Mapping the Geographic Distribution of Tungiasis in Sub-Saharan Africa
Abstract
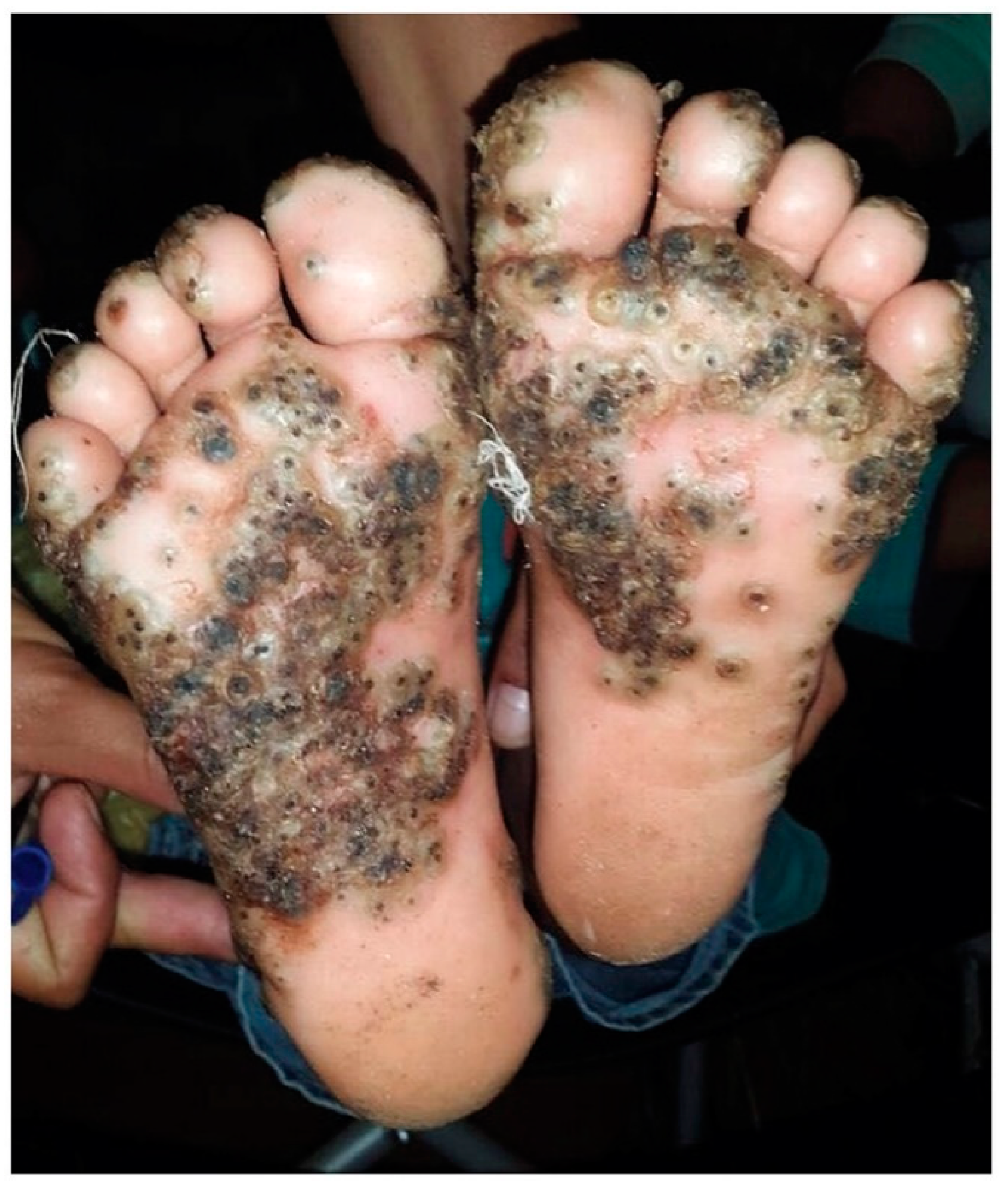
1. Introduction
2. Materials and Methods
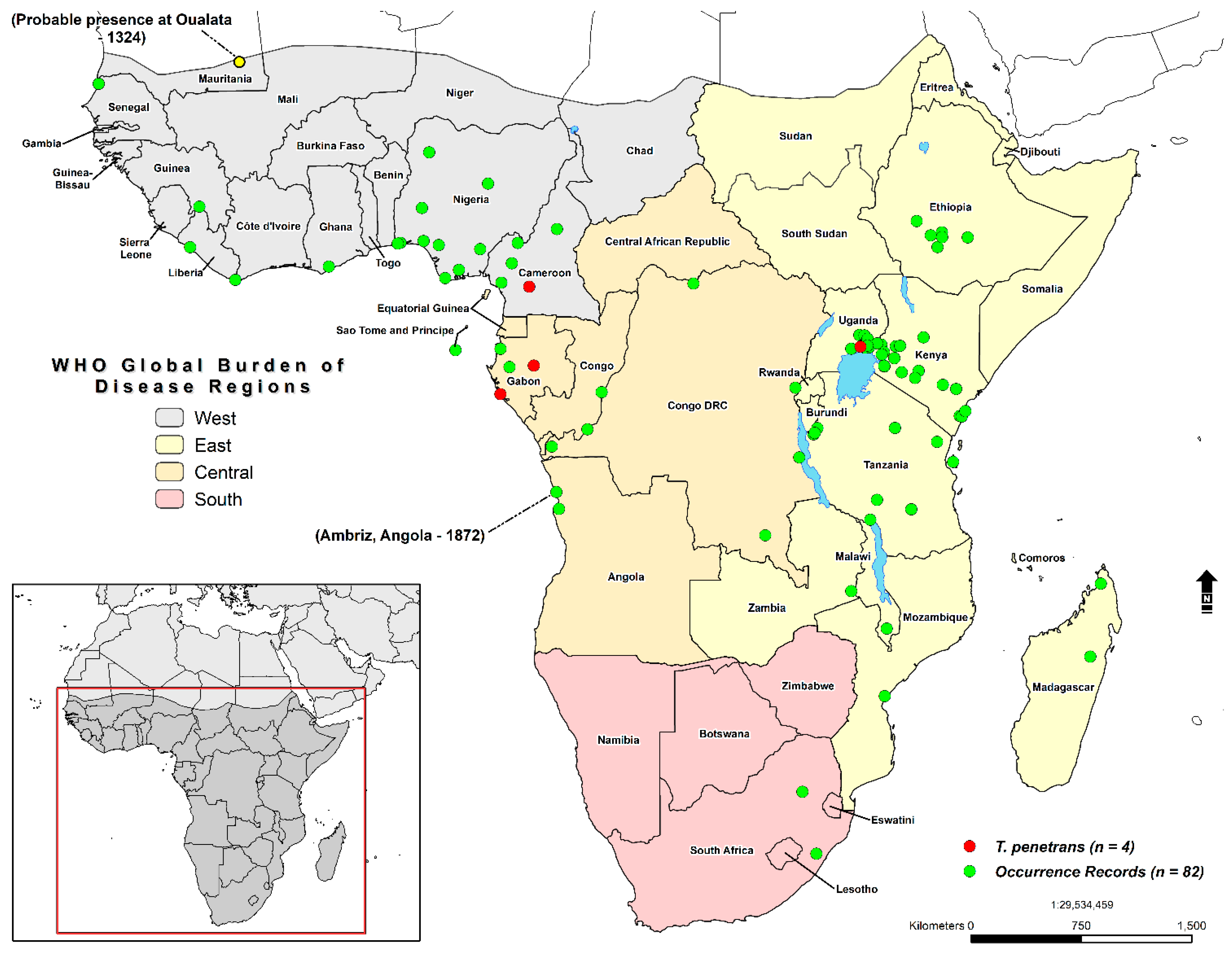
