Intestinal Schistosomiasis and Giardiasis Co-Infection in Sub-Saharan Africa: Can a One Health Approach Improve Control of Each Waterborne Parasite Simultaneously?
Abstract
1. Introduction
2. Intestinal Schistosomiasis and Giardiasis: Pathology and Epidemiology
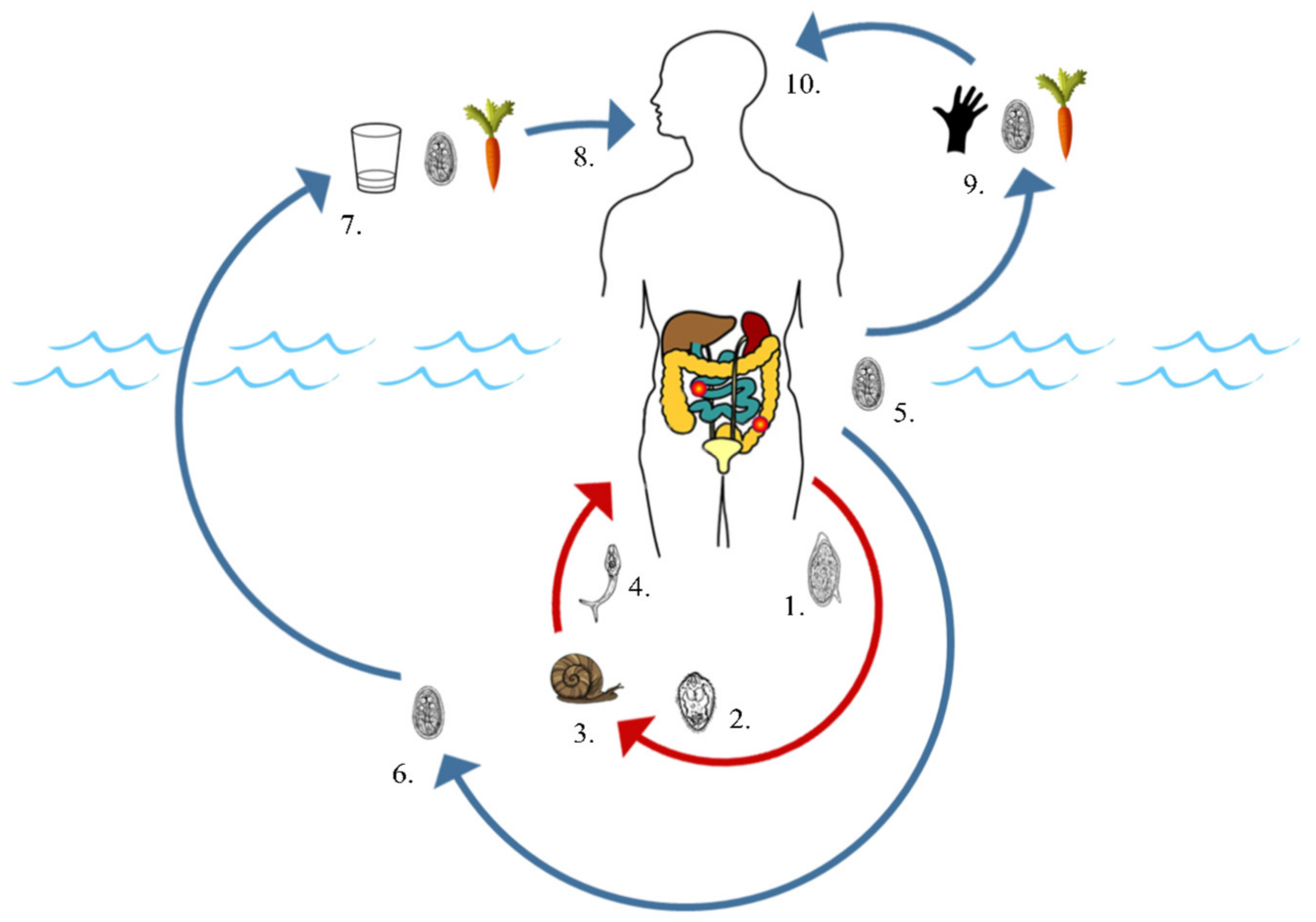
2.1. Common Modes of Environmental Contamination
2.2. Transmission via Non-Human Hosts
3. Intestinal Schistosomiasis and Giardiasis: Surveillance and Control
3.1. Diagnosis: Parasitological, Immunological and Molecular Methods
3.2. Exploring the One Health InterFace with Increased Host Surveillance
3.3. Detecting Parasitic Contamination through Water Sampling and by Environmental DNA (eDNA) Analysis
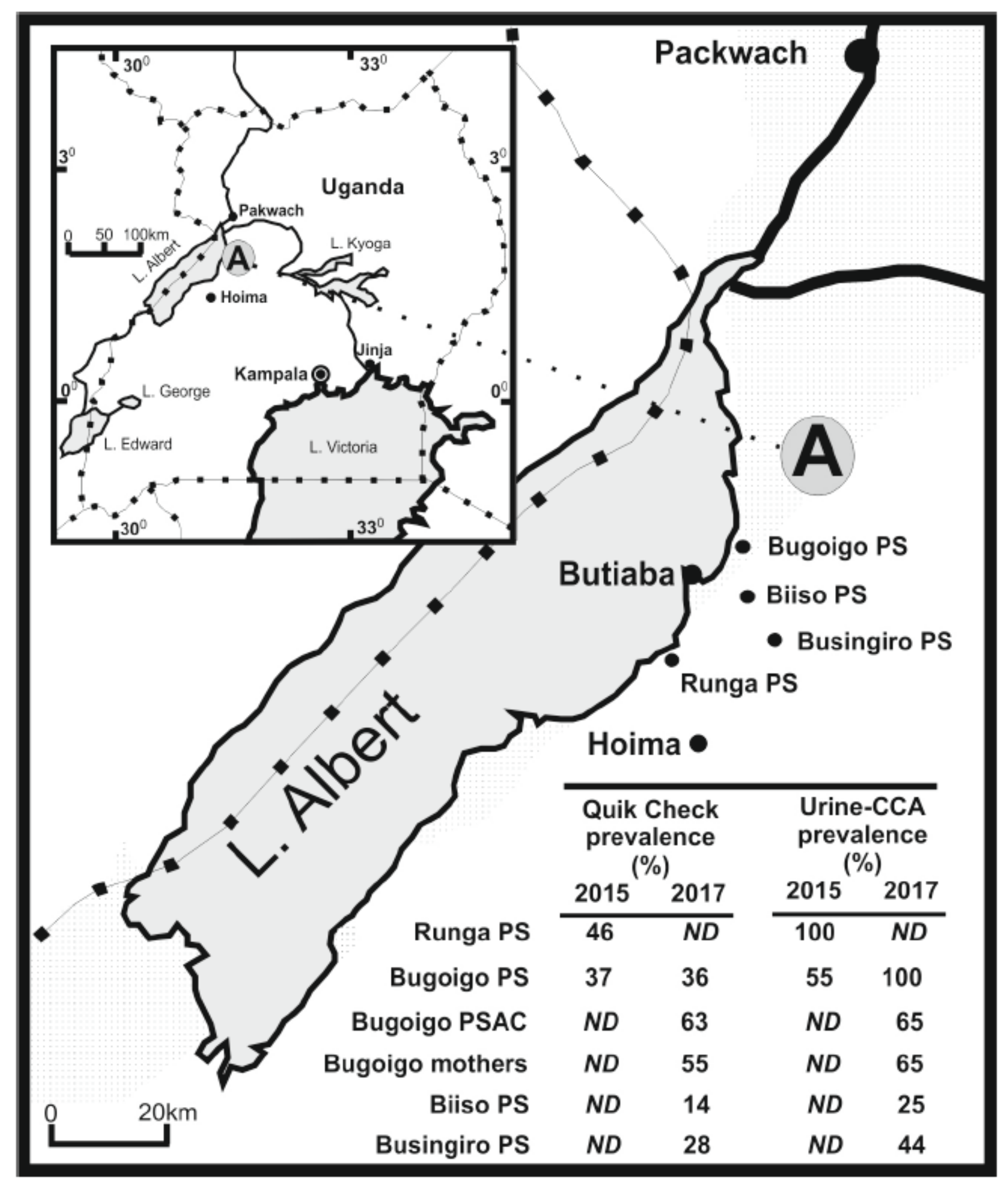
3.4. A Case Example of Co-Infection and Morbidity Surveillance in Uganda
3.5. Access to Treatment and Large-Scale Campaigns
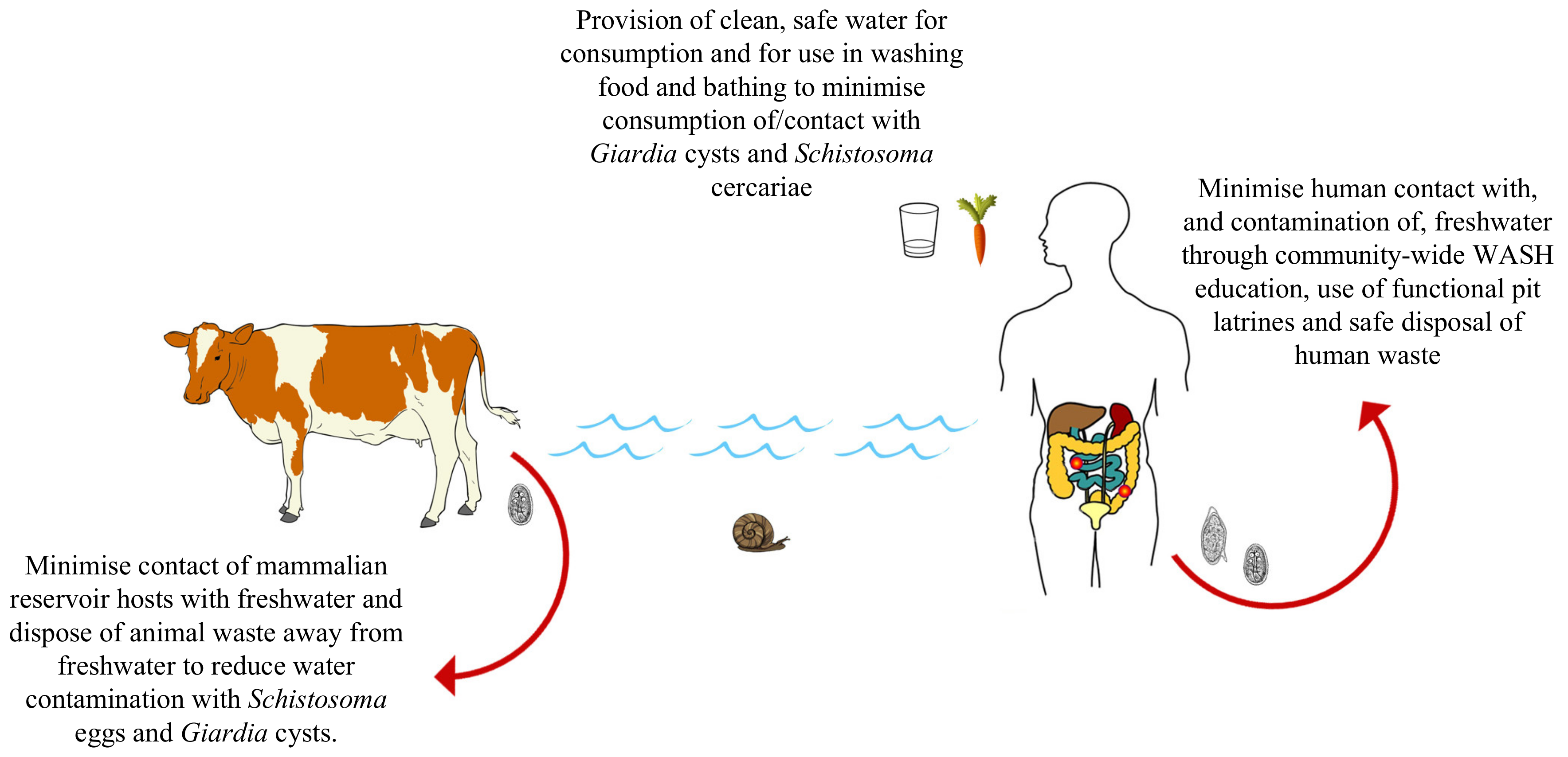
3.6. Water, Sanitation and Hygeine (WASH)
4. Intestinal Schistosomiasis and Giardiasis: Towards a One Health Approach
- Integrating screening of giardiasis endemicity and infection prevalence into existing schistosomiasis control programmes by using stool samples used for diagnosing infection with S. mansoni, and other intestinal parasites, to also record and report levels of Giardia infection in school-aged children. This can be conducted with POC-RDTs such as the Quik Chek immunoassay or using PCR/qPCR. In addition, the continued development, assessment and application of sensitive and straightforward POC-RDTs able to detect low-levels of infection in asymptomatic individuals capable of maintaining transmission of both parasites, such as the RPA, is encouraged.
- Further development and application of sensitive molecular assays to detect trace levels of species/assemblage-specific parasite DNA within freshwater snail intermediate hosts of human-infecting Schistosoma, and in faecal samples from non-human animal definitive hosts of both diseases. Further development and application of sensitive molecular assays to detect trace levels of species-/assemblage-specific parasite DNA from human-infecting Schistosoma cercariae and Giardia cysts in water samples easily collected from viable transmission sites is also encouraged.
- The upscaled provision of water, sanitation and hygiene (WASH) infrastructure and education initiatives to communities afflicted by both schistosomiasis and giardiasis to reduce environmental contamination events and to reduce contact with/consumption of contaminated water, simultaneously reducing transmission of both diseases.
- Monitoring Giardia disease prevalence and associated morbidities in tandem with schistosomiasis surveillance in school-aged children following any control programme intervention to better understand how giardiasis transmission and related pathologies can be reduced.
