Prevalence of Malaria and Leptospirosis Co-Infection among Febrile Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Information Sources
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Quality of the Included Studies
2.7. Data Synthesis and Statistical Analysis
3. Results
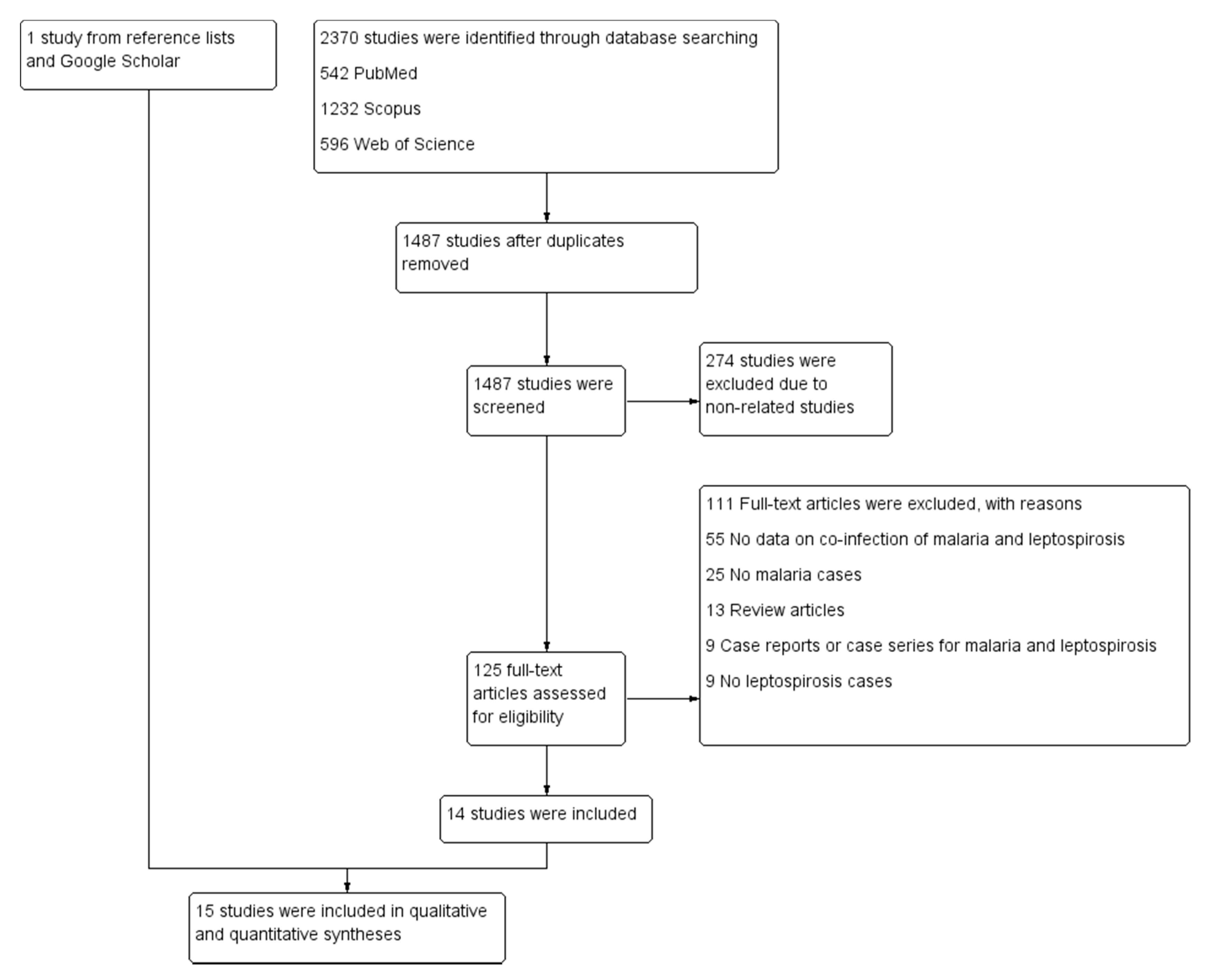
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Quality of the Included Studies
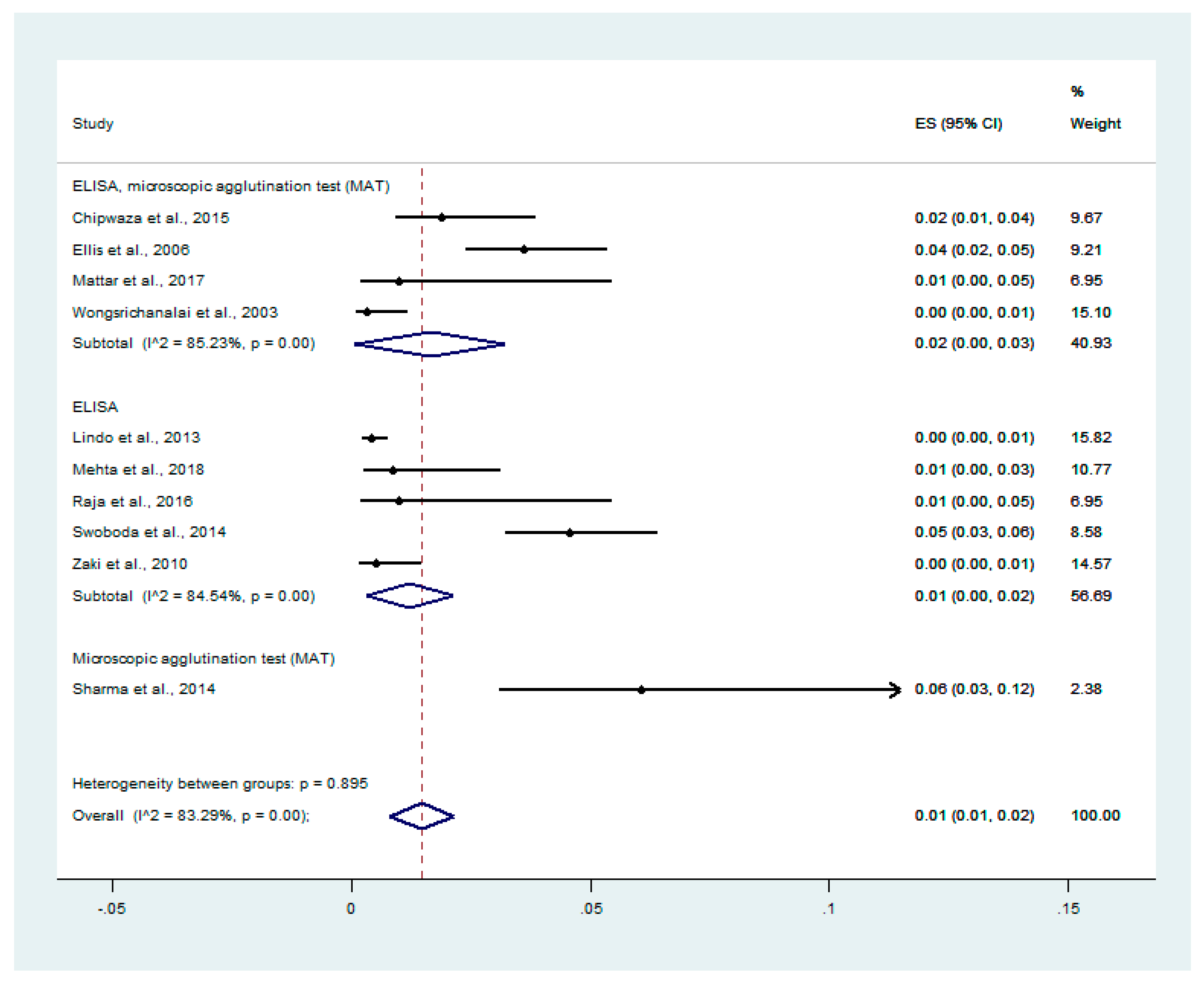
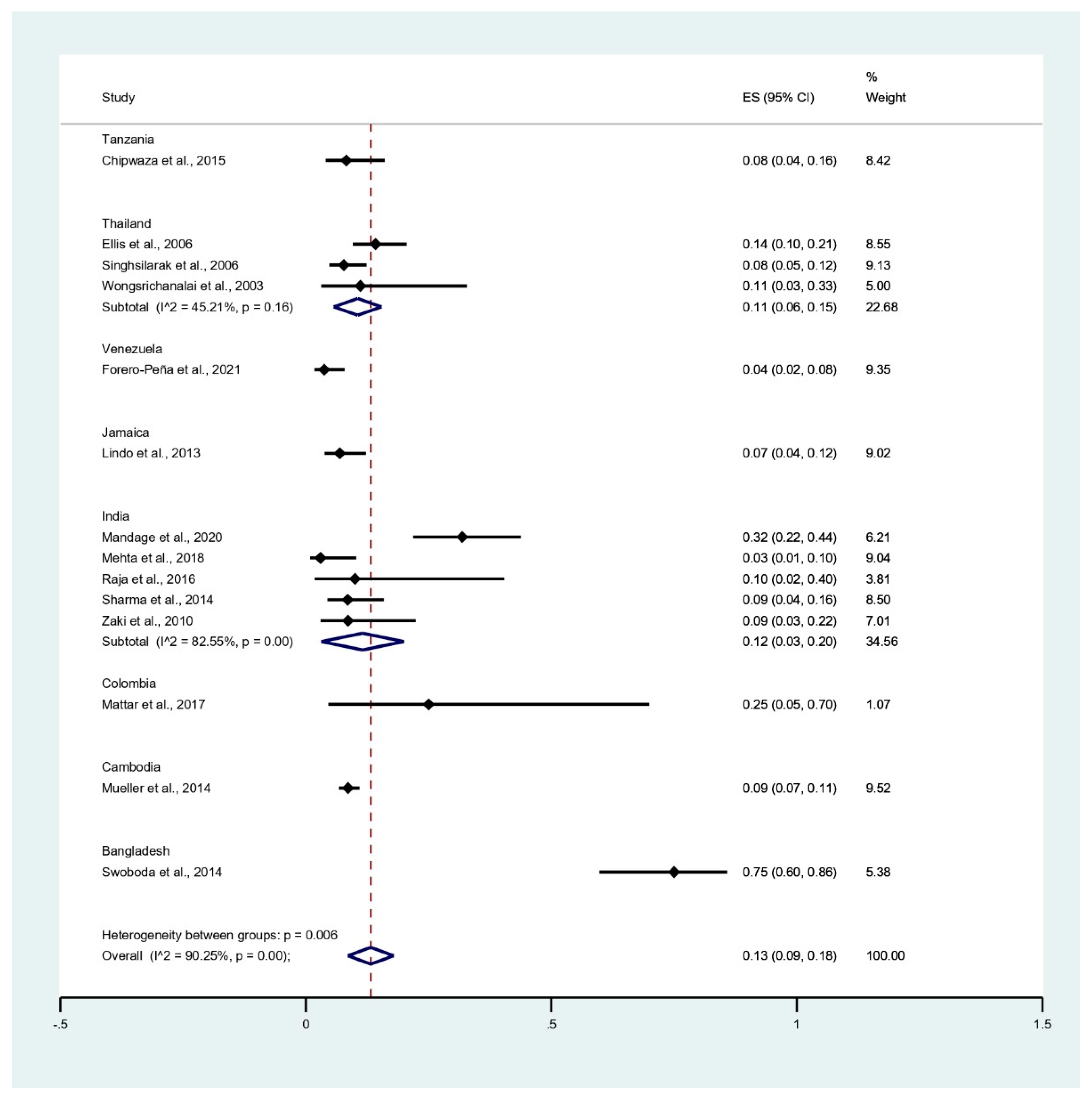
3.4. Prevalence of Malaria and Leptospirosis Co-Infection among Febrile Patients
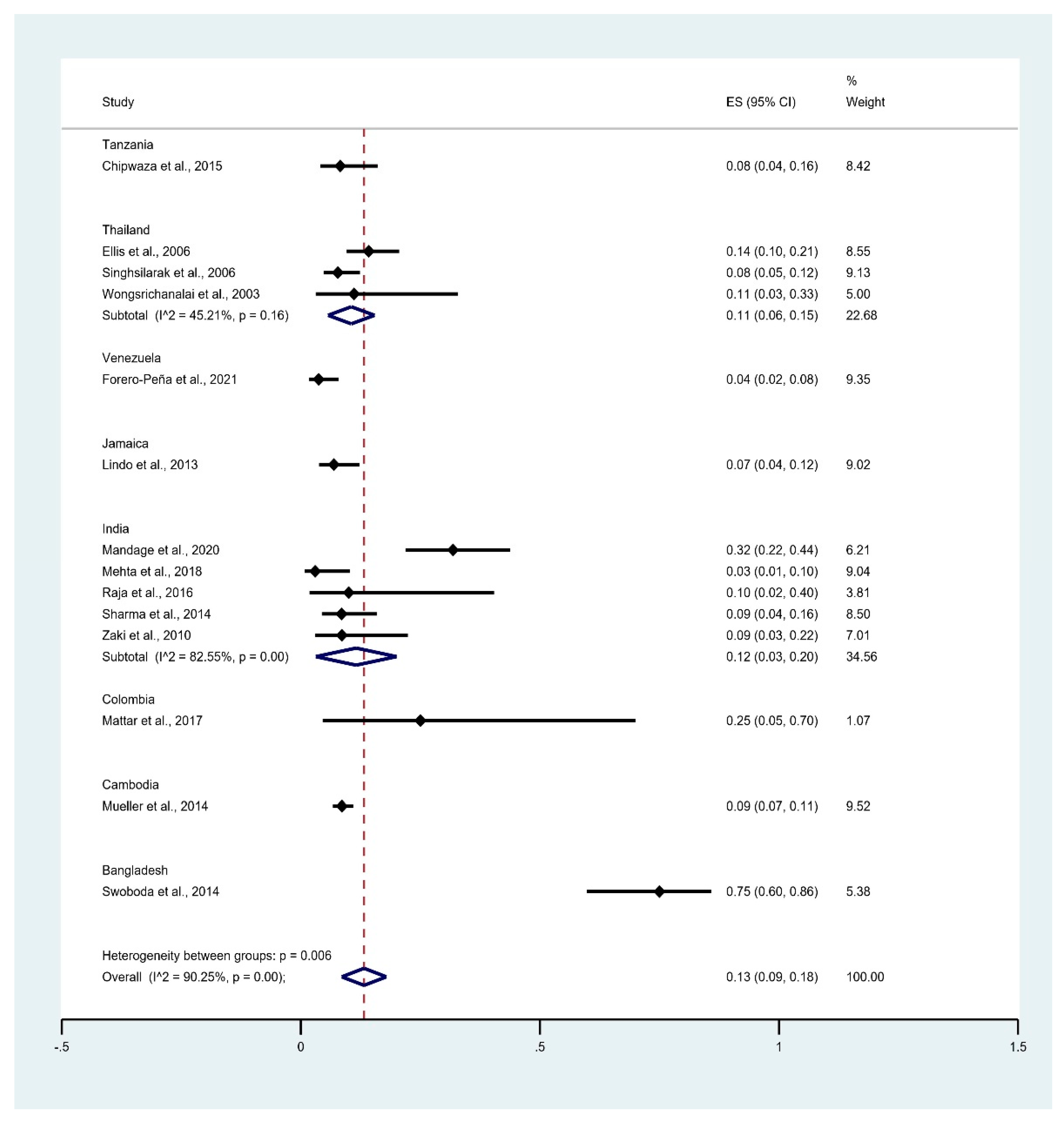
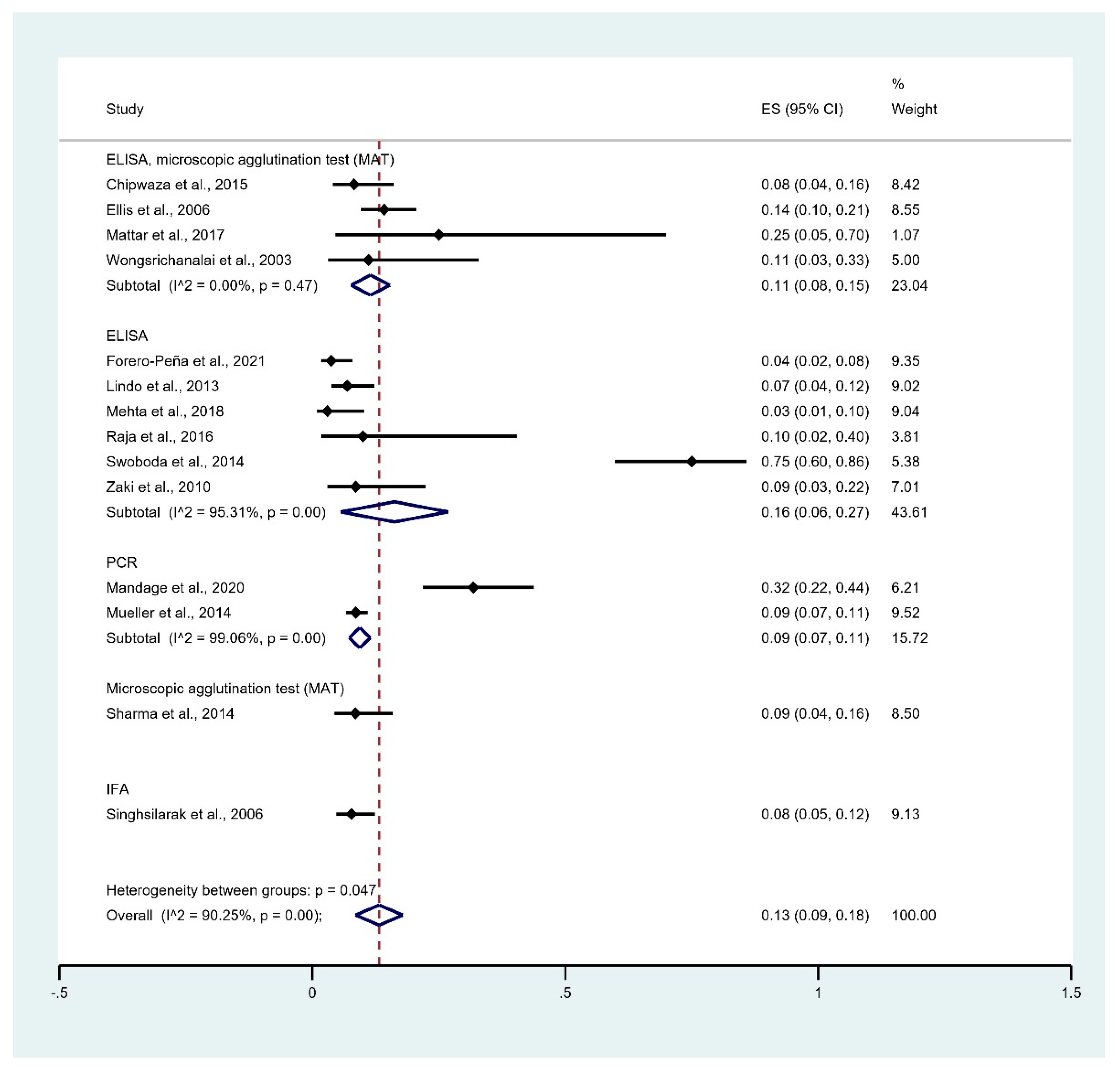
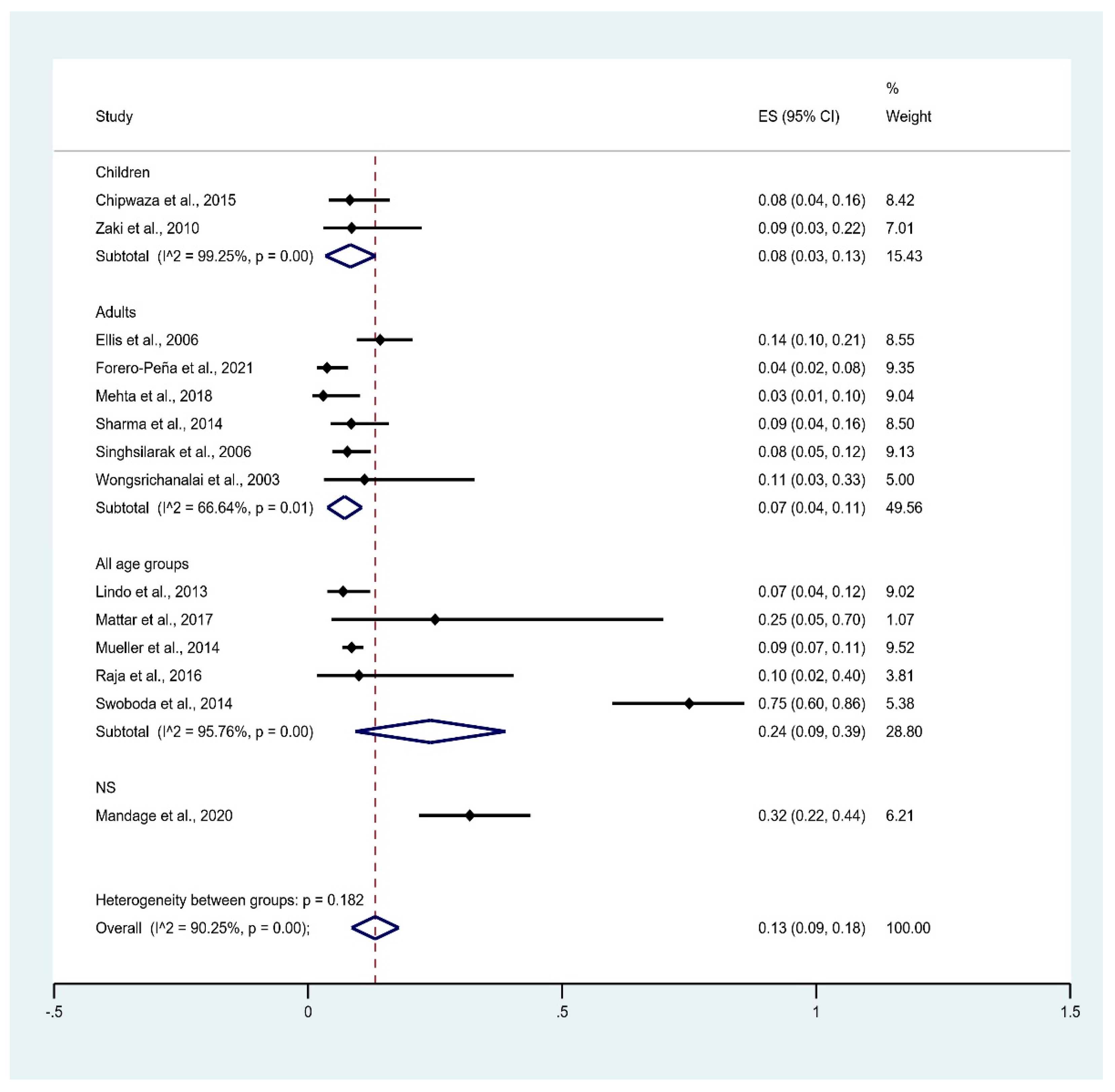
3.5. Prevalence of Leptospirosis Infection among Malaria Patients
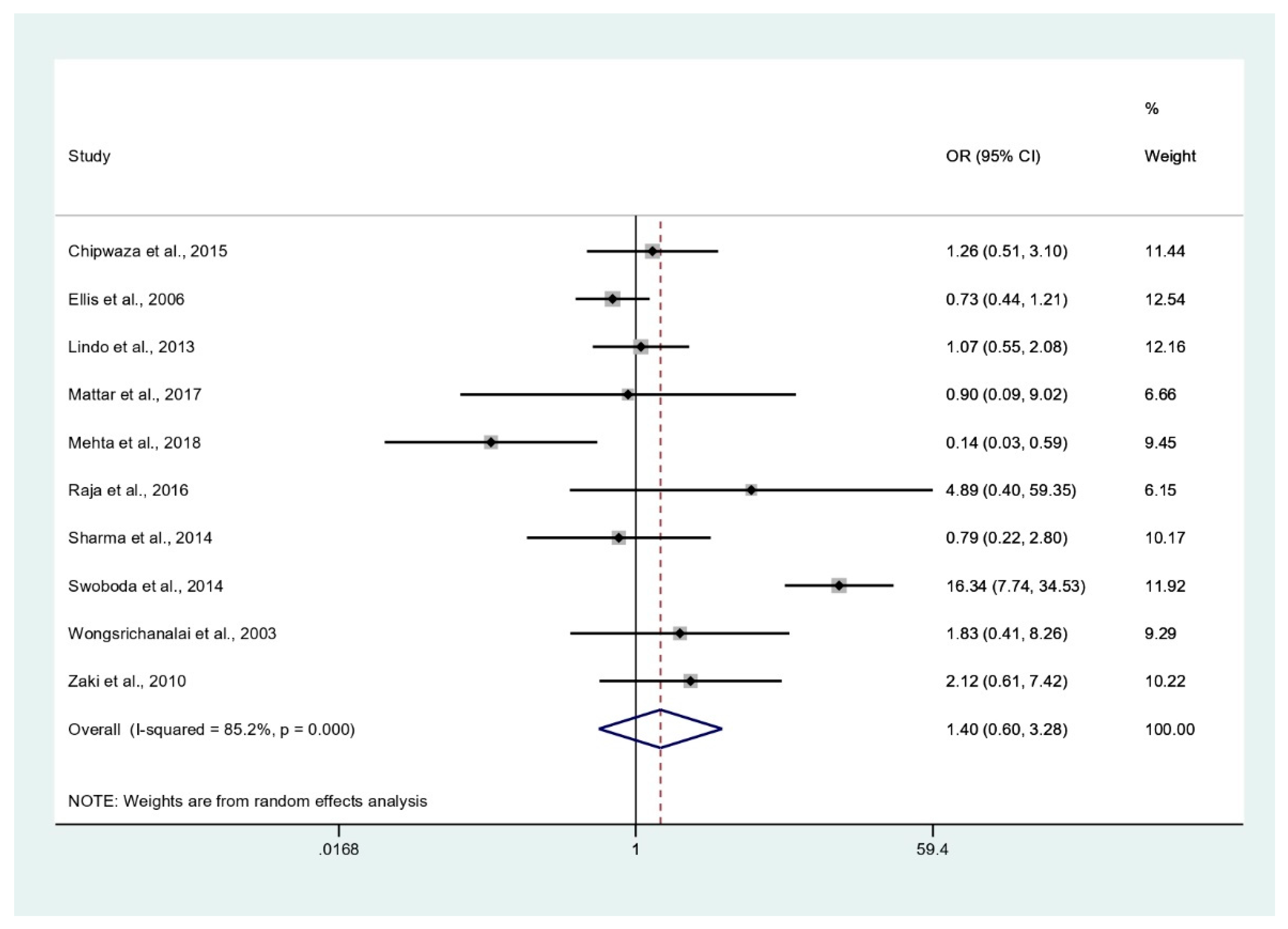
3.6. Odds of Malaria and Leptospirosis Co-Infections
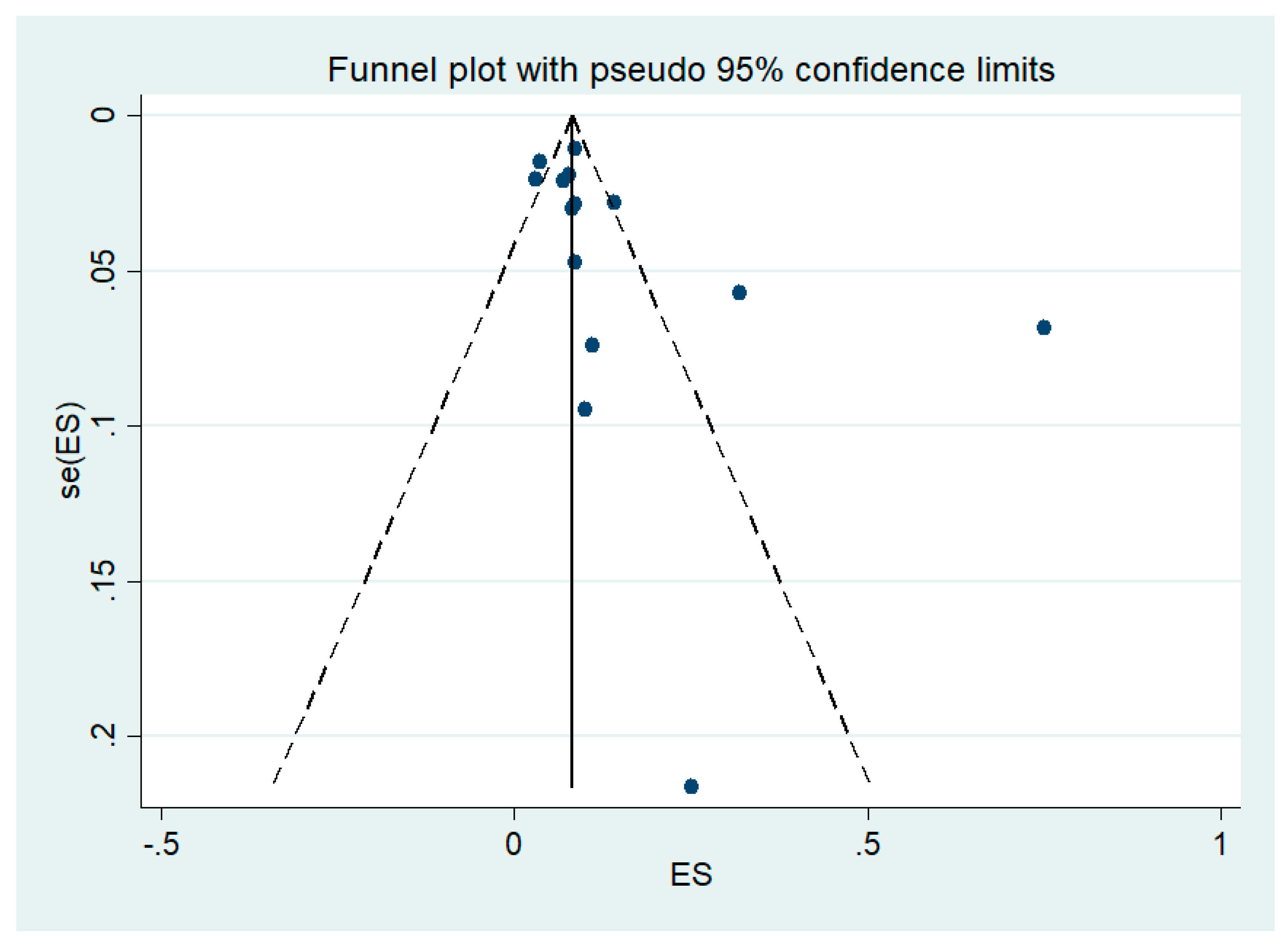
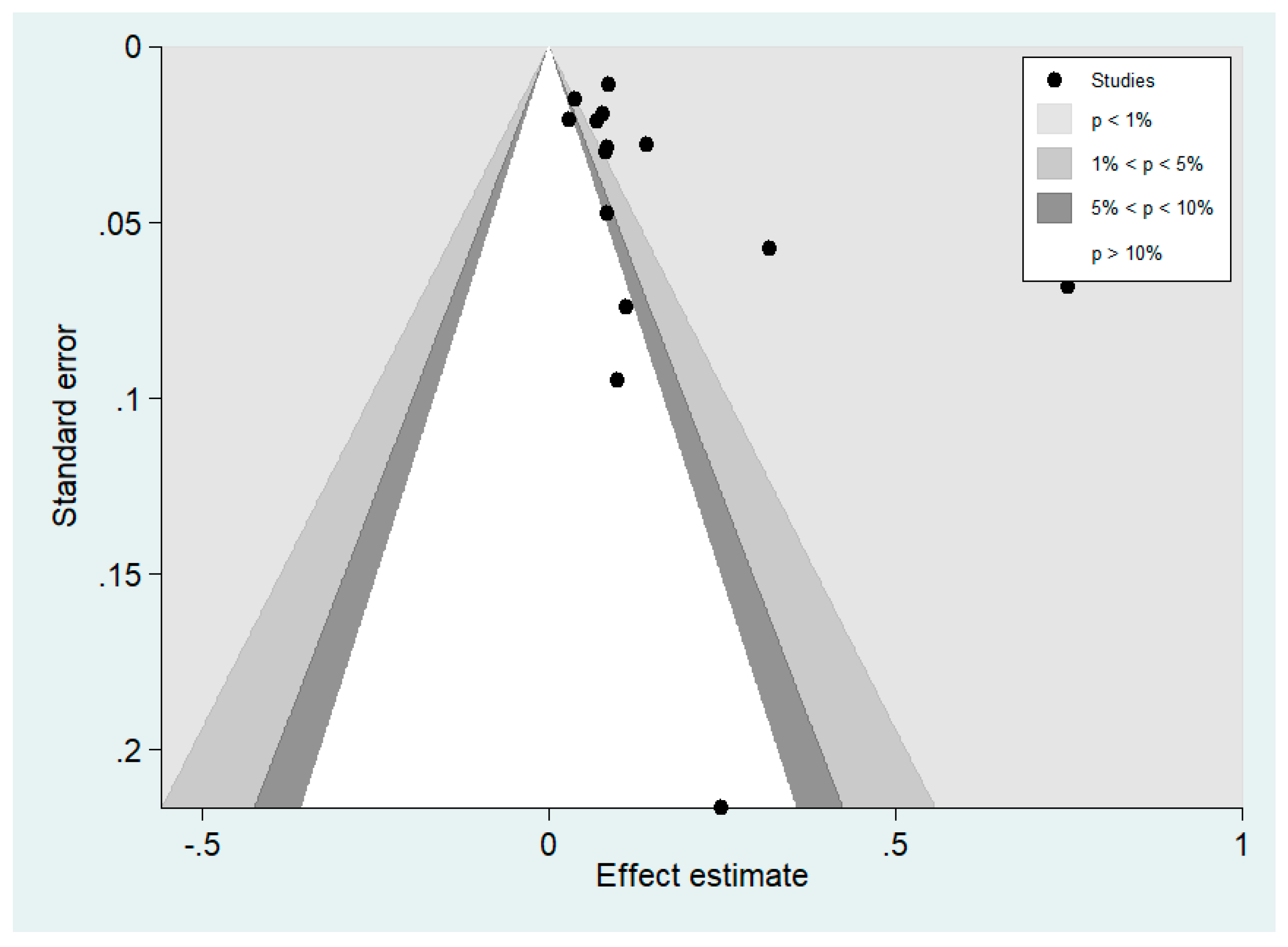
3.7. Publication Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahittikorn, A.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.J.; Kotepui, M. Comparison of Plasmodium ovale curtisi and Plasmodium ovale wallikeri infections by a meta-analysis approach. Sci. Rep. 2021, 11, 6409. [Google Scholar] [CrossRef] [PubMed]
- Imwong, M.; Madmanee, W.; Suwannasin, K.; Kunasol, C.; Peto, T.J.; Tripura, R. Asymptomatic Natural Human Infections with the Simian Malaria Parasites Plasmodium cynomolgi and Plasmodium knowlesi. J. Infect. Dis. 2019, 219, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Putaporntip, C.; Kuamsab, N.; Pattanawong, U.; Yanmanee, S.; Seethamchai, S.; Jongwutiwes, S. Plasmodium cynomolgi Co-infections among Symptomatic Malaria Patients, Thailand. Emerg. Infect. Dis 2021, 27, 590–593. [Google Scholar] [CrossRef]
