A Case of Japanese Encephalitis with a Fatal Outcome in an Australian Who Traveled from Bali in 2019
Abstract
1. Patient Case History and Clinical Presentation
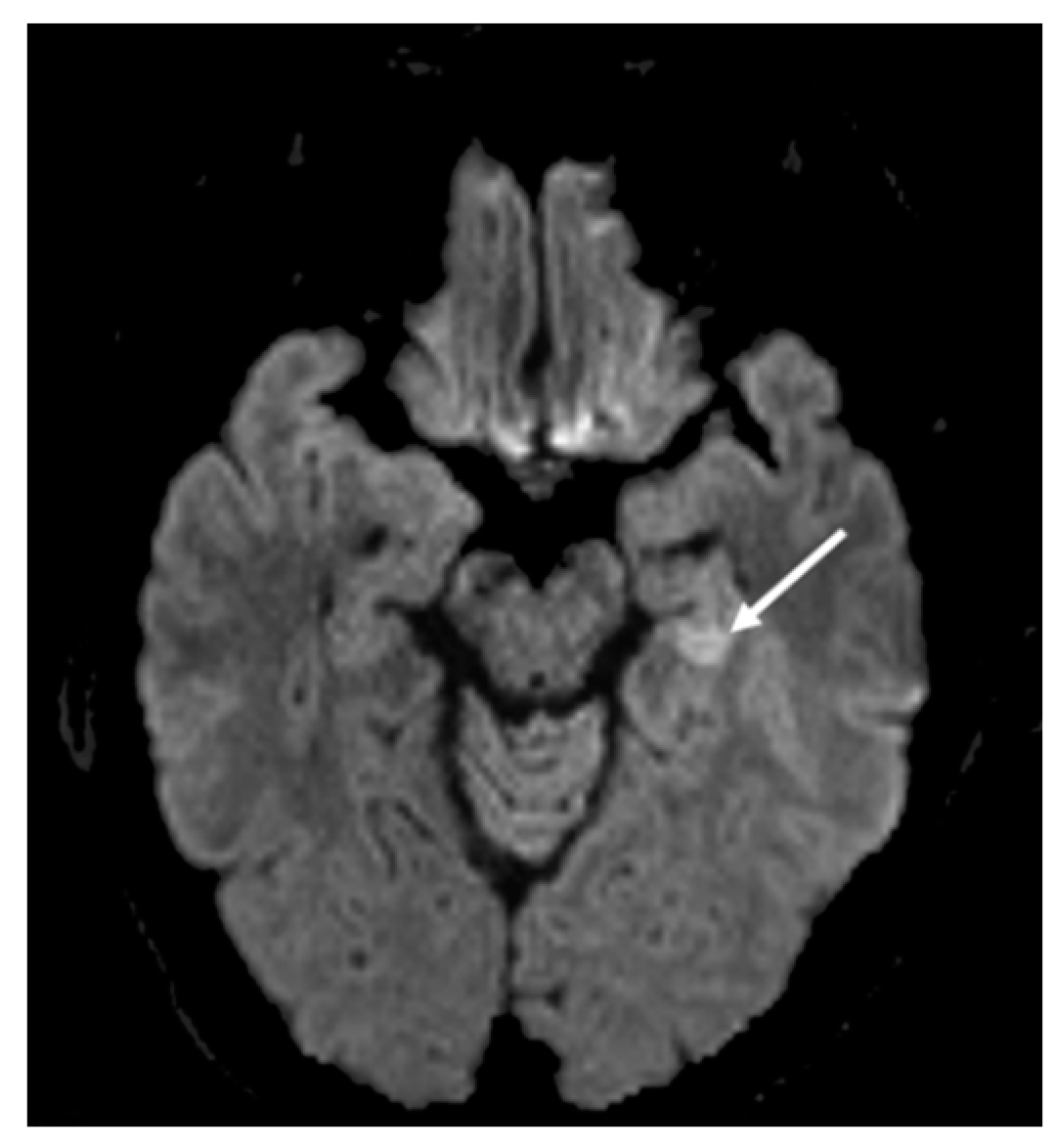
2. Hospital Examination and Laboratory Findings
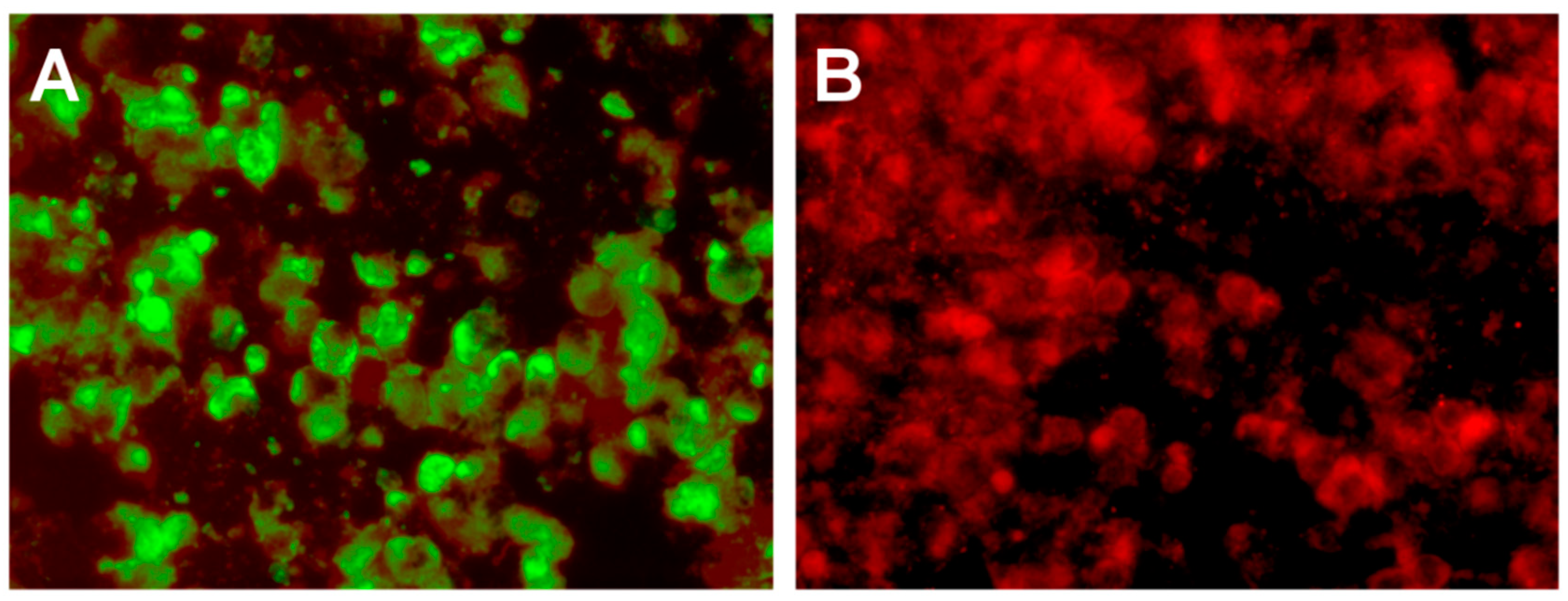
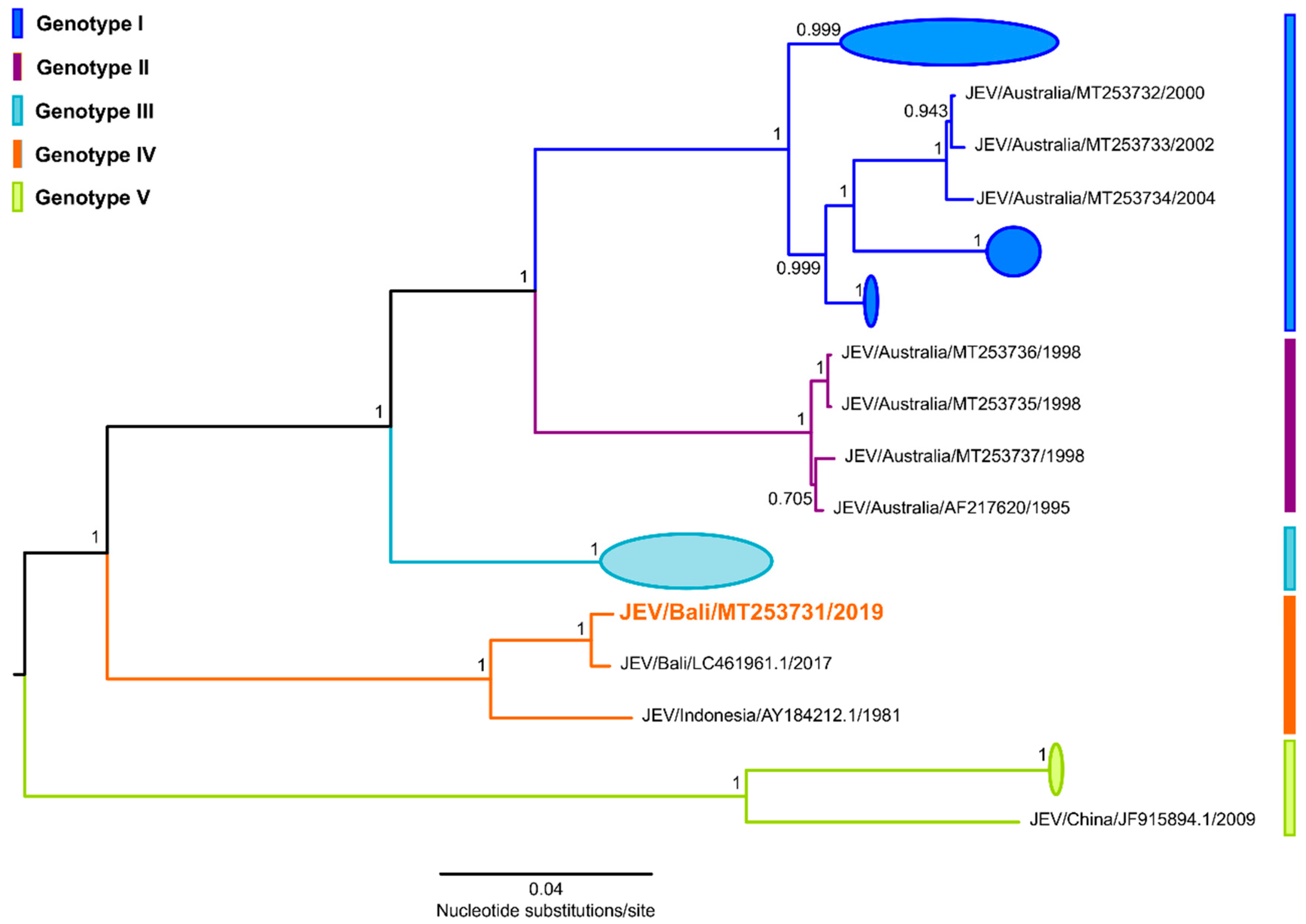
3. Whole Genome Sequencing and Phylogenetic Analysis
4. Patient Outcome
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Pyke, A.T. Japanese Encephalitis-Australia ex Indonesia: (Bali). ProMED-mail. 21st January: 20200121.6904174. Available online: http://www.promedmail.org (accessed on 24 July 2020).
- Taylor, C.T.; Mackay, I.M.; McMahon, J.L.; Wheatley, S.L.; Moore, P.R.; Finger, M.J.; Hewitson, G.R.; Moore, F.A. Detection of specific ZIKV IgM in travelers, using a 2 multiplexed flavivirus microsphere immunoassay. Viruses 2018, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Northill, J.A.; Finger, M.; Lyon, M.; Mackay, I.M. Japanese encephalitis virus real-time RT-PCR V.3. Protocolsio. 2018. Available online: https://www.protocols.io/view/japanese-encephalitis-virus-real-time-rt-pcr-r23d8gn/metrics (accessed on 24 July 2020).