- An increased focus on understanding how the transmission of intestinal schistosomiasis and giardiasis, as well as immune responses and morbidities related to both diseases, interact and are potentially exacerbated by co-infection.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| eDNA | environmental DNA |
| ELISA | enzyme-linked immunosorbent assay |
| FGS | female genital schistosomiasis |
| LAMP | loop-mediated isothermal reaction |
| LMIC | lower-middle-income countries |
| MDA | mass drug administration |
| NTD | neglected tropical disease |
| PCR | polymerase chain reaction |
| POC | point-of-care |
| qPCR | quantitative polymerase chain reaction |
| RDT | rapid diagnostic test |
| RPA | recombinase polymerase amplification |
| Th1 | T-helper 1 |
| Th2 | T-helper 2 |
| WASH | water, sanitation and hygiene |
| WHA | world health assembly |
References
- Omarova, A.; Tussupova, K.; Berndtsson, R.; Kalishev, M.; Sharapatova, K. Protozoan parasites in drinking water: A system approach for improved water, sanitation and hygiene in developing countries. Int. J. Environ. Res. Public Health 2018, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.J.; Biritwum, N.K.; Woods, G.; Velleman, Y.; Fleming, F.; Stothard, J.R. Tailoring water, sanitation, and hygiene (WASH) Targets for toil-transmitted helminthiasis and schistosomiasis control. Trends Parasitol. 2018, 34, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Reynoldson, J.A.; Sciences, B. Giardia and giardiasis. Adv. Parasitol. 1993, 32, 71–160. [Google Scholar]
- Squire, S.A.; Ryan, U. Cryptosporidium and Giardia in Africa: Current and future challenges. Parasites Vectors 2017, 10, 1–32. [Google Scholar] [CrossRef]
- Nkrumah, B.; Nguah, S. Giardia lamblia: A major parasitic cause of childhood diarrhoea in patients attending a district hospital in Ghana. Parasites Vectors 2011, 4, 1–7. [Google Scholar] [CrossRef]
- Bartelt, L.A.; Sartor, R.B. Advances in understanding Giardia: Determinants and mechanisms of chronic sequelae. F1000Prime Rep. 2015, 7, 1–14. [Google Scholar] [CrossRef]
- Naess, H.; Nyland, M.; Hausken, T.; Follestad, I.; Nyland, H.I. Chronic fatigue syndrome after Giardia enteritis: Clinical characteristics, disability and long-term sickness absence. BMC Gastroenterol. 2012, 12. [Google Scholar] [CrossRef]
- Chifunda, K.; Kelly, P. Parasitic infections of the gut in children. Paediatr. Int. Child Health 2019, 39, 65–72. [Google Scholar] [CrossRef]
- Savioli, L.; Smith, H.; Thompson, A. Giardia and Cryptosporidium join the “Neglected Diseases Initiative”. Trends Parasitol. 2006, 22, 203–208. [Google Scholar] [CrossRef]
- Al-Shehri, H.; Stanton, M.C.; LaCourse, J.E.; Atuhaire, A.; Arinaitwe, M.; Wamboko, A.; Adriko, M.; Kabatereine, N.B.; Stothard, J.R. An extensive burden of giardiasis associated with intestinal schistosomiasis and anaemia in school children on the shoreline of Lake Albert, Uganda. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 597–603. [Google Scholar] [CrossRef] [PubMed]
- La Hoz, R.M.; Morris, M.I. Intestinal parasites including Cryptosporidium, Cyclospora, Giardia, and Microsporidia, Entamoeba histolytica, Strongyloides, Schistosomiasis, and Echinococcus: Guidelines from the American Society of Transplantation Infectious Diseases Community of Pract. Clin. Transplant. 2019, 33, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.N. Prioritizing research for “One health—One world”. Infect. Dis. Poverty 2012, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Jeggo, M. One Health and Zoonoses; MDPI Books: Basel, Switzeland, 2019; ISBN 9783039212958. [Google Scholar] [CrossRef]
- Samuels, A.M.; Matey, E.; Mwinzi, P.N.M.; Wiegand, R.E.; Muchiri, G.; Ireri, E.; Hyde, M.; Montgomery, S.P.; Karanja, D.M.S.; Secor, W.E. Schistosoma mansoni morbidity among school-aged children: A SCORE Project in Kenya. Am. J. Trop. Med. Hyg. 2012, 87, 874–882. [Google Scholar] [CrossRef] [PubMed]
- De Moira, A.P.; Fulford, A.J.C.; Kabatereine, N.B.; Ouma, J.H.; Booth, M.; Dunne, D.W. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: The influence of age, sex, ethnicity and IgE. PLoS Negl. Trop. Dis. 2010, 4, e820. [Google Scholar] [CrossRef]
- Costain, A.H.; MacDonald, A.S.; Smits, H.H. Schistosome egg migration: Mechanisms, pathogenesis and host immune responses. Front. Immunol. 2018, 9, 3042. [Google Scholar] [CrossRef]
- Olveda, David Bilharzia: Pathology, diagnosis, management and control. Trop. Med. Surg. 2013, 1, 1–19. [CrossRef]
- Heyworth, M.F. Giardia duodenalis genetic assemblages and hosts. Parasite 2016, 23. [Google Scholar] [CrossRef]
- Sprong, H.; Cacciò, S.M.; Van Der Giessen, J.W.B. Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl. Trop. Dis. 2009, 3, 1–12. [Google Scholar] [CrossRef]
- Fink, M.Y.; Singer, S.M. The intersection of immune responses, microbiota, and pathogenesis in giardiasis. Trends Parasitol. 2017, 33, 901–913. [Google Scholar] [CrossRef]
- Keselman, A.; Li, E.; Maloney, J.; Singer, S.M. The microbiota contributes to CD8+ T cell activation and nutrient malabsorption following intestinal infection with Giardia duodenalis. Infect. Immun. 2016, 84, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Allain, T.; Amat, C.B.; Motta, J.P.; Manko, A.; Buret, A.G. Interactions of Giardia sp. with the intestinal barrier: Epithelium, mucus, and microbiota. Tissue Barriers 2017, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mmbaga, B.T.; Houpt, E.R. Cryptosporidium and Giardia infections in children: A Review. Pediatr. Clin. N. Am. 2017, 64, 837–850. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.; Lloyd, D. Current trends in research into the waterborne parasite Giardia. Crit. Rev. Microbiol. 2002, 28, 123–147. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC), USA: Schistosomiasis, About, Life Cycle. Available online: https://www.cdc.gov/parasites/schistosomiasis/biology.html (accessed on 1 July 2020).
- Centers for Disease Control and Prevention (CDC), USA: Giardiasis, About, Life Cycle. Available online: https://www.cdc.gov/dpdx/giardiasis/index.html#:~:text=The spectrum varies from asymptomatic carriage to severe,include diarrhea%2C abdominal pain%2C bloating%2C nausea%2C and vomiting. (accessed on 1 July 2020).
