Monitoring of the Sensitivity In Vivo of Plasmodium falciparum to Artemether-Lumefantrine in Mali
Abstract
1. Introduction
2. Materials and Methods
3. Results
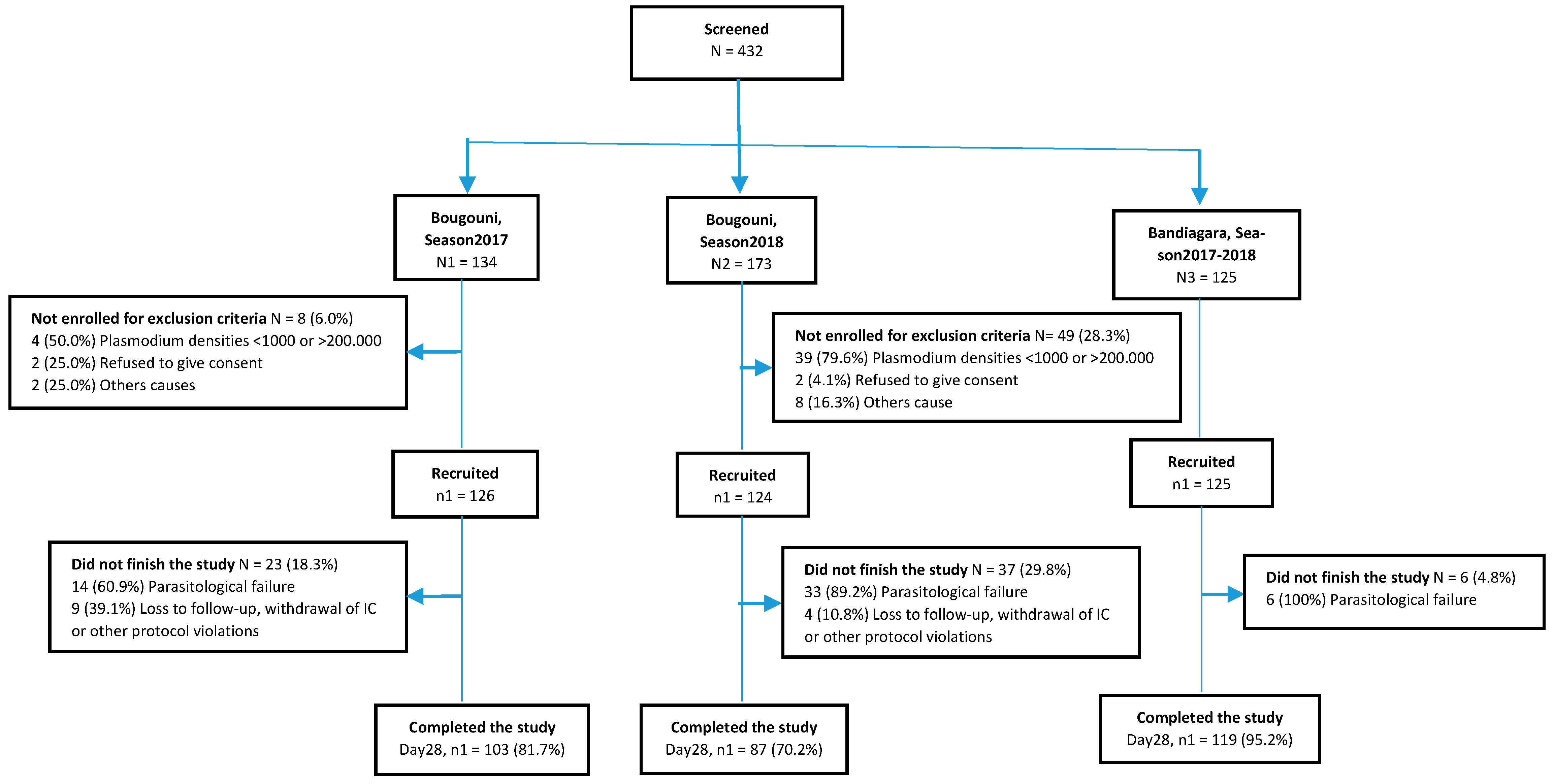
3.1. Participant Flow
3.2. Baseline Data
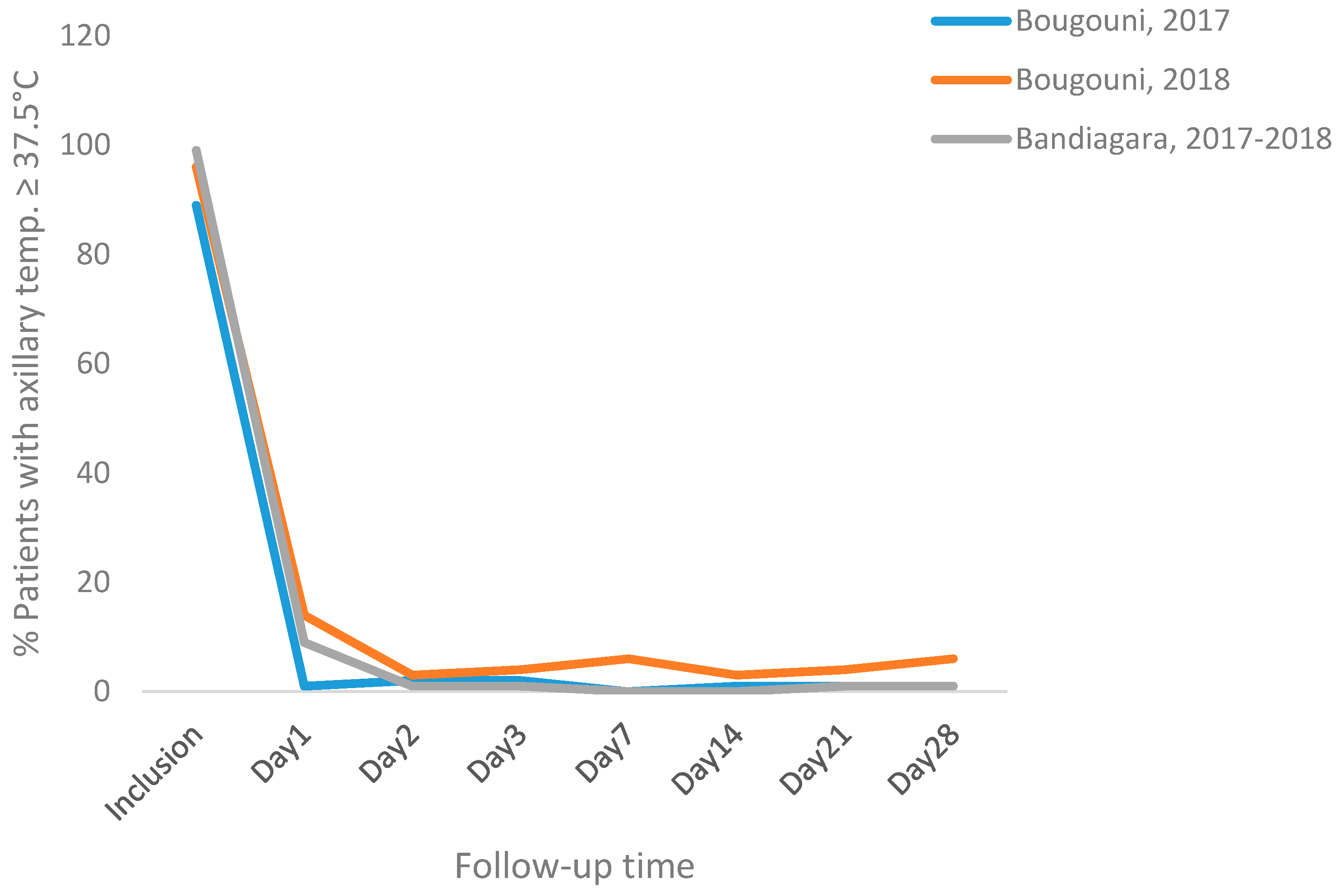
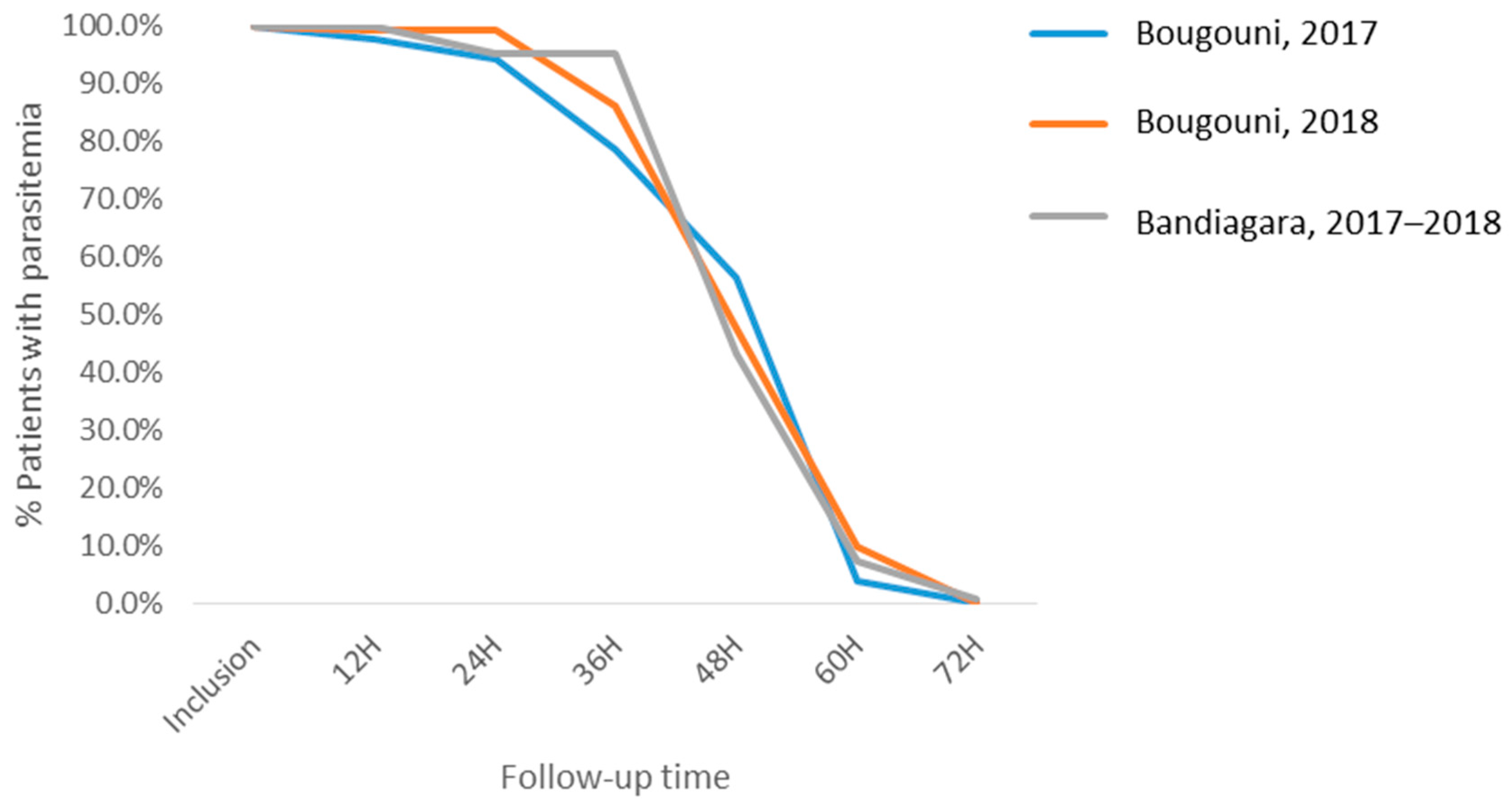
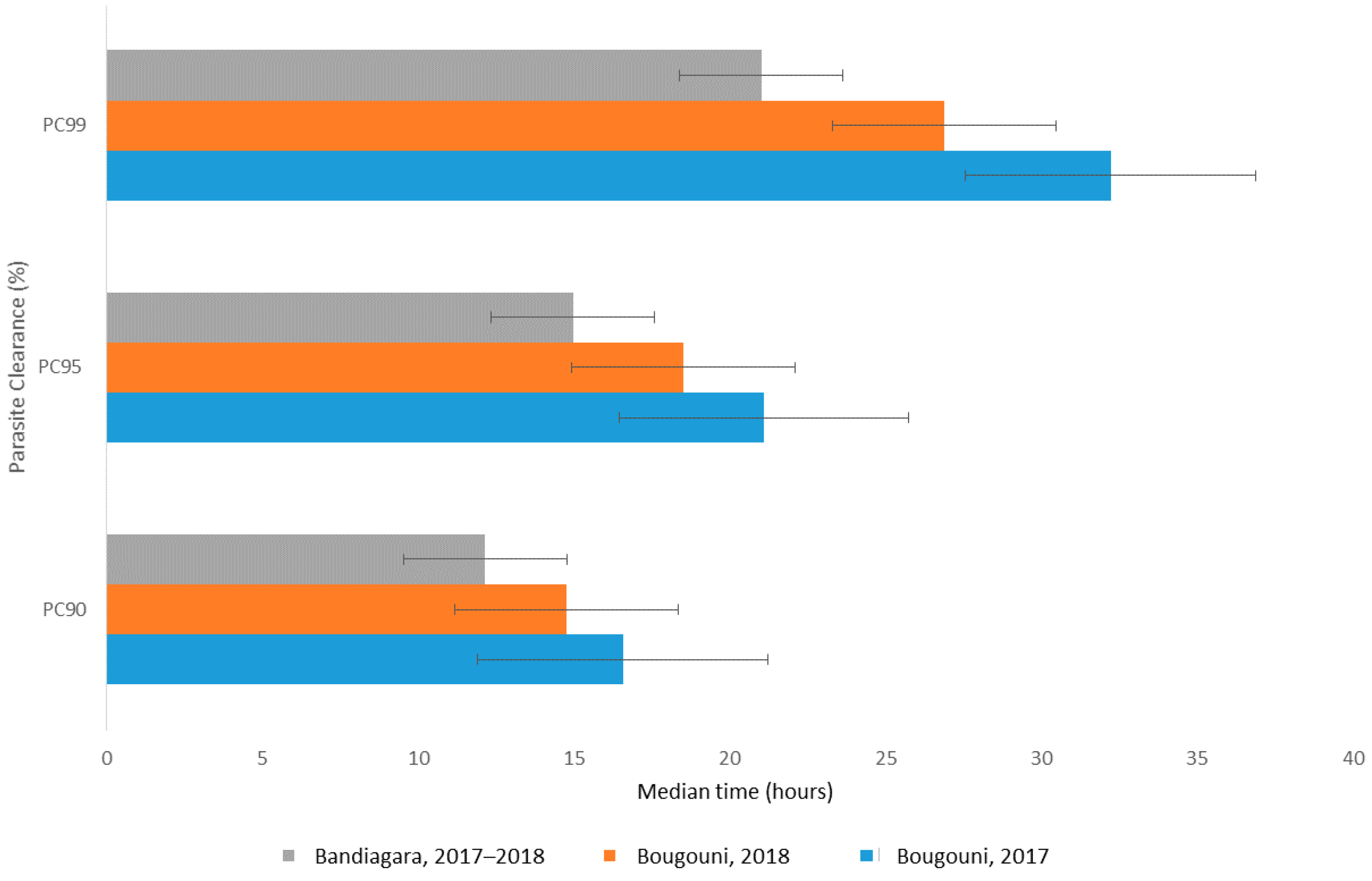
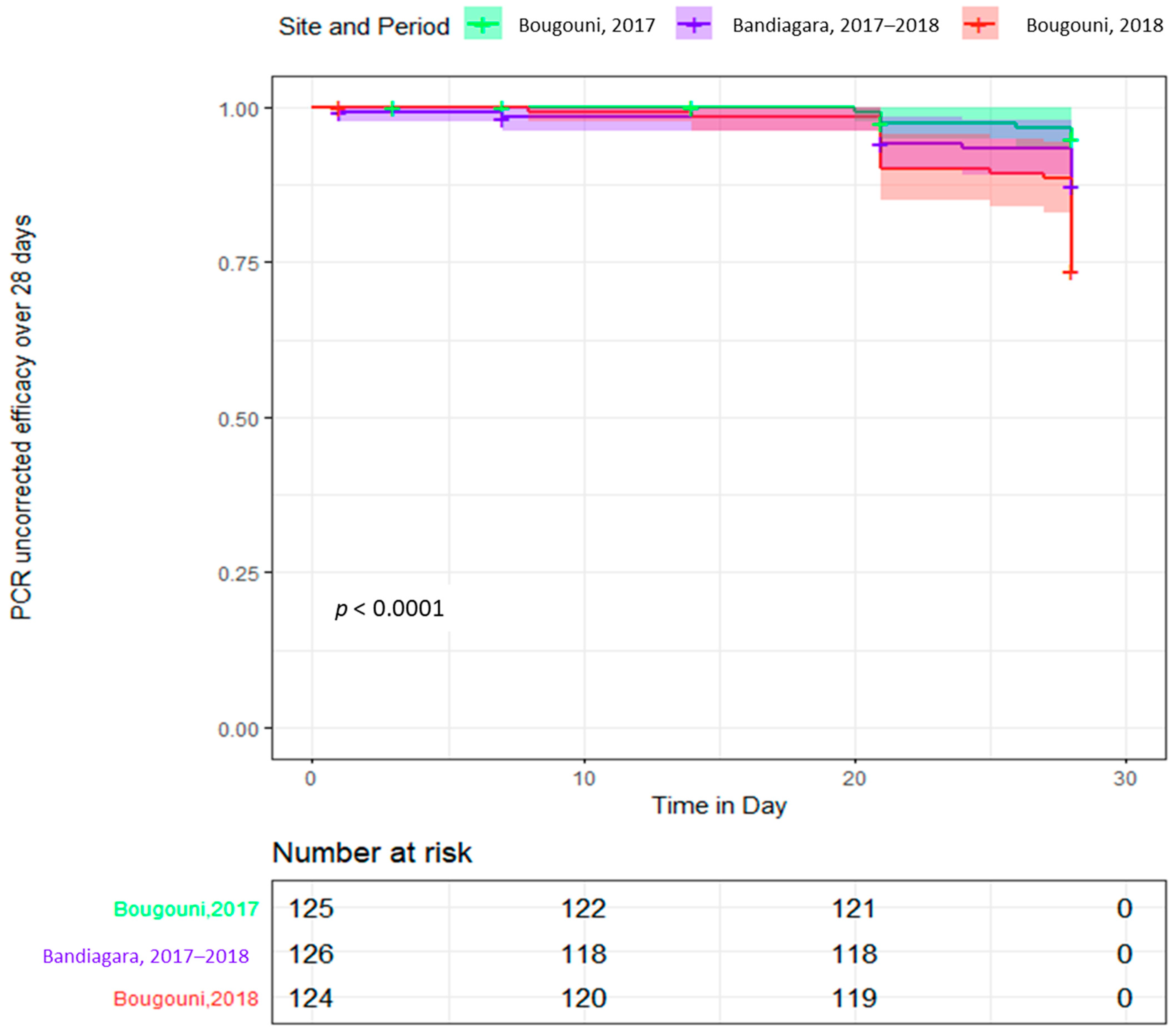
3.3. Efficacy
3.4. Tolerability and Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID Patient | Visit Days | Study Sites | Pfmsp2 | PfCA1 | PfTA99 | Outcome |
|---|---|---|---|---|---|---|
| Season 2017, Total: 14 | ||||||
| BOS006 | D0 | Bougouni | A | 1 | ||
| BOS006 | D21 | Bougouni | B | |||
| BOS009 | D0 | Bougouni | B | 1 | ||
| BOS009 | D28 | Bougouni | AC | |||
| BOS017 | D0 | Bougouni | A | 1 | ||
| BOS017 | D28 | Bougouni | B | |||
| BOS051 | D0 | Bougouni | A | 1 | ||
| BOS051 | D28 | Bougouni | BC | |||
| BAD052 | D0 | Bandiagara | B | 1 | ||
| BAD052 | UV | Bandiagara | A | |||
| BAD049 | D0 | Bandiagara | A | 1 | ||
| BAD049 | D21 | Bandiagara | B | |||
| BOS059 | D0 | Bougouni | A | 1 | ||
| BOS059 | D28 | Bougouni | B | |||
