Risk Factors for Elevated Serum Lipopolysaccharide in Acute Dengue and Association with Clinical Disease Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethics Approval
2.3. Quantification of Serum LPS Levels
2.4. Detection of Procalcitonin (PCT) and CRP
2.5. Serotyping of the DENV and Quantifying the Viral Loads
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Serum LPS Levels in Patients with Acute Dengue
3.3. Association of LPS Levels with Presence of Comorbid Illnesses
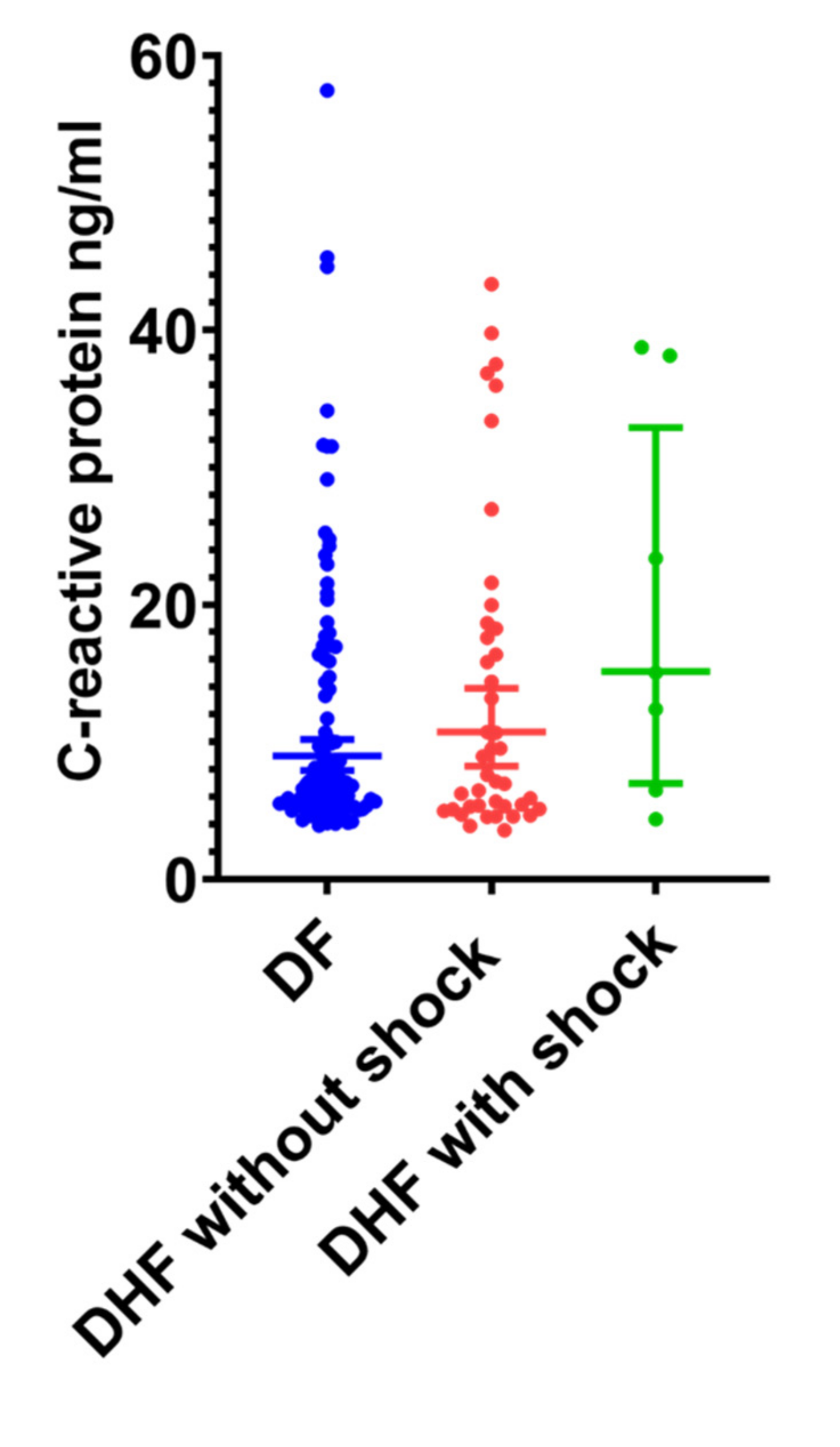
3.4. Association between Serum CRP and LPS in Patients with Acute Dengue
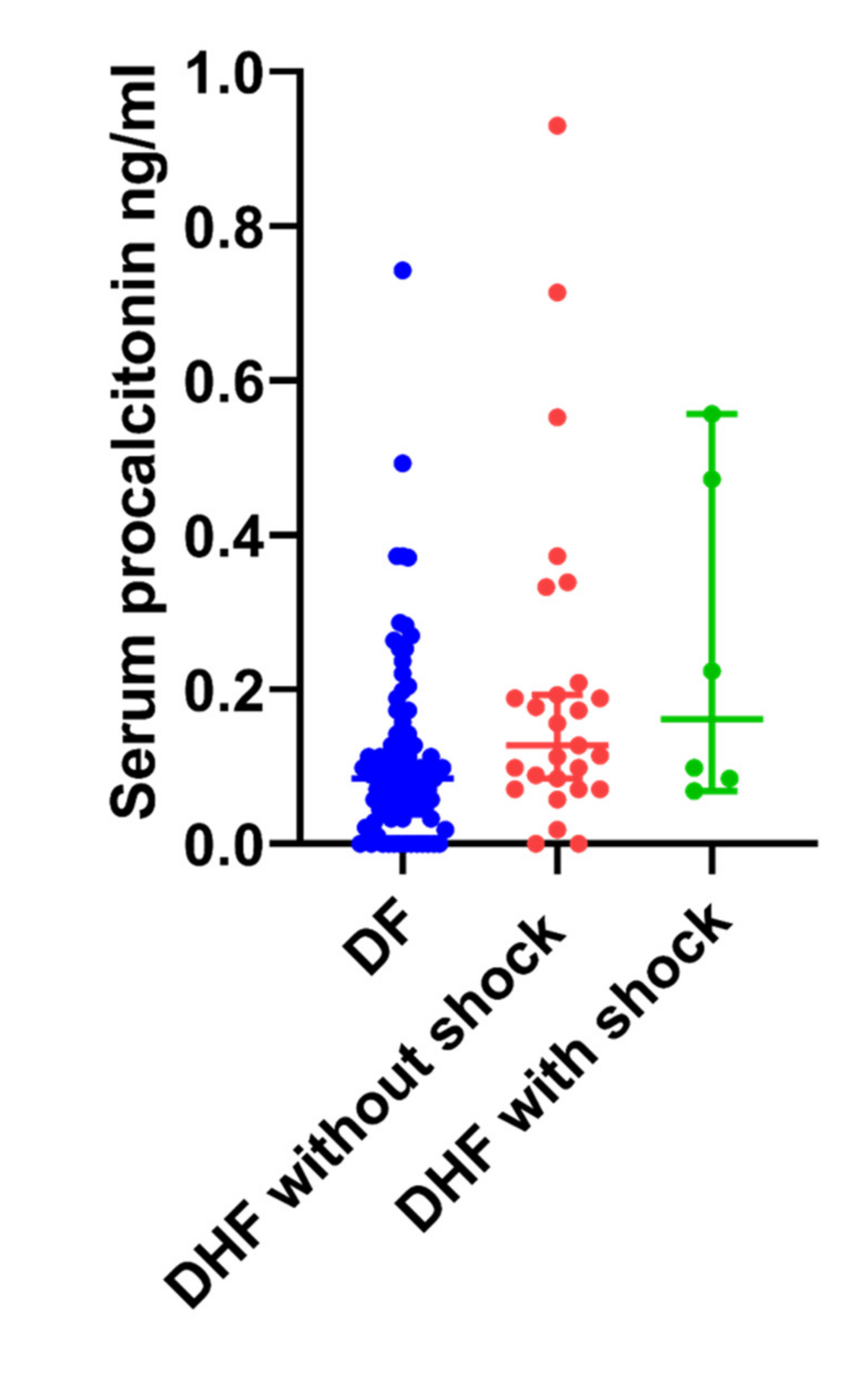
3.5. Serum Procalcitonin (PCT) Levels in Patients with Acute Dengue
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nat. Cell Biol. 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef]
- Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Stanaway, J.D. The global economic burden of dengue: A systematic analysis. Lancet Infect. Dis. 2016, 16, 935–941. [Google Scholar] [CrossRef]
- Fernando, S.; Wijewickrama, A.; Gomes, L.; Punchihewa, C.T.; Madusanka, S.D.P.; Dissanayake, H.; Jeewandara, C.; Peiris, H.; Ogg, G.S.; Malavige, G.N. Patterns and causes of liver involvement in acute dengue infection. BMC Infect. Dis. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, L.K.; Lye, D.C.; Leo, Y.S. Current management of severe dengue infection. Expert Rev. Anti Infect. Ther. 2016, 15, 67–78. [Google Scholar] [CrossRef]