- Sow, S.; Polman, K.; Vereecken, K.; Vercruysse, J.; Gryseels, B.; de Vlas, S.J. The role of hygienic bathing after defecation in the transmission of Schistosoma mansoni. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 542–547. [Google Scholar] [CrossRef]
- Vercruysse, J.; Shaw, D.J.; De Bont, J. Index of potential contamination for schistosomiasis. Trends Parasitol. 2001, 17, 256–261. [Google Scholar] [CrossRef]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The roles of water, sanitation and hygiene in reducing schistosomiasis: A review. Parasites Vectors 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Wright, C.A. Chapter 4: Fluke Life-Cycles. In Flukes and Snails; George Allen and Unwin LTD: London, UK, 1971. [Google Scholar]
- Galaktionov, K.V.; Dobrovolskij, A. The Biology and Evolution of Trematodes. An Essay on the Biology, Morphology, Life Cycles, Transmission, and Evolution of Digenetic Trematodes. Chapter 2: The Trematode Life Cycle as a System of Adaptations; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 2003. [Google Scholar]
- Fried, B.T.; Graczyk, T. Chapter 7: Host Recognition by Trematode Miracidia and Cercariae. In Advances in Trematode Biology; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Lockyer, A.E.; Jones, C.S.; Noble, L.R.; Rollinson, D. Trematodes and snails: An intimate association. Can. J. Zool. 2004, 82, 251–269. [Google Scholar] [CrossRef]
- Théron, A. Chronobiology of Trematode Cercarial Emergence: From Data Recovery to Epidemiological, Ecological and Evolutionary Implications; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 88. [Google Scholar]
- Frandsen, F.; Christensen, N. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984. [Google Scholar] [CrossRef]
- Einarsson, E.; Ma’ayeh, S.; Svärd, S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016, 34, 47–52. [Google Scholar] [CrossRef]
- Mohammed Mahdy, A.K.; Lim, Y.A.L.; Surin, J.; Wan, K.L.; Al-Mekhlafi, M.S.H. Risk factors for endemic giardiasis: Highlighting the possible association of contaminated water and food. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Ankarklev, J.; Jerlström-Hultqvist, J.; Ringqvist, E.; Troell, K.; Svärd, S.G. Behind the smile: Cell biology and disease mechanisms of Giardia species. Nat. Rev. Microbiol. 2010, 8, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Manderson, L. Schistosomiasis and the social patterning of infection. Acta Trop. 1992, 51, 175–194. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Guerrero Flórez, M.; Karanis, P. The impact of water crises and climate changes on the transmission of protozoan parasites in Africa. Pathog. Glob. Health 2018, 112, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Esrey, S.A.; Collett, J.; Miliotis, M.D.; Koornhof, H.J.; Makhale, P. The risk of infection from Giardia lamblia due to drinking water supply, use of water, and latrines among preschool children in rural Lesotho. Int. J. Epidemiol. 1989, 18, 248–253. [Google Scholar] [CrossRef]
- Campbell, S.J.; Nery, S.V.; D’Este, C.A.; Gray, D.J.; McCarthy, J.S.; Traub, R.J.; Andrews, R.M.; Llewellyn, S.; Vallely, A.J.; Williams, G.M.; et al. Water, sanitation and hygiene related risk factors for soil-transmitted helminth and Giardia duodenalis infections in rural communities in Timor-Leste. Int. J. Parasitol. 2016, 46, 771–779. [Google Scholar] [CrossRef]
- Robinson, M.W.; Dalton, J.P. Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2763–2776. [Google Scholar] [CrossRef]
- Chomel, B.B. Control and prevention of emerging parasitic zoonoses. Int. J. Parasitol. 2008, 38, 1211–1217. [Google Scholar] [CrossRef]
- MARTINS, A.V. Non-human vertebrate hosts of Schistosoma haematobium and Schistosoma mansoni. Bull. World Health Organ. 1958, 18, 931–944. [Google Scholar]
- Standley, C.J.; Dobson, A.; Dobson, A.P.; Stothard, J.R. Out of Animals and Back again: Schistosomiasis as a Zoonosis in Africa. Schistosomiasis; Rokni, M.B., Ed.; InTech Europe: Rijeka, Croatia, 2012; Available online: http://www.intechopen.com/books/schistosomiasis/out-of-animals-andback-again-schistosomiasis-as-a-zoonosis-in-africa (accessed on 13 January 2012)ISBN ISBN 978-953-307-852-6.
- Ryan, U.; Cacciò, S.M. Zoonotic potential of Giardia. Int. J. Parasitol. 2013, 43, 943–956. [Google Scholar] [CrossRef]
- Yaoyu, F.; Xiao, L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011, 24, 110–140. [Google Scholar] [CrossRef]
- Thompson, R.C.A. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet. Parasitol. 2004, 126, 15–35. [Google Scholar] [CrossRef]
- Sak, B.; Petrzelkova, K.J.; Kvetonova, D.; Mynarova, A.; Shutt, K.A.; Pomajbikova, K.; Kalousova, B.; Modry, D.; Benavides, J.; Todd, A.; et al. Long-term monitoring of Microsporidia, Cryptosporidium and Giardia Infections in western lowland gorillas (Gorilla gorilla gorilla) at different stages of habituation in Dzanga Sangha protected areas, Central African Republic. PLoS ONE 2013, 8, e71840. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, B.; Mwangi, I.N.; Kinuthia, J.M.; Maina, G.M.; Agola, L.E.; Mutuku, M.W.; Steinauer, M.L.; Agwanda, B.R.; Kigo, L.; Mungai, B.N.; et al. Schistosomes of small mammals from the Lake Victoria Basin, Kenya: New species, familiar species, and implications for schistosomiasis control. Parasitology 2010, 137, 1109–1118. [Google Scholar] [CrossRef]
- De Bont, J.; Vercruysse, J. The epidemiology and control of cattle schistosomiasis. Parasitol. Today 1997, 13, 255–262. [Google Scholar] [CrossRef]
- Olson, M.E.; O’Handley, R.M.; Ralston, B.J.; McAllister, T.A.; Thompson, R.C.A. Update on Cryptosporidium and Giardia infections in cattle. Trends Parasitol. 2004, 20, 185–191. [Google Scholar] [CrossRef]
- Savassi, B.A.E.S.; Mouahid, G.; Lasica, C.; Mahaman, S.D.K.; Garcia, A.; Courtin, D.; Allienne, J.F.; Ibikounlé, M.; Moné, H. Cattle as natural host for Schistosoma haematobium (Bilharz, 1852) Weinland, 1858 × Schistosoma bovis Sonsino, 1876 interactions, with new cercarial emergence and genetic patterns. Parasitol. Res. 2020, 1–17. [Google Scholar] [CrossRef]
- Sene-Wade, M.; Marchand, B.; Rollinson, D.; Webster, B.L. Urogenital schistosomiasis and hybridization between Schistosoma haematobium and Schistosoma bovis in adults living in Richard-Toll, Senegal. Parasitology 2018, 145, 1723–1726. [Google Scholar] [CrossRef]
- Standley, C.J.; Stothard, J.R. DNA Barcoding of schistosome cercariae reveals a novel sub-lineage within Schistosoma rodhaini from Ngamba Island chimpanzee sanctuary, Lake Victoria. J. Parasitol. 2012, 98, 1049–1051. [Google Scholar] [CrossRef]
- Catalano, S.; Sène, M.; Diouf, N.D.; Fall, C.B.; Borlase, A.; Léger, E.; Bâ, K.; Webster, J.P. Rodents as natural hosts of zoonotic schistosoma species and hybrids: An epidemiological and evolutionary perspective from West Africa. J. Infect. Dis. 2018, 218, 429–433. [Google Scholar] [CrossRef]
- Steinauer, M.L.; Hanelt, B.; Mwangi, I.N.; Maina, G.M.; Agola, L.E.; Kinuthia, J.M.; Mutuku, M.W.; Mungai, B.N.; Wilson, W.D.; Mkoji, G.M.; et al. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Mol. Ecol. 2008, 17, 5062–5074. [Google Scholar] [CrossRef] [PubMed]
- Huyse, T.; Webster, B.L.; Geldof, S.; Stothard, J.R.; Diaw, O.T.; Polman, K.; Rollinson, D. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009, 5, e1000571. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Research Priorities for Helminth Infections; WHO Technical Report Series No. 972; World Health Organisation: Geneva, Switzerland, 2012; Available online: https://apps.who.int/iris/bitstream/handle/10665/75922 (accessed on 30 June 2020).