| BOS075 | D0 | Bougouni | A | 1 | ||
| BOS075 | D21 | Bougouni | B | |||
| BOS085 | D0 | Bougouni | A | C | 1 | |
| BOS085 | D28 | Bougouni | A | AB | ||
| BOS109 | D0 | Bougouni | A | A | 1 | |
| BOS109 | D28 | Bougouni | A | B | ||
| BOS114 | D0 | Bougouni | A | 1 | ||
| BOS114 | D28 | Bougouni | B | |||
| BOS115 | D0 | Bougouni | AB | 1 | ||
| BOS115 | D21 | Bougouni | CD | |||
| BOS129 | D0 | Bougouni | A | 1 | ||
| BOS129 | D21 | Bougouni | B | |||
| BOS163 | D0 | Bougouni | AB | AB | 1 | |
| BOS163 | D21 | Bougouni | A | C | ||
| Season 2018, Total: 39 | ||||||
| BOK001 | D0 | Bougouni | A | B | 1 | |
| BOK001 | D28 | Bougouni | 9 | A | ||
| BOK002 | D0 | Bougouni | AC | A | 1 | |
| BOK002 | D21 | Bougouni | AB | B | ||
| BOK003 | D0 | Bougouni | A | A | 1 | |
| BOK003 | D21 | Bougouni | BC | B | ||
| BOK009 | D0 | Bougouni | AB | A | 1 | |
| BOK009 | D28 | Bougouni | B | B | ||
| BOK013 | D0 | Bougouni | AB | A | 1 | |
| BOK013 | D21 | Bougouni | 9 | B | ||
| BOK016 | D0 | Bougouni | A | A | 1 | |
| BOK016 | D28 | Bougouni | 9 | B | ||
| BOK019 | D0 | Bougouni | A | 1 | ||
| BOK019 | D28 | Bougouni | B | |||
| BOK020 | D0 | Bougouni | AB | B | 1 | |