- Pyke, A.T.; Gunn, W.; Taylor, C.T.; Mackay, I.M.; McMahon, J.; Jelley, L.; Waite, B.; May, F. On the home front: Specialised reference testing for dengue in the Australasian region. Trop. Med. Infect. Dis. 2019, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; McMahon, J.; Burtonclay, P.; Nair, N.; De Jong, A. Genome Sequences of chikungunya virus strains from Bangladesh and Thailand. Microbiol. Resour. Announc. 2020, 9, e01452-19. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Pyke, A.T.; Williams, D.T.; Nisbet, D.J.; van den Hurk, A.F.; Taylor, C.T.; Johansen, C.A.; Macdonald, J.; Hall, R.A.; Simmons, R.J.; Mason, R.J.; et al. The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am. J. Trop. Med. Hyg. 2001, 65, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Lee, J.M.; Hills, S.L.; van den Hurk, A.F.; Phillips, D.A.; Pyke, A.T.; Lee, J.M.; Johansen, C.A.; et al. Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 1999, 170, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.N.; Ritchie, S.A.; Phillips, D.A.; Shield, J.; Bailey, M.C.; Mackenzie, J.S.; Poidinger, M.; McCall, B.J.; Mills, P.J. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med. J. Aust. 1996, 165, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Phillips, D.; Broom, A.; Mackenzie, J.; Poidinger, M.; van den Hurk, A.F. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am. J. Trop. Med. Hyg. 1997, 56, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hurk, A.F.; Montgomery, B.L.; Northill, J.A.; Smith, I.L.; Zborowski, P.; Ritchie, S.J.; MacKenzie, J.S.; A Smith, G. Short report: The first isolation of Japanese encephalitis virus from mosquitoes collected from mainland Australia. Am. J. Trop. Med. Hyg. 2006, 75, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Hills, S.; Lindsey, N.P.; Fischer, M. Travel-Related Infectious Diseases. In CDC Yellow Book 2020: Health Information for International Travel; Brunette, G.W., Nemhauser, J.B., Eds.; Oxford University Press: New York, NY, USA, 2019; p. 720. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/japanese-encephalitis (accessed on 20 July 2020).