2.1. Study Area and Occurrence Records
2.2. Description of Environmental Covariates
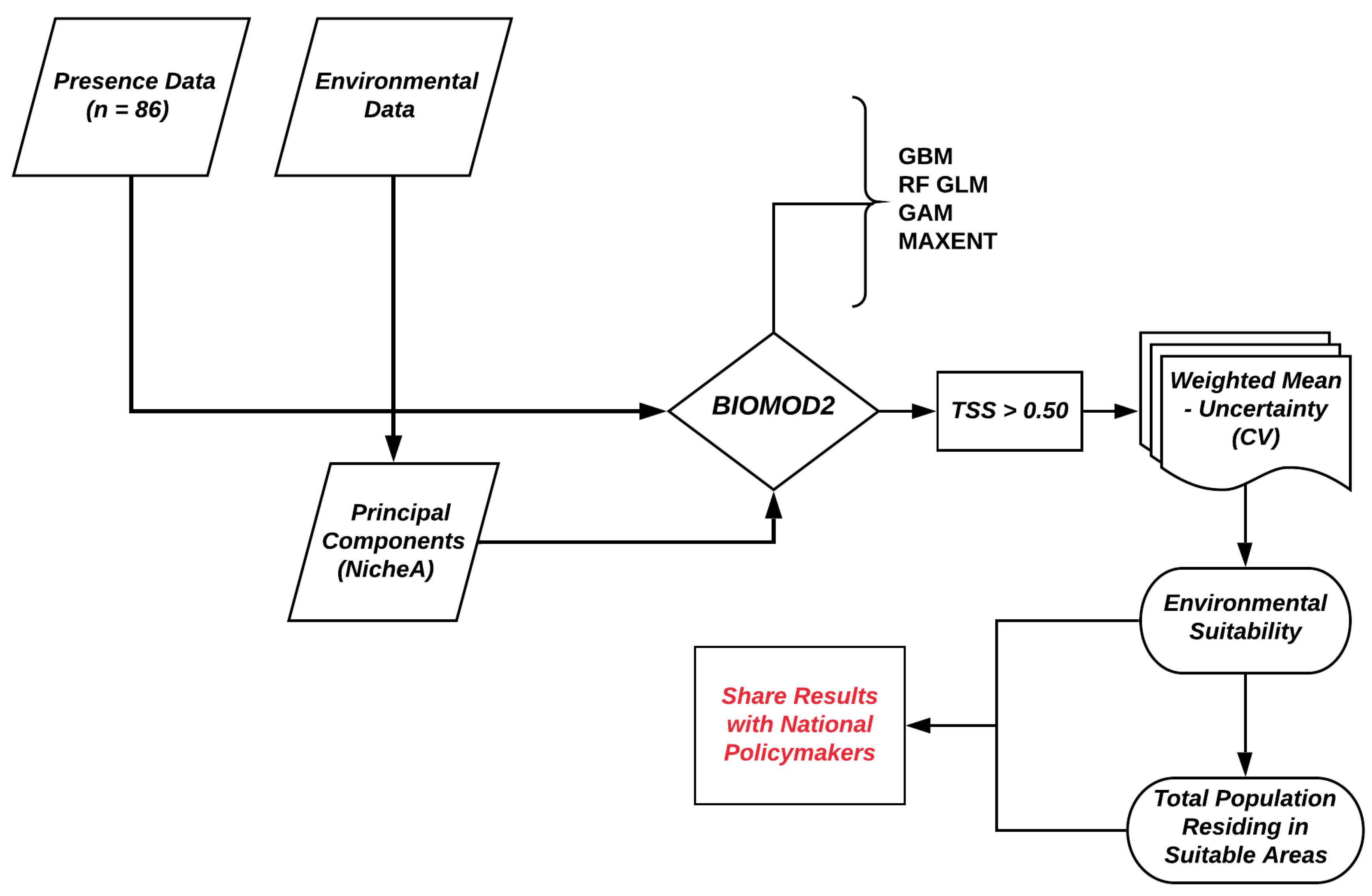
3. Data Analysis
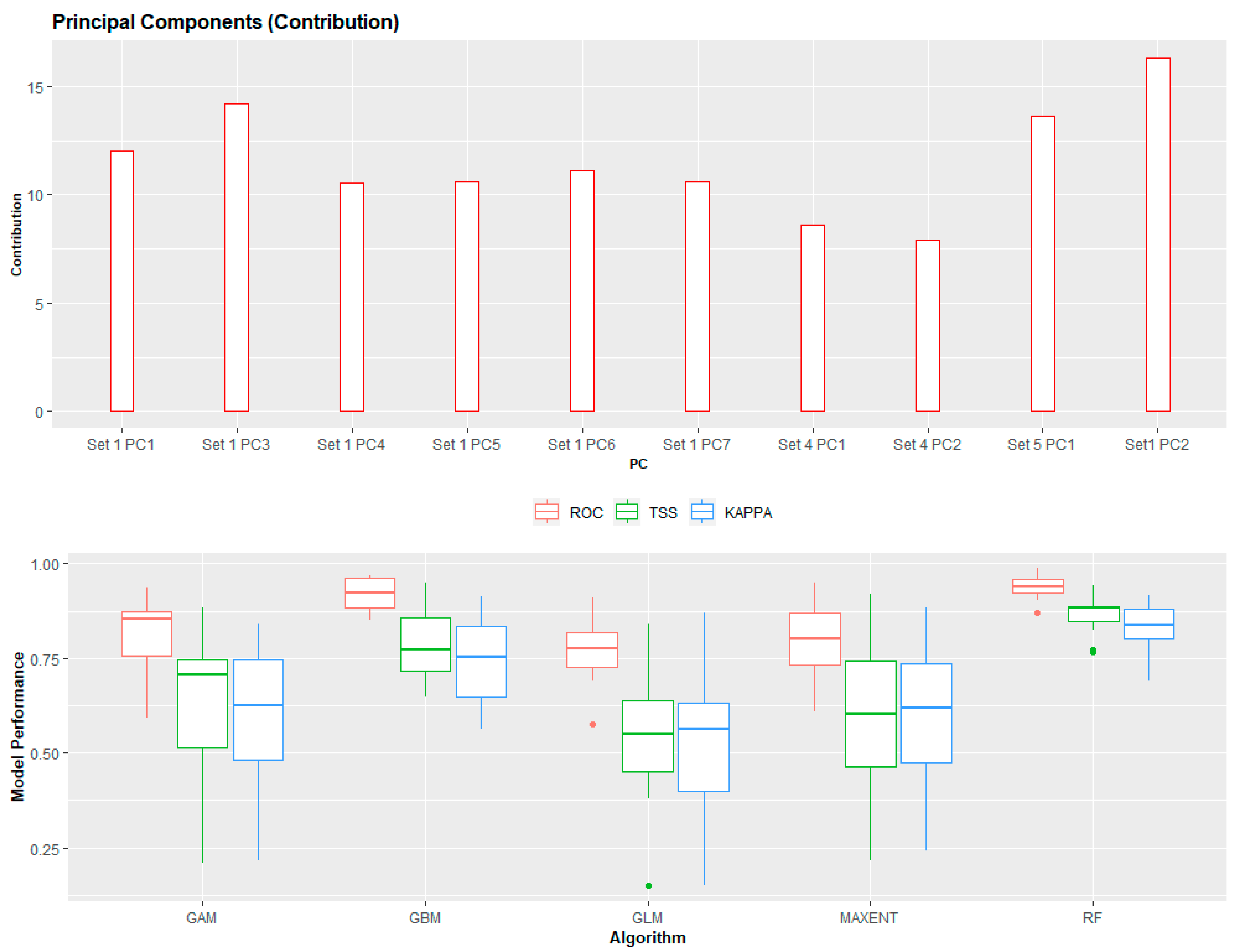
3.1. Principal Component Analysis