- Van De Weg, C.A.M.; Pannuti, C.S.; De Araújo, E.S.A.; Ham, H.-J.V.D.; Andeweg, A.C.; Boas, L.S.V.; Felix, A.C.; Carvalho, K.I.; De Matos, A.M.; Levi, J.E.; et al. Microbial Translocation Is Associated with Extensive Immune Activation in Dengue Virus Infected Patients with Severe Disease. PLoS Negl. Trop. Dis. 2013, 7, e2236. [Google Scholar] [CrossRef]
- Mariano, F.; Bussolati, B.; Migliori, M.; Russo, S.; Triolo, G.; Camussi, G. Platelet-Activating Factor Synthesis by Neutrophils, Monocytes, and Endothelial Cells is Modulated by Nitric Oxide Production. Shock 2003, 19, 339–344. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Patel, S.; Kulchitsky, V.A.; Romanovsky, A.A. Platelet-Activating Factor: A Previously Unrecognized Mediator of Fever. J. Physiol. 2003, 553, 221–228. [Google Scholar] [CrossRef]
- Kamaladasa, A.; Gomes, L.; Jeewandara, C.; Shyamali, N.; Ogg, G.S.; Malavige, G.N. Lipopolysaccharide acts synergistically with the dengue virus to induce monocyte production of platelet activating factor and other inflammatory mediators. Antivir. Res. 2016, 133, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Gomes, L.; Wickramasinghe, N.; Gutowska-Owsiak, D.; Waithe, D.; Paranavitane, S.A.; Shyamali, N.L.A.; Ogg, G.S.; Malavige, G.N. Platelet Activating Factor Contributes to Vascular Leak in Acute Dengue Infection. PLoS Negl. Trop. Dis. 2015, 9, e0003459. [Google Scholar] [CrossRef]