| BOK020 | D28 | Bougouni | B | A | ||
| BOK024 | D0 | Bougouni | A | A | 1 | |
| BOK024 | D28 | Bougouni | 9 | B | ||
| BOK025 | D0 | Bougouni | A | B | 1 | |
| BOK025 | D28 | Bougouni | A | A | ||
| BOK026 | D0 | Bougouni | A | B | 1 | |
| BOK026 | D28 | Bougouni | B | A | ||
| BOK028 | D0 | Bougouni | 9 | A | 1 | |
| BOK028 | D28 | Bougouni | A | B | ||
| BOK030 | D0 | Bougouni | B | 1 | ||
| BOK030 | D21 | Bougouni | A | |||
| BOK031 | D0 | Bougouni | BD | AC | 1 | |
| BOK031 | D21 | Bougouni | AC | B | ||
| BOK038 | D0 | Bougouni | B | A | A | 1 |
| BOK038 | D21 | Bougouni | ABC | 9 | B | |
| BOK044 | D0 | Bougouni | B | 1 | ||
| BOK044 | D28 | Bougouni | A | |||
| BOK048 | D0 | Bougouni | AB | 9 | A | 1 |
| BOK048 | D28 | Bougouni | A | A | B | |
| BOK050 | D0 | Bougouni | AB | A | 1 | |
| BOK050 | D21 | Bougouni | C | B | ||
| BOK053 | D0 | Bougouni | A | A | AC | 1 |
| BOK053 | D14 | Bougouni | 9 | A | B | |
| BOK058 | D0 | Bougouni | A | 1 | ||
| BOK058 | D28 | Bougouni | B | |||
| BOK059 | D0 | Bougouni | A | A | A | 1 |
| BOK059 | D28 | Bougouni | A | B | B | |
| BOK060 | D0 | Bougouni | ACD | A | 1 | |