- Kotepui, M.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.J. Preliminary review on the prevalence, proportion, geographical distribution, and characteristics of naturally acquired Plasmodium cynomolgi infection in mosquitoes, macaques, and humans: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 259. [Google Scholar] [CrossRef]
- Raja, T.N.; Hu, T.H.; Kadir, K.A.; Mohamad, D.S.A.; Rosli, N.; Wong, L.L.; Hii, K.C.; Simon Divis, P.C.; Singh, B. Naturally Acquired Human Plasmodium cynomolgi and P. knowlesi Infections, Malaysian Borneo. Emerg. Infect. Dis. 2020, 26, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Grignard, L.; Shah, S.; Chua, T.H.; William, T.; Drakeley, C.J.; Fornace, K.M. Natural Human Infections with Plasmodium cynomolgi and Other Malaria Species in an Elimination Setting in Sabah, Malaysia. J. Infect. Dis. 2019, 220, 1946–1949. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. WHO Guidelines for Malaria; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Severity and mortality of severe Plasmodium ovale infection: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0235014. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U.; De Jesus Milanez, G.; Masangkay, F.R. Plasmodium spp. mixed infection leading to severe malaria: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11068. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Global prevalence and mortality of severe Plasmodium malariae infection: A systematic review and meta-analysis. Malar. J. 2020, 19, 274. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Prevalence of severe Plasmodium knowlesi infection and risk factors related to severe complications compared with non-severe P. knowlesi and severe P. falciparum malaria: A systematic review and meta-analysis. Infect. Dis. Poverty 2020, 9, 106. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.J.; Masangkay, F.R. Prevalence and risk factors related to poor outcome of patients with severe Plasmodium vivax infection: A systematic review, meta-analysis, and analysis of case reports. BMC Infect. Dis. 2020, 20, 363. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, K.; Jeevan, J.; Mitra, S.; Paul, N.; Murugan, T.; Rangaraj, A.; David, S.; Hansdak, S.; Prakash, J.; Abraham, A.; et al. Acute undifferentiated febrile illness in patients presenting to a Tertiary Care Hospital in South India: Clinical spectrum and outcome. J. Global Infect. Dis. 2016, 8, 147–154. [Google Scholar] [CrossRef]
- Luvira, V.; Silachamroon, U.; Piyaphanee, W.; Lawpoolsri, S.; Chierakul, W.; Leaungwutiwong, P.; Thawornkuno, C.; Wattanagoon, Y. Etiologies of Acute Undifferentiated Febrile Illness in Bangkok, Thailand. Am. J. Trop. Med. Hyg. 2019, 100, 622–629. [Google Scholar] [CrossRef]
- Chin, V.K.; Basir, R.; Nordin, S.A.; Abdullah, M.; Sekawi, Z. Pathology and Host Immune Evasion During Human Leptospirosis: A Review. Int. Microbiol. 2020, 23, 127–136. [Google Scholar] [CrossRef]