- Wang, W.-H.; Lin, C.-Y.; Chang, K.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Lu, P.-L.; Chen, Y.-H.; Wang, S.-F. A clinical and epidemiological survey of the largest dengue outbreak in Southern Taiwan in 2015. Int. J. Infect. Dis. 2019, 88, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Hsieh, C.-J.; Lee, C.-T.; Liu, J.-W. Diabetic patients suffering dengue are at risk for development of dengue shock syndrome/severe dengue: Emphasizing the impacts of co-existing comorbidity(ies) and glycemic control on dengue severity. J. Microbiol. Immunol. Infect. 2020, 53, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Gomes, L.; Udari, S.; Paranavitane, S.; Shyamali, N.; Ogg, G.S.; Malavige, G.N. Secretory phospholipase A2 in the pathogenesis of acute dengue infection. Immunity Inflamm. Dis. 2016, 5, 7–15. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Gelb, M.H. Secretory phospholipase A2: A multifaceted family of proatherogenic enzymes. Curr. Cardiol. Rep. 2009, 11, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.L.; Coelho, J.; Couto, L.; Leite-Moreira, A.; Jr, R.R.-A. Metabolic endotoxemia: A molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 2013, 51, R51–R64. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Alizadeh, M.; Yagin, N.L.; Pasdar, Y.; Nachvak, S.M.; Abdollahzad, H.; Tabaei, A.S. New insights on atherosclerosis: A cross-talk between endocannabinoid systems with gut microbiota. J. Cardiovasc. Thorac. Res. 2018, 10, 129–137. [Google Scholar] [CrossRef]
- WHO. Comprehensive Guideline for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. 2011. Available online: https://apps.who.int/iris/handle/10665/204894 (accessed on 19 September 2020).