| BOK060 | D28 | Bougouni | AB | B | ||
| BOK062 | D0 | Bougouni | ABD | 1 | ||
| BOK062 | D28 | Bougouni | C | |||
| BOO003 | D0 | Bougouni | AD | A | 1 | |
| BOO003 | D21 | Bougouni | AB | B | ||
| BOO029 | D0 | Bougouni | AC | B | 1 | |
| BOO029 | D21 | Bougouni | B | A | ||
| BOO018 | D0 | Bougouni | ABD | A | 1 | |
| BOO018 | D28 | Bougouni | 9 | B | ||
| BOO019 | D0 | Bougouni | C | 1 | ||
| BOO019 | D28 | Bougouni | AB | |||
| BOO025 | D0 | Bougouni | AB | B | 1 | |
| BOO025 | D28 | Bougouni | A | A | ||
| BOO052 | D0 | Bougouni | AB | A | AC | 1 |
| BOO052 | D28 | Bougouni | 9 | A | B | |
| BOO045 | D0 | Bougouni | B | 1 | ||
| BOO045 | D21 | Bougouni | A | |||
| BOO057 | D0 | Bougouni | A | B | 1 | |
| BOO057 | D28 | Bougouni | AB | A | ||
| BOO072 | D0 | Bougouni | AB | A | B | 1 |
| BOO072 | D28 | Bougouni | A | 9 | A | |
| BOO073 | D0 | Bougouni | AD | 1 | ||
| BOO073 | D21 | Bougouni | B | |||
| BAD077 | D0 | Bandiagara | AB | A | 1 | |
| BAD077 | D21 | Bandiagara | B | B | ||
| BAD081 | D0 | Bandiagara | B | A | A | 1 |
| BAD081 | D26 | Bandiagara | AB | 9 | B | |
| BAD088 | D0 | Bandiagara | A | A | 1 | |
| BAD088 | D28 | Bandiagara | AB | B | ||