- Soo, Z.M.P.; Khan, N.A.; Siddiqui, R. Leptospirosis: Increasing importance in developing countries. Acta Trop. 2020, 201, 105183. [Google Scholar] [CrossRef] [PubMed]
- Samrot, A.V.; Sean, T.C.; Bhavya, K.S.; Sahithya, C.S.; Chan-Drasekaran, S.; Palanisamy, R.; Robinson, E.R.; Subbiah, S.K.; Mok, P.L. Leptospiral Infection, Pathogenesis and Its Diagnosis-A Review. Pathogens 2021, 10, 145. [Google Scholar] [CrossRef]
- Karpagam, K.B.; Ganesh, B. Leptospirosis: A neglected tropical zoonotic infection of public health importance-an updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Mohd Radi, M.F.; Hashim, J.H.; Jaafar, M.H.; Hod, R.; Ahmad, N.; Mohammed Nawi, A.; Baloch, G.M.; Ismail, R.; Farakhin Ayub, N.I. Leptospirosis Outbreak After the 2014 Major Flooding Event in Kelantan, Malaysia: A Spatial-Temporal Analysis. Am. J. Trop. Med. Hyg. 2018, 98, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Jorge, S.; Schuch, R.A.; de Oliveira, N.R.; da Cunha, C.E.P.; Gomes, C.K.; Oliveira, T.L.; Rizzi, C.; Qadan, A.F.; Pacce, V.D.; Coelho Recuero, A.L.; et al. Human and animal leptospirosis in Southern Brazil: A five-year retrospective study. Travel Med. Infect. Dis. 2017, 18, 46–52. [Google Scholar] [CrossRef]
- Thibeaux, R.; Iraola, G.; Ferres, I.; Bierque, E.; Girault, D.; Soupe-Gilbert, M.E.; Picardeau, M.; Goarant, C. Deciphering the unexplored Leptospira diversity from soils uncovers genomic evolution to virulence. Microb. Genom. 2018, 4. [Google Scholar] [CrossRef]
