Pharyngeal Carriage of Beta-Haemolytic Streptococcus Species and Seroprevalence of Anti-Streptococcal Antibodies in Children in Bouaké, Côte d’Ivoire
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Procedures
2.3. Microbiological Methods
2.4. Statistical Analysis
3. Results
3.1. Epidemiological Characteristics
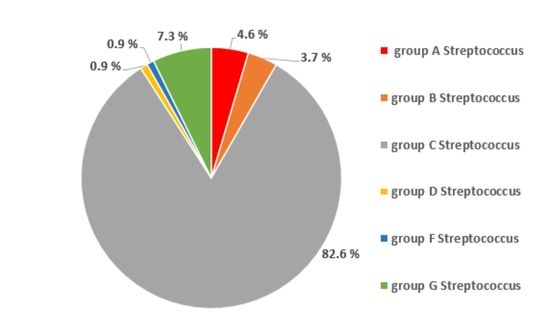
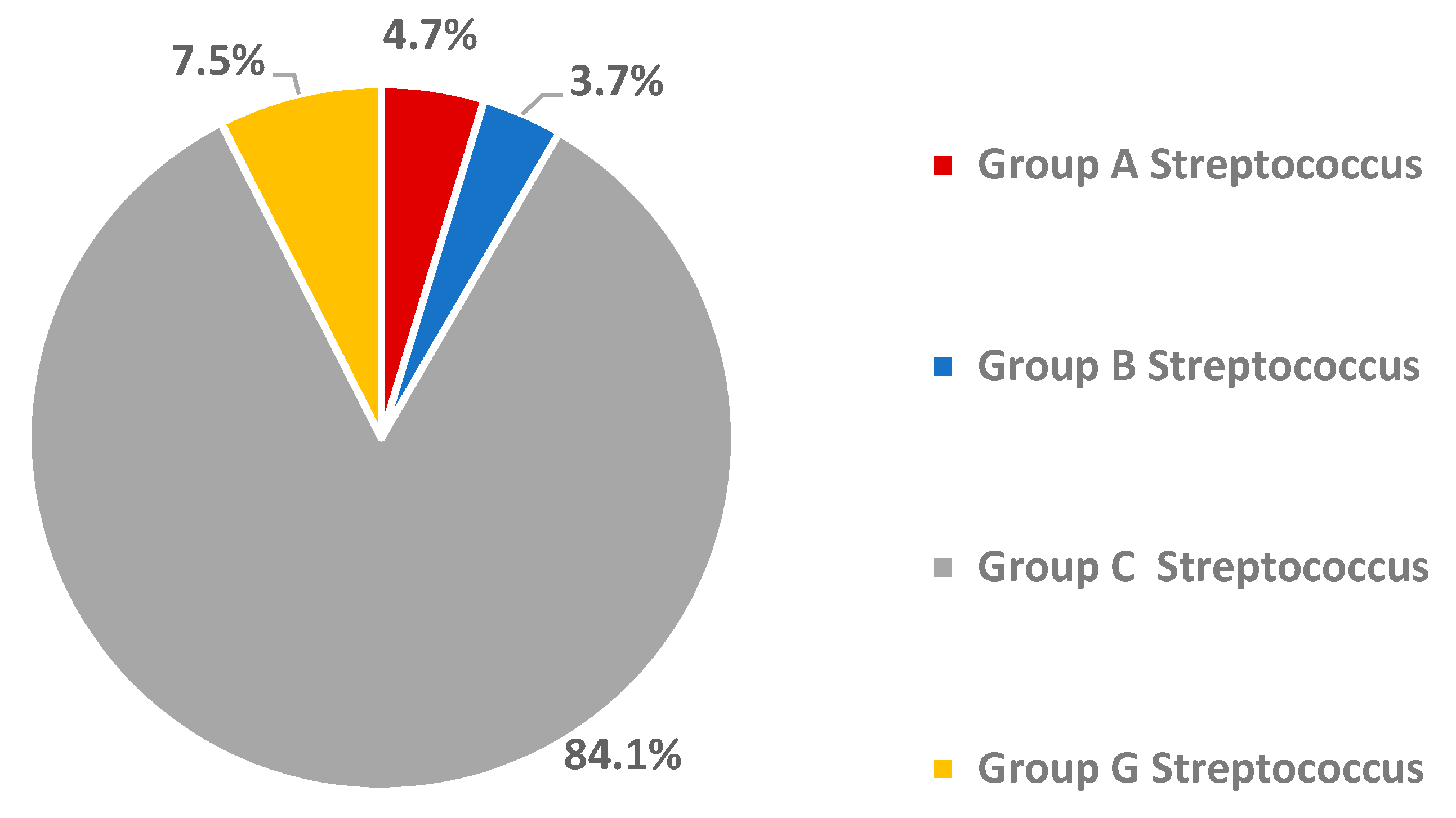
3.2. Oropharyngeal Carriage Rate of Beta-Haemolytic Streptococci
3.3. Seroprevalence of Anti-Streptococcal Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ajumobi, O.; Kwaga, J.; Ntadom, G.; Poggensee, G.; Nguku, P.; Sabitu, K.; Oyibo, W.; Maire, M.; Elizeus, R.; Nsubuga, P.; et al. Performance of an HRP-2 Rapid Diagnostic Test in Nigerian Children Less Than 5 Years of Age. Am. J. Trop. Med. Hyg. 2015, 92, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Altindis, M.; Aktepe, O.C.; Kocagoz, T. Comparison of Dio-Bacit, Bacitracin-Trimethoprim/ Sulphamethoxazole and Latex Agglutination in the Diagnosis of Group A Beta-Hemolytic Streptococci. Yonsei Med. J. 2004, 45, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Charvériat, M.; Chomarat, M.; Watson, M.; Garin, B. Étude du portage rhinopharyngé de Streptococcus pneumoniae chez les enfants sains âgés de 2 à 24 mois en Nouvelle-Calédonie. Med. Mal. Infect. 2005, 35, 500–520. (In French) [Google Scholar] [CrossRef] [PubMed]
- El-Ghany, S.M.A.; Abdelmaksoud, A.; Saber, S.M.; El Hamid, D.H.A. Group A beta-hemolytic streptococcal pharyngitis and carriage rate among Egyptian children: A case-control study. Ann. Saudi Med. 2015, 35, 377–382. [Google Scholar] [CrossRef]
- Raimond, J.; Cohen, R.; Moulin, F.; Gendrel, D.; Berche, P. Factors influencing Streptococcus pneumoniae carriage. Med. Mal. Infect. 2002, 32, 13–20. [Google Scholar] [CrossRef]
- Bogdan, M.; Muller, D.; Pin, I.; Dres, M. Exposition au tabagisme passif et santé respiratoire du nourrisson et de l’enfant. Rev. Mal. Respir. Actual. 2010, 2, 353–357. [Google Scholar] [CrossRef]
- Bélard, S.; Toepfner, N.; Arnold, B.; Alabi, A.S.; Berner, R. β-hemolytic streptococcal throat carriage and tonsillopharyngitis: A cross-sectional prevalence study in Gabon, Central Africa. Infection 2014, 43, 177–183. [Google Scholar] [CrossRef]