| BAD097 | D0 | Bandiagara | AB | 1 | ||
| BAD097 | D21 | Bandiagara | C | |||
| BAD101 | D0 | Bandiagara | A | 1 | ||
| BAD101 | D21 | Bandiagara | CD | |||
| BAD120 | D0 | Bandiagara | D | 1 | ||
| BAD120 | D28 | Bandiagara | A | |||
References
- Trape, J.F. The public health impact of chloroquine resistance in Africa. Am. J. Trop. Med. Hyg. 2001, 64, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Tekete, M.; Djimde, A.A.; Beavogui, A.H.; Maiga, H.; Sagara, I.; Fofana, B.; Ouologuem, D.; Dama, S.; Kone, A.; Dembele, D.; et al. Efficacy of chloroquine, amodiaquine and sulphadoxine-pyrimethamine for the treatment of uncomplicated falciparum malaria: Revisiting molecular markers in an area of emerging AQ and SP resistance in Mali. Malar. J. 2009, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Reyburn, H. New WHO guidelines for the treatment of malaria. Br. Med. J. Publ. Group 2010. [Google Scholar] [CrossRef] [PubMed]
- Boli, M. PNLP: Objectif, zéro paludisme au Mali à l’horizon 2030. JSTM 2019. Available online: https://www.jstm.org/pnlp-objectif-zero-paludisme-au-mali-a-lhorizon-2030/ (accessed on 11 November 2020).