- Munoz-Zanzi, C.; Groene, E.; Morawski, B.M.; Bonner, K.; Costa, F.; Bertherat, E.; Schneider, M.C. A systematic literature review of leptospirosis outbreaks worldwide, 1970–2012. Rev. Panam Salud Publica 2020, 44, e78. [Google Scholar] [CrossRef]
- Bharti, A.R.; Nally, J.E.; Ricaldi, J.N.; Matthias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gotuzzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Marquez, A.; Djelouadji, Z.; Lattard, V.; Kodjo, A. Overview of laboratory methods to diagnose Leptospirosis and to identify and to type leptospires. Int. Microbiol. 2017, 20, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Atiqah, N.; Dasiman, M.; Amran, F. Demographic, clinical and laboratory features of leptospirosis-malaria co-infections in Peninsular Malaysia. J. Med. Microbiol. 2020, 69, 451–456. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.M.Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; Mu, P.-F. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; Aromataris, E.M.Z., Ed.; JBI: Adelaide, Australia, 2020. [Google Scholar]
- Chipwaza, B.; Mhamphi, G.G.; Ngatunga, S.D.; Selemani, M.; Amuri, M.; Mugasa, J.P.; Gwakisa, P.S. Prevalence of Bacterial Febrile Illnesses in Children in Kilosa District, Tanzania. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef]
- Ellis, R.D.; Fukuda, M.M.; McDaniel, P.; Welch, K.; Nisalak, A.; Murray, C.K.; Gray, M.R.; Uthaimongkol, N.; Buathong, N.; Sriwichai, S.; et al. Causes of fever in adults on the Thai-Myanmar border. Am. J. Trop. Med. Hyg. 2006, 74, 108–113. [Google Scholar] [CrossRef]