- Katz, N.; Chaves, A.; Pellegrino, J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 1972, 14, 397–400. [Google Scholar] [PubMed]
- Lamberton, P.H.L.; Kabatereine, N.B.; Oguttu, D.W.; Fenwick, A.; Webster, J.P. Sensitivity and specificity of multiple Kato-Katz thick smears and a circulating cathodic antigen test for Schistosoma mansoni diagnosis pre- and post-repeated-praziquantel treatment. PLoS Negl. Trop. Dis. 2014, 8. [Google Scholar] [CrossRef]
- Adeyemo, F.E.; Singh, G.; Reddy, P.; Stenström, T.A. Methods for the detection of Cryptosporidium and Giardia: From microscopy to nucleic acid based tools in clinical and environmental regimes. Acta Trop. 2018, 184, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Zahan, N. A Comparison of microscopy and enzyme linked immunosorbent assay for diagnosis of Giardia lamblia in human faecal specimens. J. Clin. Diagn. Res. 2014, 8, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Barda, B.D.; Rinaldi, L.; Ianniello, D.; Zepherine, H.; Salvo, F.; Sadutshang, T.; Cringoli, G.; Clementi, M.; Albonico, M. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: Experience from the field. PLoS Negl. Trop. Dis. 2013, 7, e2344. [Google Scholar] [CrossRef]
- Barda, B.; Ianniello, D.; Zepheryne, H.; Rinaldi, L.; Cringoli, G.; Burioni, R.; Albonico, M. Parasitic infections on the shore of Lake Victoria (East Africa) detected by Mini-FLOTAC and standard techniques. Acta Trop. 2014, 137, 140–146. [Google Scholar] [CrossRef]
- Hooshyar, H.; Rostamkhani, P.; Mohsen Arbabi, M.D. Giardia lamblia infection: Review of current diagnostic strategies. Gastroenterol. Hepatol. 2019, 95, 347–349. [Google Scholar] [CrossRef]
- Glinz, D.; Silué, K.D.; Knopp, S.; Lohourignon, L.K.; Yao, K.P.; Steinmann, P.; Rinaldi, L.; Cringoli, G.; N’Goran, E.K.; Utzinger, J. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl. Trop. Dis. 2010, 4, e754. [Google Scholar] [CrossRef]
- Taye, S. Comparison of Kato-Katz and formol-ether concentration methods for the diagnosis of intestinal helminthic infections among school children of Wonji Shoa town, Eastern Ethiopia: A school based cross-sectional study. Am. J. Heal. Res. 2014, 2, 271. [Google Scholar] [CrossRef][Green Version]
- Utzinger, J.; Becker, S.L.; van Lieshout, L.; van Dam, G.J.; Knopp, S. New diagnostic tools in schistosomiasis. Clin. Microbiol. Infect. 2015, 21, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Archer, J.; Lacourse, E.J.; Webster, L.B.; Stothard, J.R. An update on non-invasive urine diagnostics for human-infecting parasitic helminths: What more could be done and how? Parasitology 2019, 147, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Binder, S.; Campbell, C.; King, C.H.; Tchuenté, L.A.T.; N’Goran, E.K.; Erko, B.; Karanja, D.M.S.; Kabatereine, N.B.; Van Lieshout, L.; et al. A five-country evaluation of a point-of-care circulating cathodic antigen urine assay for the prevalence of Schistosoma mansoni. Am. J. Trop. Med. Hyg. 2013, 88, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, K.G.; Gordon, C.A.; McManus, D.P. DNA diagnostics for schistosomiasis control. Trop. Med. Infect. Dis. 2018, 3, 81. [Google Scholar] [CrossRef]
- Minetti, C.; LaCourse, E.J.; Reimer, L.; Stothard, J.R. Focusing nucleic acid-based molecular diagnostics and xenomonitoring approaches for human helminthiases amenable to preventive chemotherapy. Parasitol. Open 2016, 2. [Google Scholar] [CrossRef]
- Mwangi, I.N.; Agola, E.L.; Mugambi, R.M.; Shiraho, E.A.; Mkoji, G.M. Development and evaluation of a loop-mediated isothermal amplification assay for diagnosis of Schistosoma mansoni infection in faecal samples. J. Parasitol. Res. 2018, 2018. [Google Scholar] [CrossRef]
- Poulton, K.; Webster, B. Development of a lateral flow recombinase polymerase assay for the diagnosis of Schistosoma mansoni infections. Anal. Biochem. 2018, 546, 65–71. [Google Scholar] [CrossRef]
- Koehler, A.V.; Jex, A.R.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. Giardia/giardiasis—A perspective on diagnostic and analytical tools. Biotechnol. Adv. 2014, 32, 280–289. [Google Scholar] [CrossRef]
- Alexander, L.C.; Niebel, M.; Jones, B. The rapid detection of Cryptosporidium and Giardia species in clinical stools using the Quik Chek immunoassay. Parasitol. Int. 2013, 62, 552–553. [Google Scholar] [CrossRef]
- Silva, R.K.N.R.; Pacheco, F.T.F.; Martins, A.S.; Menezes, J.F.; Costa-Ribeiro, H.; Ribeiro, T.C.M.; Mattos, Â.P.; Oliveira, R.R.; Soares, N.M.; Teixeira, M.C.A. Performance of microscopy and ELISA for diagnosing Giardia duodenalis infection in different pediatric groups. Parasitol. Int. 2016, 65, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.J.M.; Bhattacharyya, T.; Alshehri, H.R.; Poulton, K.; Allen, S.; Miles, M.A.; Arianitwe, M.; Tukahebwa, E.M.; Webster, B.; Stothard, J.R. Application of a recombinase polymerase amplification (RPA) assay and pilot field testing for Giardia duodenalis at Lake Albert, Uganda. Parasit. Vectors 2020, 13, 1–9. [Google Scholar] [CrossRef]
- Crannell, Z.A.; Cabada, M.M.; Castellanos-Gonzalez, A.; Irani, A.; White, A.C.; Richards-Kortum, R. Recombinase polymerase amplification-based assay to diagnose Giardia in stool samples. Am. J. Trop. Med. Hyg. 2015, 92, 583–587. [Google Scholar] [CrossRef]
- Plutzer, J.; Karanis, P. Rapid identification of Giardia duodenalis by loop-mediated isothermal amplification (LAMP) from faecal and environmental samples and comparative findings by PCR and real-time PCR methods. Parasitol. Res. 2009, 104, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- De Dood, C.J.; Hoekstra, P.T.; Mngara, J.; Kalluvya, S.E.; Van Dam, G.J.; Downs, J.A.; Corstjens, P.L.A.M. Refining diagnosis of Schistosoma haematobium infections: Antigen and antibody detection in urine. Front. Immunol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Weerakoon, K.G.A.D.; Gobert, G.N.; Cai, P.; McManus, D.P. Advances in the diagnosis of human schistosomiasis. Clin. Microbiol. Rev. 2015. [Google Scholar] [CrossRef]