- WHO. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018; Available online: http://www.who.int/malaria/publications/world-malaria-report-2018/en/ (accessed on 19 November 2020).
- WHO. Assessment of Therapeutic Efficacy of Antimalarial Drugs: For Uncomplicated Falciparum Malaria in Areas with Intense Transmission; World Health Organization: Geneva, Switzerland, 1996; Available online: https://apps.who.int/iris/handle/10665/63295 (accessed on 19 November 2020).
- Profile_mli_en.pdf. Available online: https://www.who.int/malaria/publications/country-profiles/profile_mli_en.pdf (accessed on 19 November 2020).
- Kaddouri, H.; Djimdé, A.; Dama, S.; Kodio, A.; Tekete, M.; Hubert, V.; Koné, A.; Maiga, H.; Yattara, O.; Fofana, B.; et al. Baseline in vitro efficacy of ACT component drugs on Plasmodium falciparum clinical isolates from Mali. Int. J. Parasitol. 2008, 38, 791–798. [Google Scholar] [CrossRef]
- Ouattara, A.; Kone, A.; Adams, M.; Fofana, B.; Maiga, A.W.; Hampton, S.; Coulibaly, D.; Thera, M.A.; Diallo, N.; Dara, A.; et al. Polymorphisms in the K13-Propeller Gene in Artemisinin-Susceptible Plasmodium falciparum Parasites from Bougoula-Hameau and Bandiagara, Mali. Am. J. Trop. Med. Hyg. 2015, 92, 1202–1206. [Google Scholar] [CrossRef]
- Dicko, A.; Sagara, I.; Djimdé, A.A.; Touré, S.O.; Traore, M.; Dama, S.; Diallo, A.I.; Barry, A.; Dicko, M.; Coulibaly, O.M.; et al. Molecular markers of resistance to sulphadoxine-pyrimethamine one year after implementation of intermittent preventive treatment of malaria in infants in Mali. Malar. J. 2010, 9, 9. [Google Scholar] [CrossRef]
- Djimdé, A.A.; Fofana, B.; Sagara, I.; Sidibe, B.; Toure, S.; Dembele, D.; Dama, S.; Ouologuem, D.; Dicko, A.; Doumbo, O.K. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am. J. Trop. Med. Hyg. 2008, 78, 455–461. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A.P.; Tarning, J.; Lwin, K.M.; Ariey, F.; Hanpithakpong, W.; Lee, S.J.; et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2009, 361, 455–467. [Google Scholar] [CrossRef]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M.; Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef]
- WHO. Global Plan for Artemisinin Resistance Containment—GPARC (Archived); World Health Organization: Geneva, Switzerland, 2011; Available online: http://www.who.int/malaria/publications/atoz/9789241500838/en/ (accessed on 19 November 2020).
- Flegg, J.A.; Guerin, P.J.; White, N.J.; Stepniewska, K. Standardizing the measurement of parasite clearance in falciparum malaria: The parasite clearance estimator. Malar. J. 2011, 10, 339. [Google Scholar] [CrossRef]
- Duru, V.; Witkowski, B.; Ménard, D. Plasmodium falciparum Resistance to Artemisinin Derivatives and Piperaquine: A Major Challenge for Malaria Elimination in Cambodia. Am. J. Trop. Med. Hyg. 2016, 95, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, C.J.; White, N.J. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol. Rev. 2017, 41, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Witmer, K.; Dahalan, F.A.; Delves, M.J.; Yahiya, S.; Watson, O.J.; Straschil, U.; Chiwcharoen, D.; Sornboon, B.; Pukrittayakamee, S.; Pearson, R.D.; et al. Transmission of artemisinin-resistant malaria parasites to mosquitoes under antimalarial drug pressure. Antimicrob. Agents Chemother. 2020, 16, e00898-20. [Google Scholar] [CrossRef] [PubMed]
- WHO. Methods and Techniques for Clinical Trials on Antimalarial Drug Efficacy: Genotyping to Identify Parasite Populations; World Health Organization: Geneva, Switzerland, 2008; Available online: http://www.who.int/malaria/publications/atoz/9789241596305/en/ (accessed on 19 November 2020).