- Lindo, J.; Brown, P.D.; Vickers, I.; Brown, M.; Jackson, S.T.; Lewis-Fuller, E. Leptospirosis and malaria as causes of febrile illness during a dengue epidemic in Jamaica. Pathog. Glob. Health 2013, 107, 329–334. [Google Scholar] [CrossRef]
- Mandage, R.; Kaur, C.; Pramanik, A.; Kumar, V.; Kodan, P.; Singh, A.; Saha, S.; Pandey, S.; Wig, N.; Pandey, R.M.; et al. Association of Dengue Virus and Leptospira Co-Infections with Malaria Severity. Emerg. Infect. Dis. 2020, 26, 1645–1653. [Google Scholar] [CrossRef]
- Mattar, S.; Tique, V.; Miranda, J.; Montes, E.; Garzon, D. Undifferentiated tropical febrile illness in Cordoba, Colombia: Not everything is dengue. J. Infect. Public Health 2017, 10, 507–512. [Google Scholar] [CrossRef]
- Mehta, K.; Pajai, A.; Bhurke, S.; Shirkande, A.; Bhadade, R.; D’Souza, R. Acute kidney injury of infectious etiology in monsoon season: A prospective study using acute kidney injury network criteria. Indian J. Nephrol. 2018, 28, 143–152. [Google Scholar] [CrossRef]
- Mueller, T.C.; Siv, S.; Khim, N.; Kim, S.; Fleischmann, E.; Ariey, F.; Buchy, P.; Guillard, B.; González, I.J.; Christophel, E.M.; et al. Acute undifferentiated febrile illness in rural Cambodia: A 3-year prospective observational study. PLoS ONE 2014, 9, e95868. [Google Scholar] [CrossRef] [PubMed]
- Raja, J.M.; Mary, A.; Usha, S. A Study on Dual Infections in Pyrexia Cases. Int. J. Med Res. Health Sci. 2016, 5, 150–155. [Google Scholar]