- Amoah, A.S.; Hoekstra, P.T.; Casacuberta-Partal, M.; Coffeng, L.E.; Corstjens, P.L.A.M.; Greco, B.; van Lieshout, L.; Lim, M.D.; Markwalter, C.F.; Odiere, M.R.; et al. Sensitive diagnostic tools and targeted drug administration strategies are needed to eliminate schistosomiasis. Lancet Infect. Dis. 2020, 3099, 1–8. [Google Scholar] [CrossRef]
- Pennance, T.; Person, B.; Muhsin, M.A.; Khamis, A.N.; Muhsin, J.; Khamis, I.S.; Mohammed, K.A.; Kabole, F.; Rollinson, D.; Knopp, S. Urogenital schistosomiasis transmission on Unguja Island, Zanzibar: Characterisation of persistent hot-spots. Parasites Vectors 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Stothard, J.R.; Archer, J.; Gyapong, M.; Tchuem-Tchuenté, L.A.; Bustinduy, A.L.; Kabatereine, N.B.; Al-Shehri, H. A centenary of Robert T. Leiper’s lasting legacy on schistosomiasis and a COUNTDOWN on control of neglected tropical diseases. Parasitology 2016, 144, 1602–1612. [Google Scholar] [CrossRef]
- King, C.H.; Sturrock, R.F.; Kariuki, H.C.; Hamburger, J. Transmission control for schistosomiasis-why it matters now. Trends Parasitol. 2006, 22, 575–582. [Google Scholar] [CrossRef]
- Allan, F.; Ame, S.M.; Tian-Bi, Y.-N.T.; Hofkin, B.V.; Webster, B.L.; Diakité, N.R.; N’Goran, E.K.; Kabole, F.; Khamis, I.S.; Gouvras, A.N.; et al. Snail-related contributions from the Schistosomiasis Consortium for Operational Research and Evaluation program including xenomonitoring, focal mollusciciding, biological control, and modeling. Am. J. Trop. Med. Hyg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, I.; King, C.H.; Muchiri, E.M.; Hamburger, J. Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: Identification of infected snails from early prepatency. Am. J. Trop. Med. Hyg. 2010, 83, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Gandasegui, J.; Fernández-Soto, P.; Hernández-Goenaga, J.; López-Abán, J.; Vicente, B.; Muro, A. Biompha-LAMP: A new rapid loop-mediated isothermal amplification assay for detecting Schistosoma mansoni in Biomphalaria glabrata snail host. PLoS Negl. Trop. Dis. 2016, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bartley, P.M.; Roehe, B.K.; Thomson, S.; Shaw, H.J.; Peto, F.; Innes, E.A.; Katzer, F. Detection of potentially human infectious assemblages of Giardia duodenalis in fecal samples from beef and dairy cattle in Scotland. Parasitology 2019, 146, 1123–1130. [Google Scholar] [CrossRef]
- Sawitri, D.H.; Wardhana, A.H.; Martindah, E.; Ekawasti, F.; Dewi, D.A.; Utomo, B.N.; Shibahara, T.; Kusumoto, M.; Tokoro, M.; Sasai, K.; et al. Detections of gastrointestinal parasites, including Giardia intestinalis and Cryptosporidium spp., in cattle of Banten province, Indonesia. J. Parasit. Dis. 2020, 44, 174–179. [Google Scholar] [CrossRef]
- DiGiorgio, C.L.; Gonzalez, D.A.; Huitt, C.C. Cryptosporidium and Giardia recoveries in natural waters by using environmental protection agency method 1623. Appl. Environ. Microbiol. 2002, 68, 5952–5955. [Google Scholar] [CrossRef]
- EPA United States Environmental Protection Agency. Method 1623: Cryptosporidium and Giardia in Water by filtration/IMS/FA. Available online: https://www.epa.gov/sites/production/files/2015-07/documents/epa-1623.pdf (accessed on 24 July 2020).
- Bass, D.; Stentiford, G.D.; Littlewood, D.T.J.; Hartikainen, H. Diverse applications of environmental DNA methods in parasitology. Trends Parasitol. 2015, 31, 499–513. [Google Scholar] [CrossRef]
- Sengupta, M.E.; Hellström, M.; Kariuki, H.C.; Olsen, A.; Thomsen, P.F.; Mejer, H.; Willerslev, E.; Mwanje, M.T.; Madsen, H.; Kristensen, T.K.; et al. Environmental DNA for improved detection and environmental surveillance of schistosomiasis. Proc. Natl. Acad. Sci. USA 2019, 116, 8931–8940. [Google Scholar] [CrossRef]
- Baque, R.H.; Gilliam, A.O.; Robles, L.D.; Jakubowski, W.; Slifko, T.R. A real-time RT-PCR method to detect viable Giardia lamblia cysts in environmental waters. Water Res. 2011, 45, 3175–3184. [Google Scholar] [CrossRef]
- Alzaylaee, H.; Collins, R.A.; Rinaldi, G.; Shechonge, A.; Ngatunga, B.; Morgan, E.R.; Genner, M.J. Schistosoma species detection by environmental DNA assays in african freshwaters. PLoS Negl. Trop. Dis. 2020, 14, 1–19. [Google Scholar] [CrossRef]
- Lass, A.; Szostakowska, B.; Korzeniewski, K.; Karanis, P. Detection of Giardia intestinalis in water samples collected from natural water reservoirs and wells in northern and north-eastern Poland using LAMP, real-time PCR and nested PCR. J. Water Health 2017, 15, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Alzaylaee, H.; Collins, R.A.; Shechonge, A.; Ngatunga, B.P.; Morgan, E.R.; Genner, M.J. Environmental DNA-based xenomonitoring for determining Schistosoma presence in tropical freshwaters. Parasites Vectors 2020, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mulero, S.; Boissier, J.; Allienne, J.; Quilichini, Y.; Foata, J.; Pointier, J.; Rey, O. Environmental DNA for detecting Bulinus truncatus: A new environmental surveillance tool for schistosomiasis emergence risk assessment. Environ. DNA 2020, 2, 161–174. [Google Scholar] [CrossRef]
- Coulibaly, G.; Ouattara, M.; Dongo, K.; Hürlimann, E.; Bassa, F.K.; Koné, N.; Essé, C.; Yapi, R.B.; Bonfoh, B.; Utzinger, J.; et al. Epidemiology of intestinal parasite infections in three departments of south-central Côte d’Ivoire before the implementation of a cluster-randomised trial. Parasite Epidemiol. Control 2018, 3, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Fofana, H.K.M.; Schwarzkopf, M.; Doumbia, M.N.; Saye, R.; Nimmesgern, A.; Landouré, A.; Traoré, M.S.; Mertens, P.; Utzinger, J.; Sacko, M.; et al. Prevalence of Giardia intestinalis infection in schistosomiasis-endemic areas in south-central Mali. Trop. Med. Infect. Dis. 2019, 4, 86. [Google Scholar] [CrossRef]