- World Health Organization, Communicable Diseases Cluster. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 2000, 94 (Suppl. 1), S1–S90. [Google Scholar] [CrossRef]
- Sagara, I.; Beavogui, A.H.; Zongo, I.; Soulama, I.; Borghini-Fuhrer, I.; Fofana, B.; Camara, D.; Somé, A.F.; Coulibaly, A.S.; Traore, O.B.; et al. Safety and efficacy of re-treatments with pyronaridine-artesunate in African patients with malaria: A substudy of the WANECAM randomised trial. Lancet Infect. Dis. 2016, 16, 189–198. [Google Scholar] [CrossRef]
- Mugittu, K.; Adjuik, M.; Snounou, G.; Ntoumi, F.; Taylor, W.; Mshinda, H.; Olliaro, P.; Beck, H.-P. Molecular genotyping to distinguish between recrudescents and new infections in treatment trials of Plasmodium falciparum malaria conducted in Sub-Saharan Africa: Adjustment of parasitological outcomes and assessment of genotyping effectiveness. Trop. Med. Int. Health 2006, 11, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Ranford-Cartwright, L.C.; Taylor, J.; Umasunthar, T.; Taylor, L.H.; Babiker, H.A.; Lell, B.; Schmidt-Ott, J.R.; Lehman, L.G.; Walliker, D.; Kremsner, P.G. Molecular analysis of recrudescent parasites in a Plasmodium falciparum drug efficacy trial in Gabon. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 719–724. [Google Scholar] [CrossRef]
- Dama, S.; Niangaly, H.; Djimde, M.; Sagara, I.; Guindo, C.O.; Zeguime, A.; Dara, A.; Djimde, A.A.; Doumbo, O.K. A randomized trial of dihydroartemisinin–piperaquine versus artemether–lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Malar. J. 2018, 17, 1–8. [Google Scholar] [CrossRef]
- Niaré, K.; Dara, A.; Sagara, I.; Sissoko, M.S.; Guindo, C.O.; Cissé, N.H.; Coulibaly, C.K.; Ringwald, P.; Benoit-Vical, F.; Berry, A.; et al. In Vivo Efficacy and Parasite Clearance of Artesunate + Sulfadoxine-Pyrimethamine Versus Artemether-Lumefantrine in Mali. Am. J. Trop. Med. Hyg. 2016, 94, 634–639. [Google Scholar] [CrossRef]
- WHO. Methods for Surveillance of Antimalarial Drug Efficacy; World Health Organization: Geneva, Switzerland, 2009; Available online: http://www.who.int/malaria/publications/atoz/9789241597531/en/ (accessed on 19 November 2020).
- Parasite Clearance Estimator (PCE). Worldwide Antimalarial Resistance Network. 2015. Available online: https://www.wwarn.org/parasite-clearance-estimator-pce (accessed on 11 December 2020).
- Nhama, A.; Bassat, Q.; Enosse, S.; Nhacolo, A.; Mutemba, R.; Carvalho, E.; Naueia, E.; Sevene, E.; Guinovart, C.; Warsame, M.; et al. In vivo efficacy of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated falciparum malaria in children: A multisite, open-label, two-cohort, clinical trial in Mozambique. Malar. J. 2014, 13, 309. [Google Scholar] [CrossRef]
- Myint, M.K.; Rasmussen, C.; Thi, A.; Bustos, D.; Ringwald, P.; Lin, K. Therapeutic efficacy and artemisinin resistance in northern Myanmar: Evidence from in vivo and molecular marker studies. Malar. J. 2017, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Price, R.N.; Bethell, D.; Guerin, P.J.; Stepniewska, K. Early parasitological response following artemisinin-containing regimens: A critical review of the literature. Malar. J. 2013, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Stepniewska, K.; Ashley, E.; Lee, S.J.; Anstey, N.; Barnes, K.I.; Binh, T.Q.; D’Alessandro, U.; Day, N.P.J.; de Vries, P.J.; Dorsey, G.; et al. In vivo parasitological measures of artemisinin susceptibility. J. Infect. Dis. 2010, 201, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Rouse, P.; Mkulama, M.A.; Thuma, P.E.; Mharakurwa, S. Distinction of Plasmodium falciparum recrudescence and re-infection by MSP2 genotyping: A caution about unstandardized classification criteria. Malar. J. 2008, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Felger, I.; Tavul, L.; Kabintik, S.; Marshall, V.; Genton, B.; Alpers, M.; Beck, H.P. Plasmodium falciparum: Extensive polymorphism in merozoite surface antigen 2 alleles in an area with endemic malaria in Papua New Guinea. Exp. Parasitol. 1994, 79, 106–116. [Google Scholar] [CrossRef]
- Beshir, K.B.; Sutherland, C.J.; Sawa, P.; Drakeley, C.J.; Okell, L.; Mweresa, C.K.; Omar, S.A.; Shekalaghe, S.A.; Kaur, H.; Ndaro, A.; et al. Residual Plasmodium falciparum Parasitemia in Kenyan Children After Artemisinin-Combination Therapy Is Associated With Increased Transmission to Mosquitoes and Parasite Recurrence. J. Infect. Dis. 2013, 208, 2017–2024. [Google Scholar] [CrossRef]