3.2. Ensemble ENM Approach
3.3. Estimating the Population Living in Environmentally Suitable Areas
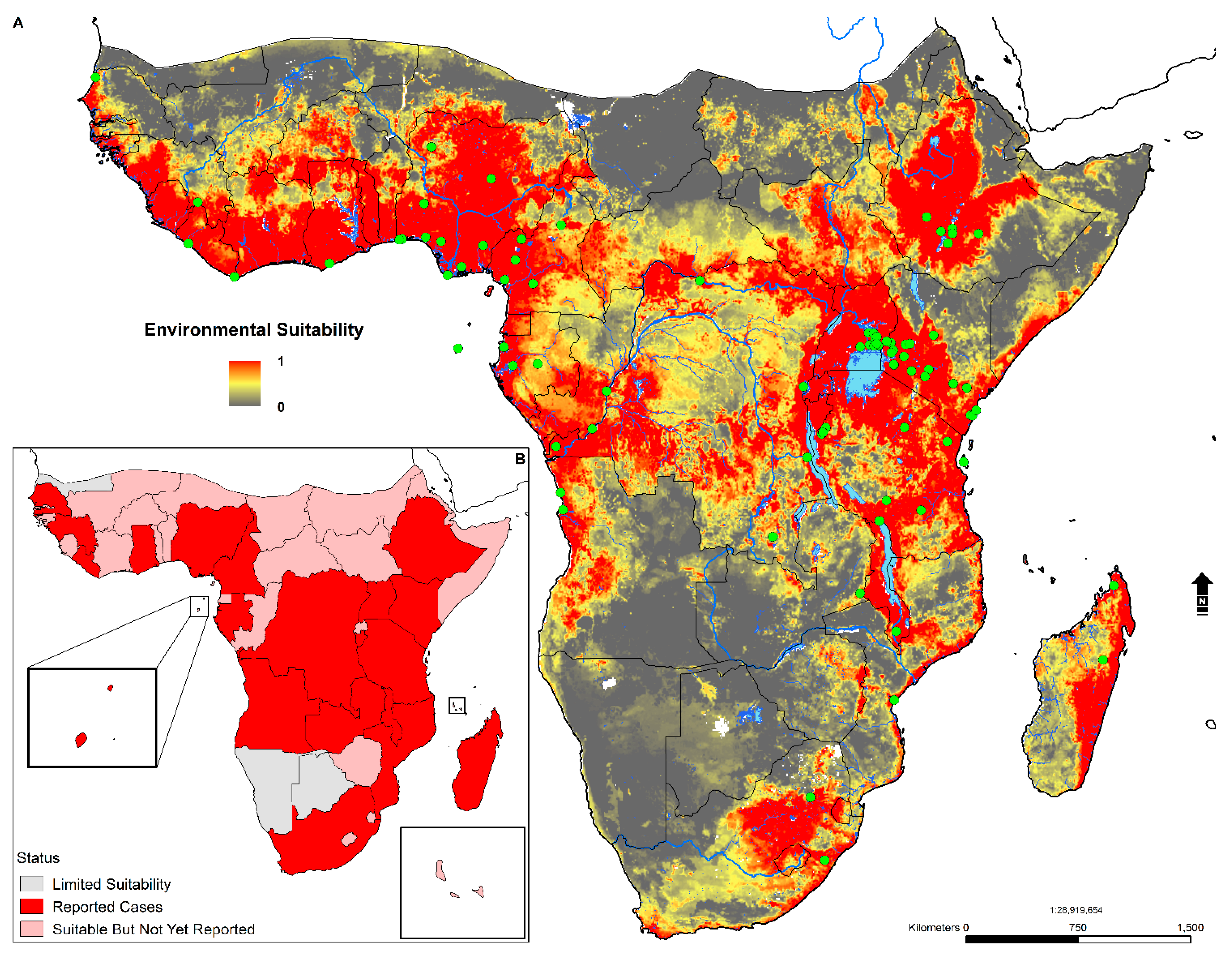
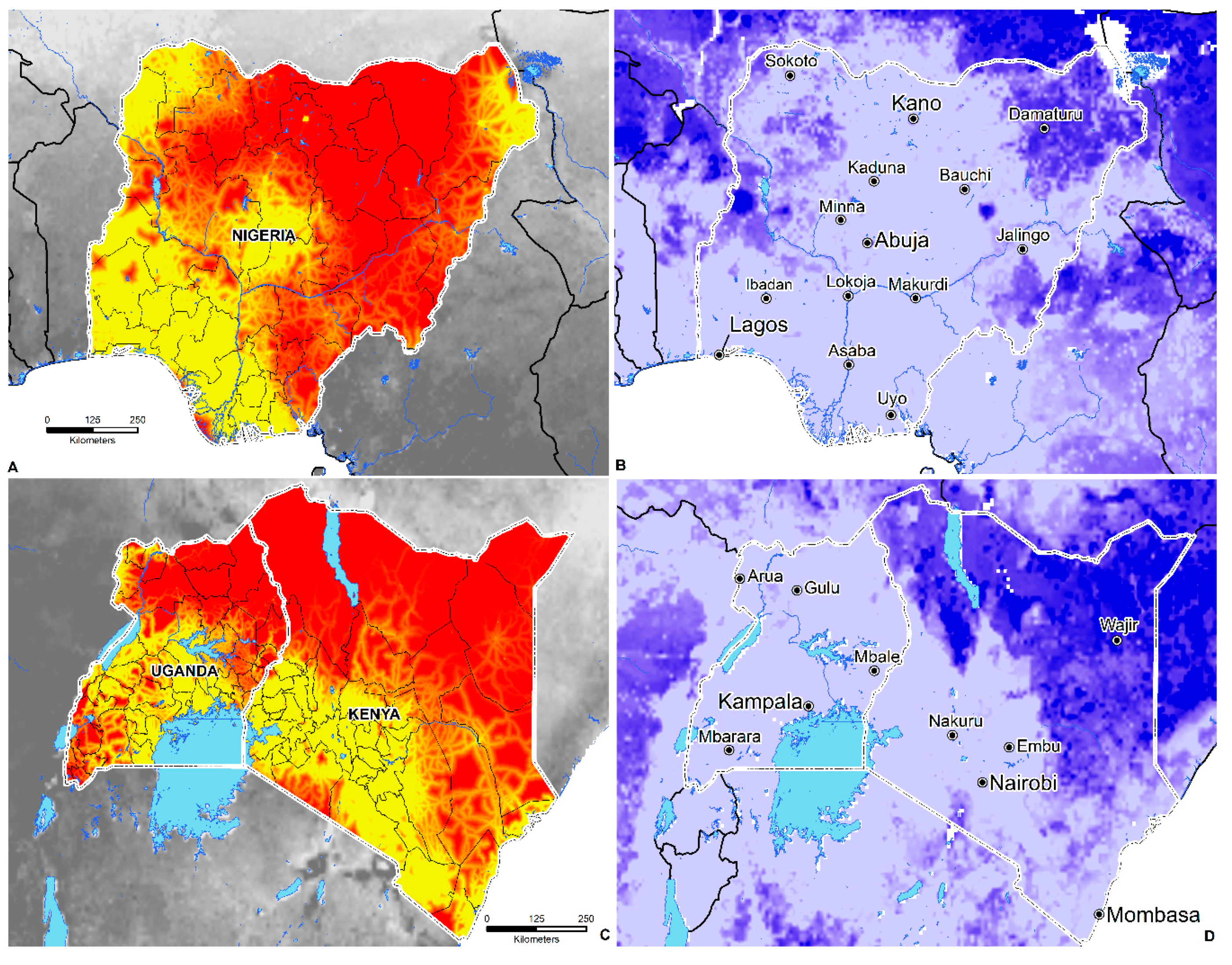
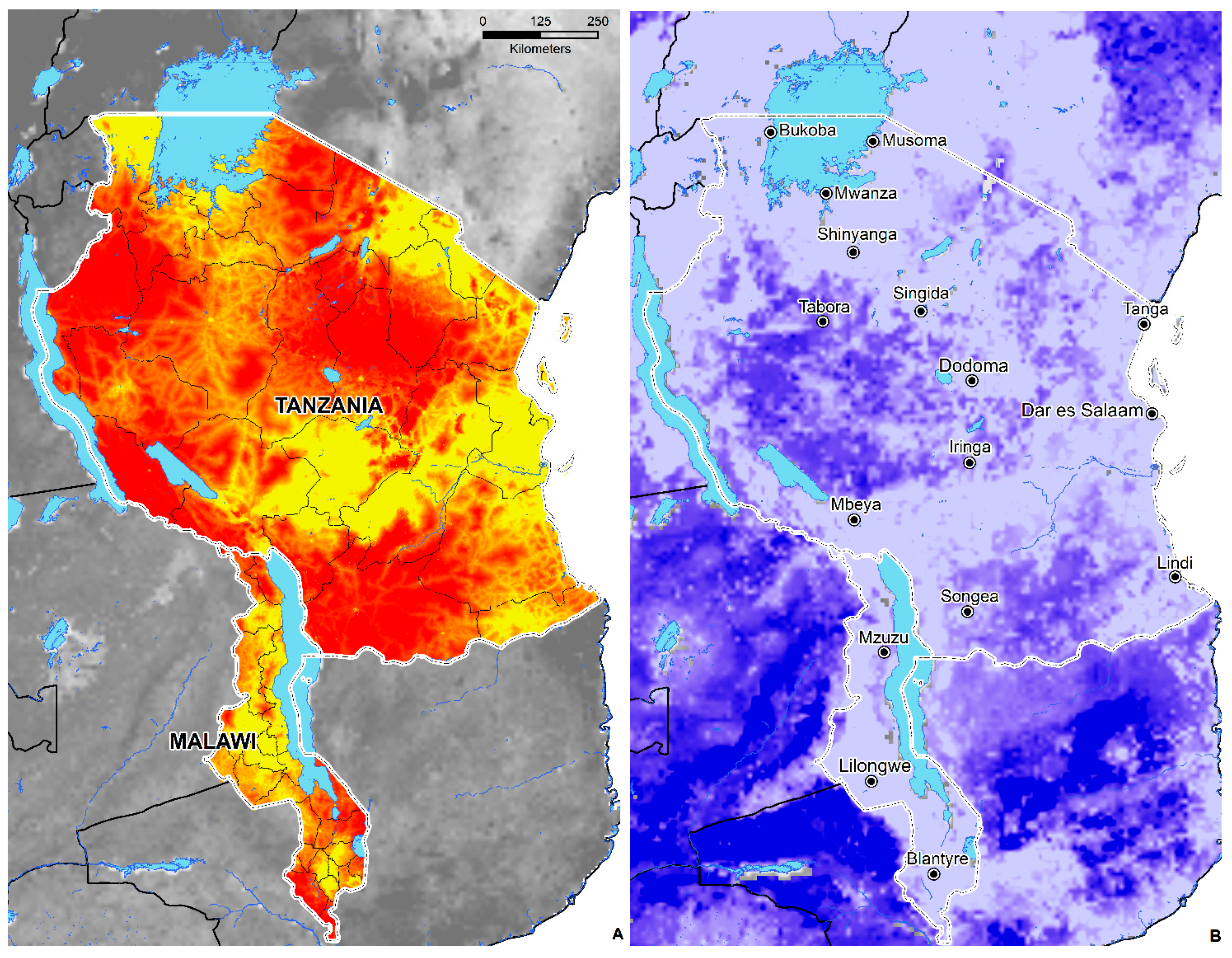
3.4. Environmental Suitability in SSA
4. Discussion
5. Conclusions
- An estimated 668 million individuals live in environmentally suitable areas: 304 million in WHO GBD East Africa and 263 million in WHO GBD West Africa.