- Sharma, S.; Mandal, A.; Vijayachari, P. Investigation of Malaria among Patients of Febrile Illness and Co-Infection with Leptospirosis in Andaman and Nicobar Islands, India. Res. J. Microbiol. 2014, 9, 104–110. [Google Scholar] [CrossRef][Green Version]
- Singhsilarak, T.; Phongtananant, S.; Jenjittikul, M.; Watt, G.; Tangpakdee, N.; Popak, N.; Chalermrut, K.; Looareesuwan, S. Possible acute coinfections in Thai malaria patients. Southeast. Asian J. Trop. Med. Public Health 2006, 37, 1–4. [Google Scholar]
- Swoboda, P.; Fuehrer, H.P.; Ley, B.; Starzengruber, P.; Ley-Thriemer, K.; Jung, M.; Matt, J.; Fally, M.A.; Mueller, M.K.; Reismann, J.A.; et al. Evidence of a major reservoir of non-malarial febrile diseases in malaria-endemic regions of Bangladesh. Am. J. Trop. Med. Hyg. 2014, 90, 377–382. [Google Scholar] [CrossRef][Green Version]
- Wongsrichanalai, C.; Murray, C.K.; Gray, M.; Miller, R.S.; McDaniel, P.; Liao, W.J.; Pickard, A.L.; Magill, A.J. Co-infection with malaria and leptospirosis. Am. J. Trop. Med. Hyg. 2003, 68, 583–585. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.A.; Shanbag, P. Clinical manifestations of dengue and leptospirosis in children in Mumbai: An observational study. Infection 2010, 38, 285–291. [Google Scholar] [CrossRef]
- Forero-Peña, D.A.; Amaya, I.; Gamardo, A.F.; Chavero, M.; Figuera, L.; Camejo-Ávila, N.A.; Marcano, M.V.; Hidalgo, M.; Arenas, C.J.; Arévalo-Herrera, M.; et al. High Prevalence of Viral and Bacterial Coinfections in Malaria in Venezuela. Available online: https://www.researchsquare.com/article/rs-332012/v1 (accessed on 19 May 2021).