- Brandt, C.M.; Spellerberg, B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin. Infect. Dis. 2009, 49, 766–772. [Google Scholar] [CrossRef]
- Nayiga, I.; Okello, E.; Lwabi, P.; Ndeezi, G. Prevalence of group a Streptococcus pharyngeal carriage and clinical manifestations in school children aged 5–15 years in Wakiso District, Uganda. BMC Infect. Dis. 2017, 5, 241–248. [Google Scholar]
- Herrmann, M.; Abdullah, S.; Alabi, A.; Alonso, P.; Friedrich, A.W.; Fuhr, G.; Germann, A.; Kern, W.V.; Kremsner, P.G.; Mandomando, I.; et al. Staphylococcal disease in Africa: Another neglected ‘tropical’ disease. Future Microbiol. 2013, 8, 17–26. [Google Scholar] [CrossRef]
- Adebanjo, T.; Lessa, F.C.; Mucavele, H.; Moiane, B.; Chaúque, A.; Pimenta, F.; Massora, S.; Carvalho, M.D.G.; Whitney, C.G.; Sigauque, B. Pneumococcal carriage and serotype distribution among children with and without pneumonia in Mozambique, 2014–2016. PLoS ONE 2018, 13, e0199363. [Google Scholar] [CrossRef] [PubMed]
- Heath, C.J.; LaBeaud, A.D.; King, C.H.; Malhotra, I.; Mukoko, D.; Nayakwadi-Singer, M.; Mutuku, F. Nasopharyngeal Carriage of Streptococcus pneumoniae in Children in Coastal Kenya. Am. J. Trop. Med. Hyg. 2018, 98, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Foster-Nyarko, E.; Kwambana-Adams, B.; Ceesay, F.; Jawneh, K.; Darboe, S.; Mulwa, S.; Ceesay, B.; Secka, O.; Adetifa, I.M.; Antonio, M. Incidence of macrolide-lincosamide-streptogramin B resistance amongst beta-haemolytic streptococci in The Gambia. BMC Res. Notes 2017, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Abdissa, A.; Asrat, D.; Kronvall, G.; Shitu, B.; Achiko, D.; Zeidan, M.; Yamuah, L.; Aseffa, A. Throat carriage rate and antimicrobial susceptibility pattern of group A Streptococci (GAS) in healthy Ethiopian school children. Ethiop. Med. J. 2011, 49, 7–10. [Google Scholar]
- Asse, K.V.; Seka, A.; Poe, P.; Diabate, C.; Brou, S.; Plo, K.J. Bacterial meningitis in children at the University hospital center of Bouaké in Côte d’Ivoire. Arch. Pediatr. 2001, 8, 1266–1267. [Google Scholar] [CrossRef]
- De Wals, P.; Gilquin, C.; De Maeyer, S.; Bouckaert, A.; Noël, A.; Lechat, M.; Lafontaine, A. Longitudinal study of asymptomatic meningococcal carriage in two Belgian populations of schoolchildren. J. Infect. 1983, 6, 147–156. [Google Scholar] [CrossRef]