- Geographically, environmental suitability is broad and diverse, ranging from tropical savanna (Aw), semi-arid (Bsh), humid subtropical (Cwa), tropical monsoon (Am), tropical rainforest (Af), and subtropical highland (Cwb) climates.
- Environmental suitability is predicted in 44 countries; these include Angola, Nigeria, Ghana, Cameroon, Cote de Ivoire, Mali, South Sudan, Sudan, Somalia, Ethiopia, the Democratic Republic of the Congo, Kenya, Gabon, Central African Republic, Uganda, Rwanda, Tanzania, Zambia, Zimbabwe, Madagascar, Mozambique, and South Africa.
- The total area of environmental suitability (weighted mean threshold > 0.438) is 8,110,107 sq. kilometers or 3,131,329 sq. miles.
Supplementary Materials
Funding
Conflicts of Interest
References
- Maco, V.; Tantaleán, M.; Gotuzzo, E. Evidence of tungiasis in pre-Hispanic America. Emerg. Infect. Dis. 2011, 17, 855. [Google Scholar] [CrossRef]
- Hoeppli, R. Early References to the Occurrence of Tunga pénétrons in Tropical Africa. Acta Trop. 1963, 20, 143–153. [Google Scholar] [PubMed]
- Heukelbach, J.; Costa, A.; Wilcke, T.; Mencke, N.; Feldmeier, H. The animal reservoir of Tunga penetrans in severely affected communities of north-east Brazil. Med. Vet. Entomol. 2004, 18, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ade-Serrano, M.A.; Ejezie, G.C. Prevalence of tungiasis in Oto-Ijanikin village, Badagry, Lagos State, Nigeria. Ann. Trop. Med. Parasitol. 1981, 75, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D. Tungiasis among five communities in south—Western Trinidad, West Indies. Ann. Trop. Med. Parasitol. 1998, 92, 107–113. [Google Scholar]
- Karunamoorthi, K. Tungiasis: A neglected epidermal parasitic skin disease of marginalized populations—A call for global science and policy. Parasitol. Res. 2013, 112, 3635–3643. [Google Scholar] [CrossRef]
- Ahadi Kenya Trust. The Jigger Menace in Kenya. In Anti Jigger Magazine Final Indd; 2011; Volume 2, Available online: http://jigger-ahadi.org/anti_jigger_magazine_year_2_final.pdf (accessed on 2 April 2020).
- Pampiglione, S.; Fioravanti, M.L.; Gustinelli, A.; Onore, G.; Mantovani, B.; Luchetti, A.; Trentini, M. Sand flea (Tunga spp.) infections in humans and domestic animals: State of the art. Med. Vet. Entomol. 2009, 23, 172–186. [Google Scholar] [CrossRef]
- Barbosa, M.M.; Barbosa, A.D. Tungiasis. N. Engl. J. Med. 2019, 380, e19. [Google Scholar] [CrossRef]
- Chen, C.-W.; Thong, H.-Y.; Jee, S.-H. Tungiasis: A case report and review of the literature. Dermatol. Sin. 2011, 29, 29–31. [Google Scholar] [CrossRef]
- Eisele, M.; Heukelbach, J.; Van Marck, E.; Mehlhorn, H.; Meckes, O.; Franck, S.; Feldmeier, H. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol. Res. 2003, 90, 87–99. [Google Scholar] [CrossRef]
- Geigy, J.R.; Herbig, A. Die hypertrophie der Organe beim Weibchen von Tunga penetrans; Verlag f. Recht u. Gesellschaft: Bethesda, MD, USA, 1949. [Google Scholar]
- Nagy, N.; Abari, E.; D’Haese, J.; Calheiros, C.; Heukelbach, J.; Mencke, N.; Feldmeier, H.; Mehlhorn, H. Investigations on the life cycle and morphology of Tunga penetrans in Brazil. Parasitol. Res. 2007, 101, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Mullen, G.R.; Durden, L.A. Medical and Veterinary Entomology; Academic press: Amsterdam, The Netherlands, 2009; ISBN 0-08-091969-3. [Google Scholar]
- Elson, L.; Wright, K.; Swift, J.; Feldmeier, H. Control of tungiasis in absence of a roadmap: Grassroots and global approaches. Trop. Med. Infect. Dis. 2017, 2, 33. [Google Scholar] [CrossRef]
- Kimotho, S.; Miller, A.N.; Ngure, P. Managing communication surrounding tungiasis stigma in Kenya. Communication 2015, 41, 523–542. [Google Scholar] [CrossRef]
- Nyangacha, R.M.; Odongo, D.; Oyieke, F.; Ochwoto, M.; Korir, R.; Ngetich, R.K.; Nginya, G.; Makwaga, O.; Bii, C.; Mwitari, P.; et al. Secondary bacterial infections and antibiotic resistance among tungiasis patients in Western, Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005901. [Google Scholar] [CrossRef] [PubMed]
- Miller, H.; Ocampo, J.; Ayala, A.; Trujillo, J.; Feldmeier, H. Very severe tungiasis in Amerindians in the Amazon lowland of Colombia: A case series. PLoS Negl. Trop. Dis. 2019, 13, e0007068. [Google Scholar] [CrossRef]
- Feldmeier, H.; Eisele, M.; Sabóia-Moura, R.C.; Heukelbach, J. Severe Tungiasis in Underprivileged Communities: Case Series from Brazil. Emerg. Infect. Dis. 2003, 9, 949–955. [Google Scholar] [CrossRef]
- Obengui, I. La tungose et le tétanos au CHU de Brazzaville. Dakar Médical 1989, 34, 44–48. [Google Scholar]