- Chirathaworn, C.; Inwattana, R.; Poovorawan, Y.; Suwancharoen, D. Interpretation of microscopic agglutination test for leptospirosis diagnosis and seroprevalence. Asian Pac. J. Trop. Biomed. 2014, 4, S162–S164. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis. Clin. Microbiol Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Ahmed, A.; Engelberts, M.F.; Boer, K.R.; Ahmed, N.; Hartskeerl, R.A. Development and validation of a real-time PCR for detection of pathogenic leptospira species in clinical materials. PLoS ONE 2009, 4, e7093. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; La Scola, B. Laboratory diagnosis of leptospirosis: A challenge. J. Microbiol. Immunol. Infect. 2013, 46, 245–252. [Google Scholar] [CrossRef]
- Guerrier, G.; Hie, P.; Gourinat, A.C.; Huguon, E.; Polfrit, Y.; Goarant, C.; D’Ortenzio, E.; Missotte, I. Association between age and severity to leptospirosis in children. PLoS Negl. Trop. Dis. 2013, 7, e2436. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Krijger, I.M.; Ahmed, A.A.A.; Goris, M.G.A.; Groot Koerkamp, P.W.G.; Meerburg, B.G. Prevalence of Leptospira Infection in Rodents from Bangladesh. Int J. Environ. Res. Public Health 2019, 16, 2113. [Google Scholar] [CrossRef]
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef]
- Haque, U.; Overgaard, H.J.; Clements, A.C.; Norris, D.E.; Islam, N.; Karim, J.; Roy, S.; Haque, W.; Kabir, M.; Smith, D.L.; et al. Malaria burden and control in Bangladesh and prospects for elimination: An epidemiological and economic assessment. Lancet. Glob. Health 2014, 2, e98–e105. [Google Scholar] [CrossRef]
| Author | Study Site | Year of Conducted | Study Design | Participants | Age | Gender (Male:Female) | Co-Infection | All Malaria Cases | Malaria without Leptospirosis | Leptospirosis without Malaria | Test for Malaria | Test for Leptospirosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mueller et al., 2014 | Cambodia | 2008–2010 | Prospective observational studies | 1193 febrile patients and 282 non-febrile individuals | 7–49 years | 801:392 | 58 | 676 | 618 | 53 | Microscopy, RDT, PCR | PCR |
| Sharma et al., 2014 | India | 2009 | Prospective observational studies | 132 febrile patients | ≥18 years | NS | 8 | 94 | 86 | 4 | RDT | Microscopic agglutination test (MAT) |
| Mehta et al., 2018 | India | 2012–2013 | Prospective observational studies | 230 patients with acute kidney injury | ≥18 years | NS | 2 | 67 | 65 | 30 | Microscopy, RDT | ELISA IgM |
| Wongsrichanalai et al., 2003 | Thailand | 1999–2002 | Prospective observational studies | 613 febrile patients | ≥20 years | NS | 2 | 18 | 16 | 38 | Microscopic agglutination test (MAT) | ELISA, microscopic agglutination test (MAT) |
| Ellis et al., 2006 | Thailand | 1999–2002 | Prospective observational studies | 370 febrile patients | 20–87 years | 325:288 | 22 | 155 | 133 | 85 | Microscopy | ELISA, microscopic agglutination test (MAT) |
| Swoboda et al., 2014 | Bangladesh | 2007–2010 | Cross-sectional study | 659 febrile patients | ≥8 years | 344:315 | 30 | 40 | 10 | 96 | Microscopy, RDT, PCR | ELISA IgM |
| Mattar et al., 2017 | Colombia | 2012–2013 | Prospective observational studies | 100 febrile patients | 1–79 years | 62:38 | 1 | 4 | 3 | 26 | Microscopy | ELISA, microscopic agglutination test (MAT) |
| Raja et al., 2016 | India | 2013–2014 | Cross-sectional study | 100 febrile patients | 5–60 years | NS | 1 | 10 | 9 | 2 | Microscopy | ELISA |
| Lindo et al., 2013 | Jamaica | 2007–2008 | Cross-sectional study | 2419 participants testing for dengue | All age groups | 1092:1327 | 10 | 145 | 135 | 147 | ELISA | ELISA IgM |
| Zaki et al., 2010 | India | 2005 | Cross-sectional study | 602 febrile patients | 1 month to 12 years | 3 | 35 | 32 | 24 | Microscopy | ELISA IgM | |
| Chipwaza et al., 2015 | Tanzania | 2013 | Cross-sectional study | 370 febrile patients | 2–13 years | 189:191 | 7 | 85 | 78 | 19 | Microscopy | ELISA, microscopic agglutination test (MAT) |
| Rao et al., 2020 | Malaysia | 2011–2014 | Retrospective observational study | 111 leptospirosis-positive patients | Adults | 107:4 | 26 | NS | NS | 85 | Microscopy | PCR, Microscopic agglutination test (MAT) |
| Singhsilarak et al., 2006 | Thailand | NS | Retrospective observational study | 194 malaria positive cases | All age | NS | 15 | 194 | NS | Microscopy | IFA | |
| Mandage et al., 2020 | India | 2017–2018 | Prospective observational studies | 66 malaria positive cases | NS | NS | 21 | 66 | 61 | NS | Microscopy, RDT, PCR | PCR |
| Forero-Peña et al., 2021 | Venezuela | 2018 | Cross-sectional study | 161 patients with P. vivax | Adults | NS | 6 | 161 | NA | NA | Microscopy | ELISA IgM/IgG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilairatana, P.; Mala, W.; Rattaprasert, P.; Kotepui, K.U.; Kotepui, M. Prevalence of Malaria and Leptospirosis Co-Infection among Febrile Patients: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2021, 6, 122. https://doi.org/10.3390/tropicalmed6030122
Wilairatana P, Mala W, Rattaprasert P, Kotepui KU, Kotepui M. Prevalence of Malaria and Leptospirosis Co-Infection among Febrile Patients: A Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease. 2021; 6(3):122. https://doi.org/10.3390/tropicalmed6030122
Chicago/Turabian StyleWilairatana, Polrat, Wanida Mala, Pongruj Rattaprasert, Kwuntida Uthaisar Kotepui, and Manas Kotepui. 2021. "Prevalence of Malaria and Leptospirosis Co-Infection among Febrile Patients: A Systematic Review and Meta-Analysis" Tropical Medicine and Infectious Disease 6, no. 3: 122. https://doi.org/10.3390/tropicalmed6030122
APA StyleWilairatana, P., Mala, W., Rattaprasert, P., Kotepui, K. U., & Kotepui, M. (2021). Prevalence of Malaria and Leptospirosis Co-Infection among Febrile Patients: A Systematic Review and Meta-Analysis. Tropical Medicine and Infectious Disease, 6(3), 122. https://doi.org/10.3390/tropicalmed6030122