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lee, I.-K.; Liu, J.-W.; Huang, S.-Y.; Wang, L. Utility of C-Reactive Protein Levels for Early Prediction of Dengue Severity in Adults. BioMed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Vuong, N.L.; Le Duyen, H.T.; Lam, P.K.; Tam, D.T.H.; Chau, N.V.V.; Van Kinh, N.; Chanpheaktra, N.; Lum, L.C.S.; Pleités, E.; Jones, N.K.; et al. C-reactive protein as a potential biomarker for disease progression in dengue: A multi-country observational study. BMC Med. 2020, 18, 1–13. [Google Scholar] [CrossRef]
- Wacker, C.; Prkno, A.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta-analysis. Lancet Infect. Dis. 2013, 13, 426–435. [Google Scholar] [CrossRef]
- Thanachartwet, V.; Desakorn, V.; Sahassananda, D.; Jittmittraphap, A.; Oer-Areemitr, N.; Osothsomboon, S.; Surabotsophon, M.; Wattanathum, A. Serum Procalcitonin and Peripheral Venous Lactate for Predicting Dengue Shock and/or Organ Failure: A Prospective Observational Study. PLoS Negl. Trop. Dis. 2016, 10, e0004961. [Google Scholar] [CrossRef]
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Schuetz, P.; Chiappa, V.; Briel, M.; Greenwald, J.L. Procalcitonin Algorithms for Antibiotic Therapy Decisions. Arch. Intern. Med. 2011, 171, 1322–1331. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia Is Associated With an Increased Risk of Incident Diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Salim, A.; Lee, V.J.; Hibberd, M.L.; Chia, K.S.; Leo, Y.S.; Lye, D.C. Diabetes with Hypertension as Risk Factors for Adult Dengue Hemorrhagic Fever in a Predominantly Dengue Serotype 2 Epidemic: A Case Control Study. PLoS Negl. Trop. Dis. 2012, 6, e1641. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.-S.; Omar, S.F.S.; Teoh, B.-T.; Abd-Jamil, J.; Abubakar, S. Review of Dengue Hemorrhagic Fever Fatal Cases Seen Among Adults: A Retrospective Study. PLoS Negl. Trop. Dis. 2013, 7, e2194. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Wijewickrama, A.; Fernando, S.; Jeewandara, C.; Ginneliya, A.; Samarasekara, S.; Madushanka, P.; Punchihewa, C.; Paranavitane, S.; Idampitiya, D.; et al. A preliminary study on efficacy of rupatadine for the treatment of acute dengue infection. Sci. Rep. 2018, 8, 3857. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.M.; Jesudhasan, P.R.; Pfeiffer, J.K. Bacterial Lipopolysaccharide Binding Enhances Virion Stability and Promotes Environmental Fitness of an Enteric Virus. Cell Host Microbe 2014, 15, 36–46. [Google Scholar] [CrossRef]
- Bandoro, C.; Runstadler, J.A. Bacterial Lipopolysaccharide Destabilizes Influenza Viruses. mSphere 2017, 2, e00267-17. [Google Scholar] [CrossRef]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial Translocation in the Pathogenesis of HIV Infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef]