- Shrivastav, S.; Adhikari, R.C. Tungiasis: A rare parasitic infestation in genitals of a native male from Kathmandu. J. Nepal Health Res. Counc. 2017, 15, 295–297. [Google Scholar] [CrossRef][Green Version]
- Kaimbo, D.K.W.; Bifuko, A.; Parys-Van Ginderdeuren, R. Upper eyelid localisation of Tunga penetrans. Ophthalmologica 2007, 221, 439–442. [Google Scholar] [CrossRef]
- Sentongo, E.; Wabinga, H. Tungiasis presenting as a soft tissue oral lesion. BMC Oral Health 2014, 14, 112. [Google Scholar] [CrossRef]
- Mutebi, F.; Krücken, J.; Feldmeier, H.; Waiswa, C.; Mencke, N.; Sentongo, E.; von Samson-Himmelstjerna, G. Animal reservoirs of zoonotic tungiasis in endemic rural villages of Uganda. PLoS Negl. Trop. Dis. 2015, 9, e0004126. [Google Scholar] [CrossRef] [PubMed]
- Ruttoh, S.K.; Ochieng’ Omondi, D.; Wanyama, N.I. Tunga penetrans A Silent Setback to Development in Kenya. J. Environ. Sci. Eng. B 2012, 1, 1. [Google Scholar]
- Anderson, C.; Lee, D.; Dean, N. Identifying clusters in Bayesian disease mapping. Biostatistics 2014, 15, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Kafadar, K. Simultaneous smoothing and adjusting mortality rates in US counties: Melanoma in white females and white males. Stat. Med. 1999, 18, 3167–3188. [Google Scholar] [CrossRef]
- Feldmeier, H.; Heukelbach, J.; Ugbomoiko, U.S.; Sentongo, E.; Mbabazi, P.; von Samson-Himmelstjerna, G.; Krantz, I. Tungiasis—A neglected disease with many challenges for global public health. PLoS Negl. Trop. Dis. 2014, 8, e3133. [Google Scholar] [CrossRef]
- Ugbomoiko, U.S.; Ofoezie, I.E.; Heukelbach, J. Tungiasis: High prevalence, parasite load, and morbidity in a rural community in Lagos State, Nigeria. Int. J. Dermatol. 2007, 46, 475–481. [Google Scholar] [CrossRef]
- Escobar, L.E.; Craft, M.E. Advances and limitations of disease biogeography using ecological niche modeling. Front. Microbiol. 2016, 7, 1174. [Google Scholar] [CrossRef]
- Samy, A.M.; van de Sande, W.W.; Fahal, A.H.; Peterson, A.T. Mapping the potential risk of mycetoma infection in Sudan and South Sudan using ecological niche modeling. PLoS Negl. Trop. Dis. 2014, 8, e3250. [Google Scholar] [CrossRef]
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Henry, A.J.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.; Smith, D.L.; Moyes, C.L.; et al. Mapping the zoonotic niche of Ebola virus disease in Africa. Elife 2014, 3, e04395. [Google Scholar] [CrossRef]
- Carlson, C.J.; Kracalik, I.T.; Ross, N.; Alexander, K.A.; Hugh-Jones, M.E.; Fegan, M.; Elkin, B.T.; Epp, T.; Shury, T.K.; Zhang, W.; et al. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat. Microbiol. 2019, 4, 1337–1343. [Google Scholar] [CrossRef]
- Deka, M.A.; Morshed, N. Mapping disease transmission risk of Nipah virus in South and Southeast Asia. Trop. Med. Infect. Dis. 2018, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Han, B.A.; Kramer, A.M.; Drake, J.M. Global patterns of zoonotic disease in mammals. Trends Parasitol. 2016, 32, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Metcalf, C.J.E.; Ferrari, M.J.; Tatem, A.J.; Lessler, J. The geography of measles vaccination in the African Great Lakes region. Nat. Commun. 2017, 8, 15585. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Patil, A.P.; Smith, D.L.; Guerra, C.A.; Elyazar, I.R.; Johnston, G.L.; Tatem, A.J.; Hay, S.I. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar. J. 2011, 10, 378. [Google Scholar] [CrossRef] [PubMed]
- Reiner, R.C., Jr.; Graetz, N.; Casey, D.C.; Troeger, C.; Garcia, G.M.; Mosser, J.F.; Deshpande, A.; Swartz, S.J.; Ray, S.E.; Blacker, B.F.; et al. Variation in childhood diarrheal morbidity and mortality in Africa, 2000–2015. N. Engl. J. Med. 2018, 379, 1128–1138. [Google Scholar] [CrossRef]
- Osgood-Zimmerman, A.; Millear, A.I.; Stubbs, R.W.; Shields, C.; Pickering, B.V.; Earl, L.; Graetz, N.; Kinyoki, D.K.; Ray, S.E.; Bhatt, S.; et al. Mapping child growth failure in Africa between 2000 and 2015. Nature 2018, 555, 41–47. [Google Scholar] [CrossRef]
- Moran, A.E.; Oliver, J.T.; Mirzaie, M.; Forouzanfar, M.H.; Chilov, M.; Anderson, L.; Morrison, J.L.; Khan, A.; Zhang, N.; Haynes, N.; et al. Assessing the global burden of ischemic heart disease: Part 1: Methods for a systematic review of the global epidemiology of ischemic heart disease in 1990 and 2010. Glob. Heart 2012, 7, 315–329. [Google Scholar] [CrossRef]
- GBIF. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.xcpprz (accessed on 13 May 2020).