- Sawa, P.; Shekalaghe, S.A.; Drakeley, C.J.; Sutherland, C.J.; Mweresa, C.K.; Baidjoe, A.Y.; Manjurano, A.; Kavishe, R.A.; Beshir, K.B.; Yussuf, R.U.; et al. Malaria Transmission After Artemether-Lumefantrine and Dihydroartemisinin-Piperaquine: A Randomized Trial. J. Infect. Dis. 2013, 207, 1637–1645. [Google Scholar] [CrossRef]
- Beshir, K.B.; Diallo, N.; Sutherland, C.J. Identifying Recrudescent Plasmodium falciparum in Treated Malaria Patients by Real-time PCR and High Resolution Melt Analysis of Genetic Diversity. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
| Parameters at Inclusion | Bougouni 2017 N = 126 | Bougouni 2018 N = 124 | Bandiagara 2017–2018 N = 125 |
|---|---|---|---|
| Age (Year) Average (range) | 10.6 (2–40) | 11.0 (2–60) | 11.5 (1–36) |
| Age groups, n (%) | |||
| ≤5 years | 17 (15.6) | 23 (18.5) | 13 (10.4) |
| 6–12 years | 64 (58.7) | 69 (55.6) | 75 (60.0) |
| ≥13 years | 28 (25.7) | 32 (25.8) | 37 (29.6) |
| Sex, n (%) | |||
| Male | 39 (36.1) | 57 (46.0) | 35 (36.5) |
| Female | 69 (63.9) | 67 (54.0) | 61 (63.5) |
| Average weight (kg) (range) | 30.1 (6.0–96.3) | 29.5 (8.6–83.1) | 34.1 (10–90) |
| Average temperature (°C) (range) | 37.6 (35.7–40) | 38.2 (35.8–41.3) | 38.2 (35.8–40.7) |
| Fever (≥37.5 °C), n (%) | 89 (70.6) | 96 (77.4) | 99 (79.2) |
| Vomiting, n (%) | 7 (5.6) | 21 (16.9) | 50 (40.0) |
| Parasite density geometric mean (range) | 33.200 (1000–190.000) | 50.426 (1040–199.320) | 41.485 (1040–181.320) |
| Gametocytemia, n (%) | 0 | 5 (4.0) | 7 (5.6) |
| Average hemoglobin (g/dL), (range) | 12.2 (9.1–16) | 11.8 (8.1–17.7) | 12.2 (8.0–16) |
| Anemia (Hb < 11 g/dL), n (%) | 31 (24.6) | 41 (33.1) | 22 (17.6) |
| Artemether-lumefantrine | Bougouni 2017 | Bougouni 2018 | Bandiagara 2017–2018 | Total |
|---|---|---|---|---|
| Variable | N = 126 | N = 124 | N = 125 | N = 375 |
| ACPR a (uncorrected) n | 103 | 87 | 119 | 309 |
| ETF b n | 0 | 0 | 0 | 0 |
| LCF c n | 0 | 0 | 0 | 0 |
| LPF d n | 14 | 33 | 6 | 53 |
| New infections (with PCR) n | 14 | 33 | 6 | 53 |
| Recrudescences (with PCR) n | 0 | 0 | 0 | 0 |
| No treatment outcome (loss to follow-up or withdrawn) n | 9 | 4 | 0 | 13 |
| PP e day-28 efficacy (PCR-uncorrected) n/N (%, IC) | 103/117 (88.0, 82.1–93.9) | 87/120 (72.5, 64.5–80.5) | 119/125 (95.0, 91.2–98.8) | 309/362 (85.4, 81.8–98.4) |
| PP day-28 efficacy (PCR-corrected) n/N (%) | 103/103 (100) | 87/87 (100) | 119/119 (100) | 309/309 (100) |
| Artemether-Lumefantrine | Study Sites | Total N = 375 | ||
|---|---|---|---|---|
| Bougouni, 2017 N = 126 | Bougouni, 2018 N = 124 | Bandiagara, 2017–2018 N = 125 | ||
| Vomiting post-dosing 1, 2 or 3 n (%) | 1 (0.8) | 4 (3.2) | 9 (7.2) | 14 (3.7) |
| Diarrhea n (%) | 3 (2.4) | 1 (0.8) | 7 (5.6) | 11 (2.9) |
| Abdominal pain n (%) | 3 (2.4) | 8 (6.5) | 10 (8.0) | 21 (5.6) |
| Headaches n (%) | 0 (0) | 8 (6.5) | 4 (3.2) | 12 (3.2) |
| Sites | Anemia | McNemar’s Test p Value | |
|---|---|---|---|
| Day 0 | Day 28 | ||
| Bougouni, 2017 % (n/N) | 24.6 (31/126) | 16.0 (17/106) | <0.001 |
| Bougouni, 2018 % (n/N) | 33.1 (41/124) | 28.8 (32/111) | <0.001 |
| Bandiagara, 2017–2018 % (n/N) | 17.6 (22/125) | 9.7 (11/113) | <0.001 |
| Total % (n/N) | 25.1 (94/375) | 18.2 (60/330) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diarra, M.; Coulibaly, D.; Tapily, A.; Guindo, B.; Sanogo, K.; Koné, D.; Koné, Y.; Koné, K.; Bathily, A.; Yattara, O.; et al. Monitoring of the Sensitivity In Vivo of Plasmodium falciparum to Artemether-Lumefantrine in Mali. Trop. Med. Infect. Dis. 2021, 6, 13. https://doi.org/10.3390/tropicalmed6010013
Diarra M, Coulibaly D, Tapily A, Guindo B, Sanogo K, Koné D, Koné Y, Koné K, Bathily A, Yattara O, et al. Monitoring of the Sensitivity In Vivo of Plasmodium falciparum to Artemether-Lumefantrine in Mali. Tropical Medicine and Infectious Disease. 2021; 6(1):13. https://doi.org/10.3390/tropicalmed6010013
Chicago/Turabian StyleDiarra, Modibo, Drissa Coulibaly, Amadou Tapily, Boureima Guindo, Koualy Sanogo, Diakalia Koné, Youssouf Koné, Karim Koné, Aboudramane Bathily, Oumar Yattara, and et al. 2021. "Monitoring of the Sensitivity In Vivo of Plasmodium falciparum to Artemether-Lumefantrine in Mali" Tropical Medicine and Infectious Disease 6, no. 1: 13. https://doi.org/10.3390/tropicalmed6010013
APA StyleDiarra, M., Coulibaly, D., Tapily, A., Guindo, B., Sanogo, K., Koné, D., Koné, Y., Koné, K., Bathily, A., Yattara, O., Thera, M. A., Dicko, A., Djimdé, A. A., & Sagara, I. (2021). Monitoring of the Sensitivity In Vivo of Plasmodium falciparum to Artemether-Lumefantrine in Mali. Tropical Medicine and Infectious Disease, 6(1), 13. https://doi.org/10.3390/tropicalmed6010013