- Matera, G.; Quirino, A.; Giancotti, A.; Pulicari, M.C.; Rametti, L.; Rodríguez, M.L.; Liberto, M.C.; Focà, A. Procalcitonin neutralizes bacterial LPS and reduces LPS-induced cytokine release in human peripheral blood mononuclear cells. BMC Microbiol. 2012, 12, 68. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lu, J.; Cheng, Y.; Zhang, F.; Fan, X.; Weng, Y.; Zhu, J. NF-κB increases LPS-mediated procalcitonin production in human hepatocytes. Sci. Rep. 2018, 8, 8913. [Google Scholar] [CrossRef] [PubMed]

| DHF (n = 64) | DF (n = 129) | |
|---|---|---|
| Clinical features | ||
| Vomiting (%) | 15 (23) | 21 (16) |
| Abdominal pain (%) | 31 (48) | 21 (16) |
| Diarrhoea (%) | 13 (20) | 27 (21) |
| Hepatomegaly (%) | 18 (28) | 1 (0.7) |
| Bleeding manifestations (%) | 3 (5) | 0 |
| Pleural effusion (%) | 26 (41) | 0 |
| Ascites (%) | 56 (87) | 0 |
| Shock (%) | 7 (11) | 0 |
| WBC count | ||
| <4 × 109/L (%) | 41 (64) | 94 (73) |
| Lowest platelet count | ||
| <20,000 (%) | 36 (56) | 5 (4) |
| 20,000–50,000 (%) | 21 (33) | 26 (20) |
| 50,000–100,000 (%) | 7 (11) | 73 (56) |
| >100,000 (%) | 0 | 25 (19) |
| Clinical Characteristic | Patients with Detectable LPS n = 107 | Patients without LPS n = 86 | Odds ratio (95% Confidence Intervals | p Value |
|---|---|---|---|---|
| DHF (n = 64) | 44 (68.8%) | 20 (31.2%) | 2.3 (1.2 to 4.2) | 0.009 |
| Shock (n = 7) | 6 (85.7%) | 1 (14.3%) | 7.2 (1.1 to 83.3) | 0.05 |
| Severe thrombocytopenia (<20,000 cells/mm3) | 16 (80%) | 4 (20%) | 5.4 (1.8 to 15.3) | 0.002 |
| Metabolic disease (n = 29) | 22 (75.8%) | 7 (24.1%) | 2.9 (1.2 to 7.6) | 0.02 |
| Allergic diseases (n = 22) | 17 (77.3%) | 5 (22.8%) | 3.1 (1.1 to 7.8) | 0.04 |
| Any comorbidity (n = 47) | 34 (72.3%) | 13 (27.7) | 2.6 (1.2 to 5.1) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyamali, N.L.A.; Mahapatuna, S.D.; Gomes, L.; Wijewickrama, A.; Ogg, G.S.; Malavige, G.N. Risk Factors for Elevated Serum Lipopolysaccharide in Acute Dengue and Association with Clinical Disease Severity. Trop. Med. Infect. Dis. 2020, 5, 170. https://doi.org/10.3390/tropicalmed5040170
Shyamali NLA, Mahapatuna SD, Gomes L, Wijewickrama A, Ogg GS, Malavige GN. Risk Factors for Elevated Serum Lipopolysaccharide in Acute Dengue and Association with Clinical Disease Severity. Tropical Medicine and Infectious Disease. 2020; 5(4):170. https://doi.org/10.3390/tropicalmed5040170
Chicago/Turabian StyleShyamali, N. L. Ajantha, Sameera D. Mahapatuna, Laksiri Gomes, Ananda Wijewickrama, Graham S. Ogg, and Gathsaurie Neelika Malavige. 2020. "Risk Factors for Elevated Serum Lipopolysaccharide in Acute Dengue and Association with Clinical Disease Severity" Tropical Medicine and Infectious Disease 5, no. 4: 170. https://doi.org/10.3390/tropicalmed5040170
APA StyleShyamali, N. L. A., Mahapatuna, S. D., Gomes, L., Wijewickrama, A., Ogg, G. S., & Malavige, G. N. (2020). Risk Factors for Elevated Serum Lipopolysaccharide in Acute Dengue and Association with Clinical Disease Severity. Tropical Medicine and Infectious Disease, 5(4), 170. https://doi.org/10.3390/tropicalmed5040170