- Ames, C.G. Gazetteers of the Northern Provinces of Nigeria: The Highland Chieftaincies (Plateau Province); Cambridge University Press: Cambridge, UK, 1934; Volume 4. [Google Scholar]
- Partridge, C. Cross River Natives: Being Some Notes on the Primitive Pagans of Obubura Hill District, Southern Nigeria, Including a Description of the Circles of Upright Sculptured Stones on the Left Bank of the Aweyong River; Forgotten Books: London, UK, 1905. [Google Scholar]
- Bindloss, H. In the Niger Country; W. Blackwood and Sons: Edinburgh, London, UK, 1898. [Google Scholar]
- Ramaswamy, V. Assessment of socioeconomic status and the prevalence of Tungiasis in Jimma and Wolaita Sodo, Ethiopia. Int. J. Intgr. Med. Sci. 2016, 3, 211–215. [Google Scholar]
- Tadele, H. Clinical profile and outcome of pediatrics tetanus: The experience of a tertiary hospital in Ethiopia. Ethiop. J. Health Sci. 2017, 27, 559–564. [Google Scholar] [CrossRef]
- Walker, S.L.; Lebas, E.; De Sario, V.; Deyasso, Z.; Doni, S.N.; Marks, M.; Roberts, C.H.; Lambert, S.M. The prevalence and association with health-related quality of life of tungiasis and scabies in schoolchildren in southern Ethiopia. PLoS Negl. Trop. Dis. 2017, 11, e0005808. [Google Scholar] [CrossRef]
- Chelimo, J.J. Risk Factors Associated with Jigger Infestation in Kitany Location, Keiyo Marakwet County, Kenya. Master’s Thesis, Moi University, Kesses, Kenya, 2015. [Google Scholar]
- Mørkve, Å. "Getting Rid of the Plague": Jiggers Removal Program in Bungoma, Kenya. Community and Health Workers Perspectives on Tungiasis in a High Prevalence Area; The University of Bergen: Bergen, Norway, 2013. [Google Scholar]
- Wafula, S.T.; Ssemugabo, C.; Namuhani, N.; Musoke, D.; Ssempebwa, J.; Halage, A.A. Prevalence and risk factors associated with tungiasis in Mayuge district, Eastern Uganda. Pan Afr. Med. J. 2016, 24, 24. [Google Scholar] [CrossRef]
- Nyangacha, R.M.; Odongo, D.; Oyieke, F.; Bii, C.; Muniu, E.; Chasia, S.; Ochwoto, M. Spatial distribution, prevalence and potential risk factors of Tungiasis in Vihiga County, Kenya. PLoS Negl. Trop. Dis. 2019, 13, e0007244. [Google Scholar] [CrossRef]
- Zabron, W. Tungiasis Risk Factors in Rural Community in Murang’ a County. Master’s Thesis, Kenyatta University, Nairobi, Kenya, 2017. [Google Scholar]
- Mwai, J.; Mutai, J.; Karanja, S.; Karani, G. Factors Influencing Practices Towards Water, Sanitation and Hygiene with Occurrence of Tungiasis Among Pupils in Schools with a Feeding Programme in Ganze Sub County, Kenya. Glob. J. Health Sci. 2018, 3, 1–23. [Google Scholar]
- Mazigo, H.D.; Behamana, E.; Zinga, M.; Heukelbach, J. Tungiasis infestation in Tanzania. J. Infect. Dev. Ctries. 2010, 4, 187–189. [Google Scholar] [CrossRef][Green Version]
- Mwakanyamale, J.G.; Towett, R.K.; Mtango, F.; Bundala, J.; Kisanga, F. Contributions of socioeconomic and cultural factors in Tungiasis at Kwakombo village in Korogwe district, Tanzania. Imtu. Med. J. 2015, 6, 54–61. [Google Scholar]
- Jeffreys, M. Pulex penetrans: The jigger’s arrival and spread in Africa. S. Afr. J. Sci. 1952, 48, 249–255. [Google Scholar]
- Ugbomoiko, U.S.; Ariza, L.; Ofoezie, I.E.; Heukelbach, J. Risk factors for tungiasis in Nigeria: Identification of targets for effective intervention. PLoS Negl. Trop. Dis. 2007, 1, e87. [Google Scholar] [CrossRef]
- Ugbomoiko, U.S.; Ariza, L.; Heukelbach, J. Parasites of importance for human health in Nigerian dogs: High prevalence and limited knowledge of pet owners. BMC Vet. Res. 2008, 4, 49. [Google Scholar] [CrossRef]
- Mulambya, N.L.; Sakubita, P.; Hamoonga, R.; Mulubwa, B.; Namafente, O.; Mutengo, M.; Yard, E. Tungiasis Outbreak Investigation In Masaiti District, Zambia. Health Press Zamb. Bull 2018, 2, 8–16. [Google Scholar]
- Nájera Villagrana, S.M.; García Naranjo Santisteban, A. Tungiasis: A highly neglected disease among neglected diseases. Case series from Nduta refugee camp (Tanzania). Oxf. Med. Case Rep. 2019, 2019, 49. [Google Scholar] [CrossRef]
- Thielecke, M.; Raharimanga, V.; Rogier, C.; Stauss-Grabo, M.; Richard, V.; Feldmeier, H. Prevention of tungiasis and tungiasis-associated morbidity using the plant-based repellent Zanzarin: A randomized, controlled field study in rural Madagascar. PLoS Negl. Trop. Dis. 2013, 7, e2426. [Google Scholar] [CrossRef]
- Richardson, D.J.; Mangili, A.M. Infection with the Sand Flea Tunga penetrans (Tungiasis) in a Traveller Returning from Cameroon, Africa. JAAS 2016, 70, 199. [Google Scholar]
- Bourée, P.; Simeni Njonnou, R.; Takougang, I.; Kaptue, L. Tungiasis in Bangou (West Cameroon). Med Sante Trop. 2012, 22, 440–443. [Google Scholar] [CrossRef]
- Arene, F.O.I. The prevalence of sand flea (Tunga penetrans) among primary and post-primary school pupils in Choba area of the Niger Delta. Public Health 1984, 98, 282–283. [Google Scholar] [CrossRef]
- Collins, G.; McLeod, T.; Njilah Issac, K.; Lamnyam, C.; Ngarka, L.; Leo Njamnshi, N. Tungiasis: A eglected Health Problem in Rural Cameroon. Int. J. Collab. Res. Intern. Med. Public Health 2009, 1, 2–10. [Google Scholar]
- Bentley, W.H. Pioneering on the Congo; Religious tract society: London, UK, 1900; Volume 1. [Google Scholar]
- Monteiro, J.J. Angola and the River Congo; Macmillan and Company: London, UK, 1876. [Google Scholar]
- Ukonu, B.A.; Eze, E.U. Pattern of Skin Diseases at University of Benin Teaching Hospital, Benin City, Edo State, South-South Nigeria: A 12 Month Prospective Study. Glob. J. Health Sci. 2012, 4, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Stanley, H.M. Through the Dark Continent: Or, the Sources of the Nile, Around the Great Lakes of Equatorial Africa, and Down the Livingstone River to the Atlantic Ocean; Sampson, Low: London, UK, 1889. [Google Scholar]