- Kacou-N’Douba, A.; Guessend Kouadio, N.; Kouassi-M’Bengué, A.; Dosso, M. Evolution de la résistance de Streptococcus pneumoniae aux antibiotiques à Abidjan: Enquêtes de portage nasopharyngé de 1997 à 2001. Med. Mal. Infect. 2004, 34, 83–85. (In French) [Google Scholar] [CrossRef]
- Thamlikitkul, V.; Danchaivijitr, S.; Kongpattanakul, S.; Ckokloikaew, S. Impact of an Educational Program on Antibiotic Use in a Tertiary Care Hospital in a Developing Country. J. Clin. Epidemiol. 1998, 51, 773–778. [Google Scholar] [CrossRef]
- Bramhachari, P.V.; Kaul, S.Y.; McMillan, D.J.; Shaila, M.S.; Karmarkar, M.G.; Sriprakash, K.S. Disease burden due to Streptococcus dysgalactiae subsp. equisimilis (group G and C streptococcus) is higher than that due to Streptococcus pyogenes among Mumbai school children. J. Med. Microbiol. 2010, 59, 220–223. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Farrimond, F.; De Matos, J.A.; Reis, J.N.; Ramos, R.T.T.; Andrade, A.N.; Dos Reis, M.G.; Riley, L.W. Inverse Association between Lancefield Group G Streptococcus Colonization and Sore Throat in Slum and Nonslum Settings in Brazil. J. Clin. Microbiol. 2011, 49, 409–412. [Google Scholar] [CrossRef][Green Version]
- Parks, T.; Smeesters, P.; Curtis, N.N.; Steer, A.C. ASO titer or not? When to use streptococcal serology: A guide for clinicians. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Mahendrappa, K.B. Rajendra Upper limit of normal antistreptolysin-O titer in healthy school children. Indian Pediatr. 2010, 47, 629. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Number | % | |
|---|---|---|---|
| Age (years) | 3–6 | 81 | 20.2 |
| 7–10 | 199 | 49.7 | |
| 11–14 | 120 | 30.1 | |
| Sex | Female | 205 | 51.2 |
| Male | 195 | 48.8 | |
| Schooling level | Kindergarten | 28 | 7.0 |
| Preparatory class | 114 | 28.5 | |
| Elementary or intermediate class | 258 | 44.5 | |
| Number of people living in the same household | 1–5 persons | 50 | 12.5 |
| 6–12 persons | 350 | 87.5 | |
| Exposure to tobacco | Presence of smokers at home | 189 | 47.3 |
| Absence of smokers at home | 211 | 52.7 | |
| Marital status of parents | Divorced | 48 | 12.0 |
| Widower | 13 | 3.3 | |
| Living together | 339 | 84.7 | |
| Profession | Public sector worker (e.g., teacher, manager) | 70 | 17.5 |
| Informal sector worker (e.g., traders, unskilled workers) | 157 | 39.2 | |