- Stothard, J.R.; Sousa-Figueiredo, J.C.; Betson, M.; Bustinduy, A.; Reinhard-Rupp, J. Schistosomiasis in African infants and preschool children: Let them now be treated! Trends Parasitol. 2013, 29, 197–205. [Google Scholar] [CrossRef]
- Al-Shehri, H.; James LaCourse, E.; Klimach, O.; Kabatereine, N.B.; Stothard, J.R. Molecular characterisation and taxon assemblage typing of giardiasis in primary school children living close to the shoreline of Lake Albert, Uganda. Parasite Epidemiol. Control 2019, 4, e00074. [Google Scholar] [CrossRef]
- Levitz, S.; Standley, C.J.; Adriko, M.; Kabatereine, N.B.; Stothard, J.R. Environmental epidemiology of intestinal schistosomiasis and genetic diversity of Schistosoma mansoni infections in snails at Bugoigo village, Lake Albert. Acta Trop. 2013, 128, 284–291. [Google Scholar] [CrossRef]
- Tchuem Tchuenté, L.A.; Momo, S.C.; Stothard, J.R.; Rollinson, D. Efficacy of praziquantel and reinfection patterns in single and mixed infection foci for intestinal and urogenital schistosomiasis in Cameroon. Acta Trop. 2013, 128, 275–283. [Google Scholar] [CrossRef]
- Stothard, J.R.; Sousa-Figueiredo, J.C.; Navaratnam, A.M.D. Advocacy, policies and practicalities of preventive chemotherapy campaigns for African children with schistosomiasis. Expert Rev. Anti. Infect. Ther. 2013, 11, 733–752. [Google Scholar] [CrossRef]
- Park, S.K.; Marchant, J.S. The journey to discovering a flatworm target of praziquantel: A long TRP. Trends Parasitol. 2020, 36, 182–194. [Google Scholar] [CrossRef]
- Lo, N.C.; Addiss, D.G.; Hotez, P.J.; King, C.H.; Stothard, J.R.; Evans, D.S.; Colley, D.G.; Lin, W.; Coulibaly, J.T.; Bustinduy, A.L.; et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: The time is now. Lancet Infect. Dis. 2017, 17, e64–e69. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Liang, Y.S. Susceptibility or resistance of praziquantel in human schistosomiasis: A review. Parasitol. Res. 2012, 111, 1871–1877. [Google Scholar] [CrossRef]
- Bustinduy, A.L.; Friedman, J.F.; Kjetland, E.F.; Ezeamama, A.E.; Kabatereine, N.B.; Stothard, J.R.; King, C.H. Expanding praziquantel (PZQ) access beyond mass drug administration programs: Paving a way forward for a pediatric PZQ formulation for schistosomiasis. PLoS Negl. Trop. Dis. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Bergquist, R.; Utzinger, J.; Keiser, J. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect. Dis. Poverty 2017, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Doenhoff, M.J.; Cioli, D.; Utzinger, J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr. Opin. Infect. Dis. 2008, 21, 659–667. [Google Scholar] [CrossRef]
- Hill, D.R.; Timothy, B.G. Treatment of giardiasis. Curr. Treat. Options Gastroenterol. 2005, 8, 13–17. [Google Scholar] [CrossRef]
- Ce, G.; Reveiz, L.; Lg, U.; Cp, C. Drugs for treating giardiasis. Cochrane Database Syst. Rev. 2012, 12. [Google Scholar] [CrossRef]
- Carter, E.R.; Nabarro, L.E.; Hedley, L.; Chiodini, P.L. Nitroimidazole-refractory giardiasis: A growing problem requiring rational solutions. Clin. Microbiol. Infect. 2018, 24, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Behnke, J.M.; Albonico, M.; Ame, S.M.; Angebault, C.; Bethony, J.M.; Engels, D.; Guillard, B.; Hoa, N.T.V.; Kang, G.; et al. Assessment of the anthelmintic efficacy of albendazole in school children in seven countries where soil-transmitted helminths are endemic. PLoS Negl. Trop. Dis. 2011, 5, e948. [Google Scholar] [CrossRef] [PubMed]
- Hoerauf, A.; Pfarr, K.; Mand, S.; Debrah, A.Y.; Specht, S. Filariasis in Africa-treatment challenges and prospects. Clin. Microbiol. Infect. 2011, 17, 977–985. [Google Scholar] [CrossRef]
- Solaymani-Mohammadi, S.; Genkinger, J.M.; Loffredo, C.A.; Singer, S.M. A meta-analysis of the effectiveness of albendazole compared with metronidazole as treatments for infections with Giardia duodenalis. PLoS Negl. Trop. Dis. 2010, 4, e682. [Google Scholar] [CrossRef]
- Pickering, A.J.; Njenga, S.M.; Steinbaum, L.; Swarthout, J.; Lin, A.; Arnold, B.F.; Stewart, C.P.; Dentz, H.N.; Mureithi, M.; Chieng, B.; et al. Effects of single and integrated water, sanitation, handwashing, and nutrition interventions on child soil-transmitted helminth and Giardia infections: A cluster-randomized controlled trial in rural Kenya. PLoS Med. 2019, 16, 1–21. [Google Scholar] [CrossRef]
- Aw, J.Y.H.; Clarke, N.E.; McCarthy, J.S.; Traub, R.J.; Amaral, S.; Huque, M.H.; Andrews, R.M.; Gray, D.J.; Clements, A.C.A.; Vaz Nery, S. Giardia duodenalis infection in the context of a community-based deworming and water, sanitation and hygiene trial in Timor-Leste. Parasites Vectors 2019, 12, 4–13. [Google Scholar] [CrossRef]
- Roche, R.; Bain, R.; Cumming, O. A long way to go-Estimates of combined water, sanitation and hygiene coverage for 25 sub-Saharan African countries. PLoS ONE 2017, 12, 1–24. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Water, Sanitation & Hygiene for Accelerating and Sustaining Progress on Neglected Tropical Diseases. Available online: https://www.who.int/water_sanitation_health/publications/wash-and-ntd-strategy/en/ (accessed on 2 July 2020).
- World Health Organisation. Integrating Neglected Tropical Diseases into Global Health and Development; World Health Organisation: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/255011/9789241565448-eng.pdf (accessed on 17 June 2020).