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; A Marfin, A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health. Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef] [PubMed]
- van den Hurk, A.F.; Pyke, A.T.; Mackenzie, J.S.; Hall-Mendelin, S.; Ritchie, S.A. Japanese encephalitis virus in Australia: From known known to known unknown. Trop. Med. Infect. Dis. 2019, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Garjito, T.A.; Anggraeni, Y.M.; Alfiah, S.; Satoto, T.B.T.; Farchanny, A.; Samaan, G.; Afelt, A.; Manguin, S.; Frutos, R.; Aditama, T.Y. Japanese encephalitis in Indonesia: An update on epidemiology and transmission ecology. Acta. Trop. 2018, 187, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Garjito, T.A.; Prihatin, M.T.; Susanti, L.; Prastowo, D.; Sa’adah, S.R.; Taviv, Y.; Satoto, T.B.T.; Waluyo, J.; Manguin, S.; Frutos, R. First evidence of the presence of genotype-1 of Japanese encephalitis virus in Culex gelidus in Indonesia. Parasit. Vectors. 2019, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Wittesjo, B.; Eitrem, R.; Niklasson, B.; Vene, S.; Mangiafico, J.A. Japanese encephalitis after a 10-day holiday in Bali. Lancet 1995, 345, 856–857. [Google Scholar] [CrossRef]
- Buhl, M.R.; Black, F.T.; Andersen, P.L.; Laursen, A. Fatal Japanese encephalitis in a Danish tourist visiting Bali for 12 days. Scand. J. Infect. Dis. 1996, 28, 189. [Google Scholar] [CrossRef]
- Macdonald, W.B.; Tink, A.R.; Ouvrier, R.A.; Menser, M.A.; de Silva, L.M.; Naim, H.; Hawkes, R.A. Japanese encephalitis after a two-week holiday in Bali. Med. J. Aust. 1989, 150, 334–336. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyke, A.T.; Choong, K.; Moore, F.; Schlebusch, S.; Taylor, C.; Hewitson, G.; McMahon, J.; Nair, N.; Moore, P.; Finger, M.; et al. A Case of Japanese Encephalitis with a Fatal Outcome in an Australian Who Traveled from Bali in 2019. Trop. Med. Infect. Dis. 2020, 5, 133. https://doi.org/10.3390/tropicalmed5030133
Pyke AT, Choong K, Moore F, Schlebusch S, Taylor C, Hewitson G, McMahon J, Nair N, Moore P, Finger M, et al. A Case of Japanese Encephalitis with a Fatal Outcome in an Australian Who Traveled from Bali in 2019. Tropical Medicine and Infectious Disease. 2020; 5(3):133. https://doi.org/10.3390/tropicalmed5030133
Chicago/Turabian StylePyke, Alyssa T., Keat Choong, Frederick Moore, Sanmarié Schlebusch, Carmel Taylor, Glen Hewitson, Jamie McMahon, Neelima Nair, Peter Moore, Mitchell Finger, and et al. 2020. "A Case of Japanese Encephalitis with a Fatal Outcome in an Australian Who Traveled from Bali in 2019" Tropical Medicine and Infectious Disease 5, no. 3: 133. https://doi.org/10.3390/tropicalmed5030133
APA StylePyke, A. T., Choong, K., Moore, F., Schlebusch, S., Taylor, C., Hewitson, G., McMahon, J., Nair, N., Moore, P., Finger, M., Burtonclay, P., & Wheatley, S. (2020). A Case of Japanese Encephalitis with a Fatal Outcome in an Australian Who Traveled from Bali in 2019. Tropical Medicine and Infectious Disease, 5(3), 133. https://doi.org/10.3390/tropicalmed5030133