| Manual worker | 125 | 31.2 | |
| Unemployed | 34 | 8.5 | |
| Siblings | 1–2 | 78 | 19.5 |
| 3–5 | 256 | 64.0 | |
| ≥6 | 66 | 16.5 | |
| ASO 1 titre | Positive (≥200 IU/mL) | 69 | 17.2 |
| Negative (<200 IU/mL) | 331 | 82.8 |
| Bacteria | Number | % |
|---|---|---|
| Beta-haemolytic Streptococcus spp. | 107 | 26.8 |
| Non-haemolytic Streptococcus spp. | 39 | 9.8 |
| Coagulase-negative Staphylococcus spp. | 33 | 8.3 |
| Streptococcus pneumoniae | 10 | 2.5 |
| Staphylococcus aureus | 1 | 0.3 |
| Negative cultures | 210 | 52.5 |
| Total | 400 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monemo, P.; Demba, N.; Touré, F.S.; Traoré, A.; Avi, C.; N’Guessan, M.A.; Tadet, J.O.; Gobey, A.R.; Anoh, A.E.; Diarrassouba, A.; et al. Pharyngeal Carriage of Beta-Haemolytic Streptococcus Species and Seroprevalence of Anti-Streptococcal Antibodies in Children in Bouaké, Côte d’Ivoire. Trop. Med. Infect. Dis. 2020, 5, 177. https://doi.org/10.3390/tropicalmed5040177
Monemo P, Demba N, Touré FS, Traoré A, Avi C, N’Guessan MA, Tadet JO, Gobey AR, Anoh AE, Diarrassouba A, et al. Pharyngeal Carriage of Beta-Haemolytic Streptococcus Species and Seroprevalence of Anti-Streptococcal Antibodies in Children in Bouaké, Côte d’Ivoire. Tropical Medicine and Infectious Disease. 2020; 5(4):177. https://doi.org/10.3390/tropicalmed5040177
Chicago/Turabian StyleMonemo, Pacôme, Nadia Demba, Fidèle S. Touré, Adjartou Traoré, Christelle Avi, Micheline A. N’Guessan, Juste O. Tadet, Arthur R. Gobey, Augustin E. Anoh, Abdoulaye Diarrassouba, and et al. 2020. "Pharyngeal Carriage of Beta-Haemolytic Streptococcus Species and Seroprevalence of Anti-Streptococcal Antibodies in Children in Bouaké, Côte d’Ivoire" Tropical Medicine and Infectious Disease 5, no. 4: 177. https://doi.org/10.3390/tropicalmed5040177
APA StyleMonemo, P., Demba, N., Touré, F. S., Traoré, A., Avi, C., N’Guessan, M. A., Tadet, J. O., Gobey, A. R., Anoh, A. E., Diarrassouba, A., Tuo, M. N., Cissé, A., Saric, J., Utzinger, J., Tia, H., Kouassi-N’Djeundo, J., Becker, S. L., & Akoua-Koffi, C. (2020). Pharyngeal Carriage of Beta-Haemolytic Streptococcus Species and Seroprevalence of Anti-Streptococcal Antibodies in Children in Bouaké, Côte d’Ivoire. Tropical Medicine and Infectious Disease, 5(4), 177. https://doi.org/10.3390/tropicalmed5040177