ISIM drugs were prescribed | |
| Multiple sclerosis | 282 (69.4%) |
| Rheumatic disease | 35 (8.5%) |
| Dermatological disease | 14 (3.4%) |
| Inflammatory bowel disease | 7 (1.7%) |
| Organ transplantation | 17 (4.2%) |
| Other: including pre transplantation screening | 54 (13.3%) |
| Immunosuppression at the time of the Quantiferon test, n (%) | 184 (52%) ◊ |
| Immunosuppression at the time of the serological screening, n (%) | 220 (57.6%) ⌂ |
| Pathogen | Number of Test Done | Positive n (%) | 95% CI |
|---|---|---|---|
| Mycobacterium tuberculosis | 353 | 24 (6.7%) | 4.6–10.0 |
| Strongyloides spp. | |||
| • Strongyloides spp. in–house ELISA IgG | 368 | 6 (1.6%) | 0.7–3.6 |
| • Strongyloides spp. ELISA IgG Euroimmune® (Confirmation test) | 6 | 5 (83.3%) | 2.3–9.8 |
| • Baermann test and stool culture | 38 | 0 | - |
| • a Definition of positive: in-house > 0.7 or Euroimmune® positive | 368 | 8 (2.2%) | 0.9–4.2 |
| Entamoeba histolytica | 32 | 1 (3.1%) | 0.1–16 |
| Trypanosoma cruzi | 64 | 0 | - |
| Echinococcus multilocularis | 56 | 0 | - |
| Leishmania spp. | 299 | 1 (0.3%) | <0.1–2.3 |
| Characteristic | Positive | Negative  | Crude PR (95% CI) | Adjusted PR * (95% CI) |
|---|---|---|---|---|
| LTBI | ||||
| Originate from high prevalence regions | ||||
| No | 8 (3.3%) | 231 | Ref | Ref |
| Yes | 16 (14.0%) | 98 | 4.2 (1.8–9.5) | 4.0 (1.8–8.9) |
| Travel | ||||
| No | 6 (3.1%) | 184 | Ref | Ref |
| Yes | 18 (11.0%) | 145 | 3.5 (1.4–8.6) | 3.4 (1.4–8.2) |
| Immunosuppression | ||||
| No | 15 (8.9%) | 154 | Ref | Ref |
| Yes | 9 (4.9%) | 175 | 0.5 (0.2–1.2) | |
| Age | - | - | 1.0 (0.9–1.0) | 1.0 (0.9–1) |
| Sex | ||||
| Female | 17 (7.5%) | 208 | Ref | Ref |
| Male | 7 (5.5%) | 121 | 0.7 (0.3–1.7) | 0.7 (0.3–1.6) |
| Strongyloidiasis | ||||
| Originate from endemic country | ||||
| No | 3 (1.2%) | 240 | Ref | - |
| Yes | 5 (4.0%) | 120 | 3.2 (0.8–13.4) | - |
| Travel | - | |||
| No | 3 (1.6%) | 183 | Ref | - |
| Yes | 5 (2.7%) | 177 | 1.7 (0.4–7.0) | |
| Immunosuppression | ||||
| No | 7 (3.2%) | 211 | Ref | - |
| Yes | 1 (0.6%) | 149 | 0.1 (0.01–0.8) | - |
| Age | - | - | 1.0 (0.9–1.0) | - |
| Sex | ||||
| Female | 3 (1.3%) | 226 | Ref | |
| Male | 5 (3.6%) | 132 | 2.8 (0.7–11.5) | - |
 For TB indeterminate results considered as negative.
For TB indeterminate results considered as negative.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carnino, L.; Schwob, J.-M.; Neofytos, D.; Lazo-Porras, M.; Chappuis, F.; Eperon, G. Screening for Parasitic Infection and Tuberculosis in Immunosuppressed and Pre-Immunosuppressed Patients: An Observational Study. Trop. Med. Infect. Dis. 2021, 6, 170. https://doi.org/10.3390/tropicalmed6030170
Carnino L, Schwob J-M, Neofytos D, Lazo-Porras M, Chappuis F, Eperon G. Screening for Parasitic Infection and Tuberculosis in Immunosuppressed and Pre-Immunosuppressed Patients: An Observational Study. Tropical Medicine and Infectious Disease. 2021; 6(3):170. https://doi.org/10.3390/tropicalmed6030170
Chicago/Turabian StyleCarnino, Luisa, Jean-Marc Schwob, Dionysios Neofytos, Maria Lazo-Porras, François Chappuis, and Gilles Eperon. 2021. "Screening for Parasitic Infection and Tuberculosis in Immunosuppressed and Pre-Immunosuppressed Patients: An Observational Study" Tropical Medicine and Infectious Disease 6, no. 3: 170. https://doi.org/10.3390/tropicalmed6030170
APA StyleCarnino, L., Schwob, J.-M., Neofytos, D., Lazo-Porras, M., Chappuis, F., & Eperon, G. (2021). Screening for Parasitic Infection and Tuberculosis in Immunosuppressed and Pre-Immunosuppressed Patients: An Observational Study. Tropical Medicine and Infectious Disease, 6(3), 170. https://doi.org/10.3390/tropicalmed6030170