- Campbell, S.J.; Savage, G.B.; Gray, D.J.; Atkinson, J.A.M.; Soares Magalhães, R.J.; Nery, S.V.; McCarthy, J.S.; Velleman, Y.; Wicken, J.H.; Traub, R.J. Water, sanitation, and hygiene (WASH): A critical component for sustainable soil-transmitted helminth and schistosomiasis control. PLoS Negl. Trop. Dis. 2014, 8, 1–5. [Google Scholar] [CrossRef]
- Spear, R.C. Commentary by Spear, R. on “Integration of water, sanitation, and hygiene for the prevention and control of Neglected Tropical Diseases: A rationale for inter-sectoral collaboration:” Can the control of NTDs profit from a good WASH? PLoS Negl. Trop. Dis. 2013, 7, e2473. [Google Scholar] [CrossRef]
- Rollinson, D.; Knopp, S.; Levitz, S.; Stothard, J.R.; Tchuem Tchuenté, L.A.; Garba, A.; Mohammed, K.A.; Schur, N.; Person, B.; Colley, D.G.; et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013, 128, 423–440. [Google Scholar] [CrossRef]
- Reed, S.L.; Mckerrow, J.H. Why funding for Neglected Tropical Diseases should be a global priority. Clin. Infect. Dis. 2018, 18, 323–326. [Google Scholar] [CrossRef]
- Van den Berg, H.; Kelly-Hope, L.A.; Lindsay, S.W. Malaria and lymphatic filariasis: The case for integrated vector management. Lancet Infect. Dis. 2013, 13, 89–94. [Google Scholar] [CrossRef]
- Kelly-Hope, L.A.; Molyneux, D.H.; Bockarie, M.J. Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in Africa; Capturing a window of opportunity? Parasites Vectors 2013, 6, 1–12. [Google Scholar] [CrossRef]
- Knipes, A.K.; Lemoine, J.F.; Monestime, F.; Fayette, C.R.; Direny, A.N.; Desir, L.; Beau de Rochars, V.E.; Streit, T.G.; Renneker, K.; Chu, B.K.; et al. Partnering for impact: Integrated transmission assessment surveys for lymphatic filariasis, soil transmitted helminths and malaria in Haiti. PLoS Negl. Trop. Dis. 2017, 11, e0005387. [Google Scholar] [CrossRef]
- Bronzan, R.N.; Dorkenoo, A.M.; Agbo, Y.M.; Halatoko, W.; Layibo, Y.; Adjeloh, P.; Teko, M.; Sossou, E.; Yakpa, K.; Tchalim, M.; et al. Impact of community-based integrated mass drug administration on schistosomiasis and soil-transmitted helminth prevalence in Togo. PLoS Negl. Trop. Dis. 2018, 12, e0006551. [Google Scholar] [CrossRef]
| Species | Life-Stage | Survival Post-Defecation in the Stool or in Freshwater | Reference(s) |
|---|---|---|---|
| S. mansoni | Eggs | ~8 days in stool prior to reaching freshwater | [30] |
| Miracidia | <6 h in freshwater | [31,32,33] | |
| Cercariae | ~1–3 days in freshwater | [32,33,35,36] | |
| G. duodenalis (Assemblages A and B) | Cysts | Up to eight weeks in stool or in freshwater | [4,37,39] |
| Species | Humans | Non-Human Primates | Ruminants | Rodents | Other Mammals | Fish | References |
|---|---|---|---|---|---|---|---|
| S. mansoni | + | + | - | + | - | - | [46,47] |
| G. duodenalis (assemblage A) | + | + | + | + | + | + | [20,48,49,50,51] |
| G. duodenalis (assemblage B) | + | + | + | + | + | + | [20,48,49,50] |
| G. duodenalis (assemblage C) | - * | - | + | - | + | - | [19,20] |
| G. duodenalis (assemblage D) | - * | - | + | - | + | - | [19,20] |
| G. duodenalis (assemblage E) | - * | - | + | - | + | - | [19,20] |
| G. duodenalis (assemblage F) | - * | - | - | - | + | - | [19,20] |
| G. duodenalis (assemblage G) | - | - | - | + | - | - | [19,20] |
| G. duodenalis (assemblage H) | - | - | - | - | + | - | [19,20] |
| Species | Direct Diagnosis | Antigen Detection | Molecular Diagnosis |
|---|---|---|---|
| S. mansoni | Identification of ova in concentrated faecal smear via Kato-Katz technique [62,71] | Detection of circulating cathodic antigen (CCA) or circulating anodic antigen (CAA) in urine samples using ELISA or lateral-flow test strips [72,73] | Detection and amplification of species-specific DNA in faecal samples using PCR/qPCR [74], (LAMP and RPA assays have also been developed [71,75,76,77]). |
| G. duodenalis | Identification of cysts in concentrated faecal smear via formalin/ether concentration techniques, flotation techniques or immunofluorescent antibody microscopy [68] | Detection of species-specific antigens in faecal samples using ELISA or lateral-flow test strips [11,78,79,80] | Detection and amplification of species-specific DNA in faecal samples using PCR/qPCR [78], (LAMP and RPA assays have also been developed [81,82,83]). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Archer, J.; O’Halloran, L.; Al-Shehri, H.; Summers, S.; Bhattacharyya, T.; Kabaterine, N.B.; Atuhaire, A.; Adriko, M.; Arianaitwe, M.; Stewart, M.; et al. Intestinal Schistosomiasis and Giardiasis Co-Infection in Sub-Saharan Africa: Can a One Health Approach Improve Control of Each Waterborne Parasite Simultaneously? Trop. Med. Infect. Dis. 2020, 5, 137. https://doi.org/10.3390/tropicalmed5030137
Archer J, O’Halloran L, Al-Shehri H, Summers S, Bhattacharyya T, Kabaterine NB, Atuhaire A, Adriko M, Arianaitwe M, Stewart M, et al. Intestinal Schistosomiasis and Giardiasis Co-Infection in Sub-Saharan Africa: Can a One Health Approach Improve Control of Each Waterborne Parasite Simultaneously? Tropical Medicine and Infectious Disease. 2020; 5(3):137. https://doi.org/10.3390/tropicalmed5030137
Chicago/Turabian StyleArcher, John, Lisa O’Halloran, Hajri Al-Shehri, Shannan Summers, Tapan Bhattacharyya, Narcis B. Kabaterine, Aaron Atuhaire, Moses Adriko, Moses Arianaitwe, Martyn Stewart, and et al. 2020. "Intestinal Schistosomiasis and Giardiasis Co-Infection in Sub-Saharan Africa: Can a One Health Approach Improve Control of Each Waterborne Parasite Simultaneously?" Tropical Medicine and Infectious Disease 5, no. 3: 137. https://doi.org/10.3390/tropicalmed5030137
APA StyleArcher, J., O’Halloran, L., Al-Shehri, H., Summers, S., Bhattacharyya, T., Kabaterine, N. B., Atuhaire, A., Adriko, M., Arianaitwe, M., Stewart, M., LaCourse, E. J., Webster, B. L., Bustinduy, A. L., & Stothard, J. R. (2020). Intestinal Schistosomiasis and Giardiasis Co-Infection in Sub-Saharan Africa: Can a One Health Approach Improve Control of Each Waterborne Parasite Simultaneously? Tropical Medicine and Infectious Disease, 5(3), 137. https://doi.org/10.3390/tropicalmed5030137