- Thielecke, M.; Raharimanga, V.; Stauss-Grabo, M.; Rogier, C.; Richard, V.; Feldmeier, H. Regression of severe tungiasis-associated morbidity after prevention of re-infestation: A case series from rural Madagascar. Am. J. Trop. Med. Hyg. 2013, 89, 932–936. [Google Scholar] [CrossRef]
- Hesse, P. Die Ausbreitung des Sandflohs in Afrika. Ein tiergeographischer Versuch. Geogr. Z. 1899, 5, 522–530. [Google Scholar]
- Heukelbach, J.; Ugbomoiko, U.S. Tungiasis in the past and present: A dire need for intervention. Niger. J. Parasitol. 2007, 28, 1–5. [Google Scholar] [CrossRef]
- Dias, J.R. Famine and disease in the history of Angola c. 1830–1930. J. Afr. Hist. 1981, 22, 349–378. [Google Scholar] [CrossRef]
- Barbot, J. A description of the Coasts of Guinea in Churchill’s A Collection of Voyages and Travels; British Library: London, UK, 1732. [Google Scholar]
- Hutton, W. A Voyage to Africa: Including a Narrative of an Embassy to One of the Interior Kingdoms, in the Year 1820, with Remarks on the Course and Termination of the Niger, and Other Principal Rivers in that Country; Longman, Hurst, Rees, Orme, and Brown: London, UK, 1821. [Google Scholar]
- comte de Mollien, G.T. Travels in the Interior of Africa, to the Sources of the Senegal and Gambia: Performed by Command of the French Government, in the Year 1818; Henry Colburn & Company: London, UK, 1820. [Google Scholar]
- Johnston, H. Liberia; Hutchinson & Company: London, UK, 1906; Volume 1. [Google Scholar]
- Johnston, H. The River Congo, from Its Mouth to Bolobo: A General Description of the Natural History and Anthropology of Its Western Basin; Sampson Low, Marston & Company: London, UK, 1895. [Google Scholar]
- Crawford, D. Thinking Black: 22 Years without a Break in the Long Grass of Central Africa; Morgan and Scott: London, UK, 1912. [Google Scholar]
- Decle, L. Three Years in Savage Africa; Methuen & Company: London, UK, 1898. [Google Scholar]
- Spencer, H.A. Chigger Flea or" Chigoe" in the Transvaal. TMJ 1912, 8, 833. [Google Scholar]
- Mockler-Ferryman, A.F. British West Africa: Its Rise and Progress; Swan Sonnenshein: Oxford, UK, 1900. [Google Scholar]
- Mwangi, M.M. Factors Influencing Participation of Stakeholders in Eradication of Jiggers: A case of Kandara sub County. Master’s Thesis, University of Nairobi, Muranga County, Kenya, 2015. [Google Scholar]
- Pampiglione, S.; Trentini, M.; Gentili, F.M.; Mendes, J.; Pampiglione, C.; Rivasi, F. Tunga penetrans (Insecta: Siphonaptera) in pigs in Sao Tomé (Equatorial Africa): Epidemiological, clinical, morphological and histopathological aspects. Rev. Elev. Med. Vet. Pays Trop. 1998, 51, 201–205. [Google Scholar]
- Gibbons, A.S.H. Africa from South to North through Marotseland; Lane, J., Ed.; Oxford University Press: Oxford, UK, 1904; Volume 2. [Google Scholar]
- Girma, M.; Astatkie, A.; Asnake, S. Prevalence and risk factors of tungiasis among children of Wensho district, southern Ethiopia. BMC Infect. Dis. 2018, 18, 456. [Google Scholar] [CrossRef] [PubMed]
- Gadisa, E.; Jote, K. Prevalence and factors associated with intestinal parasitic infection among under-five children in and around Haro Dumal Town, Bale Zone, Ethiopia. BMCP 2019, 385, 1–8. [Google Scholar] [CrossRef]
- Reiss, F. Tungiasis in New York City. Arch. Dermatol. 1966, 93, 404–407. [Google Scholar] [CrossRef]
- Dassoni, F.; Polloni, I.; Margwe, S.B.; Veraldi, S. Tungiasis in northern Tanzania: A clinical report from Qameyu village, Babati District, Manyara Region. J. Infect. Dev. Ctries. 2014, 8, 1456–1460. [Google Scholar] [CrossRef][Green Version]
- Karuga, J. Factors contributing to prevalence of jigger infestation among community members of Mugumoini sublocation. Ph.D. Thesis, Kenya Medical Training College, Gatanga District, Kenya, 2013. [Google Scholar]
- Mazigo, H.D.; Bahemana, E.; Dyegura, O.; Mnyone, L.L.; Kweka, E.J.; Zinga, M.; Konje, E.T.; Waihenya, R.; Heukelbach, J. Severe tungiasis in Western Tanzania: Case series. J. Public Health Afr. 2011, 2, 21. [Google Scholar] [CrossRef]
- Okoth, A.A. Morbidity, Risk Factors, and flea species responsible for Tungiasis in selected villages in Kisumu County. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2015. [Google Scholar]
- Proctor, E.M. Tunga penetrans Acquired while Traveling in Africa. Can. J. Infect. Dis. 1970, 5, 82–83. [Google Scholar] [CrossRef]
- Alfred, K. Factors Associated with Jigger Infestation in Kituro, Baringo Central District. A Rapid Appraisal Report, Kisumo, Kenya, 2009. [Google Scholar]
- Waruguru, C.; Mwaniki, P.; Karama, M.; Muthami, L. Prevalence of tungiasis and its associated factors among residents of Kipkelion west sub-county; Kericho county, Kenya. Int. J. Health Sci. Res. 2015, 5, 434–445. [Google Scholar]
- Weise, S.; Feldmeier, H.; Larson, L.; Mambo, B. Household-related risk factors of tungiasis and severe disease in Kilifi County, Kenya. TMIH 2015, 20, 114–115. [Google Scholar]
- Lugard, L. A Tropical Dependency; J. Nisbet & Co. Limited: London, UK, 1905. [Google Scholar]
- Wiener, L. Africa and the Discovery of America; Innes & Sons: Brooklyn, NY, USA, 1922; Volume 3. [Google Scholar]
- Hooton, E.A. Apes, Men, and Morons; GP Putnam’s Sons: New York, NY, USA, 1937. [Google Scholar]
- ESRI. ArcGIS Desktop: Release 10.7.1; Environmental Systems Research Institute: Redlands, CA, USA, 2019. [Google Scholar]
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Linardi, P.; Calheiros, C.; Campelo-Junior, E.; Duarte, E.; Heukelbach, J.; Feldmeier, H. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann. Trop. Med. Parasitol. 2010, 104, 337–345. [Google Scholar] [CrossRef]
- Dent, D. International Soil Reference and Information Centre (ISRIC). In Encyclopedia of Soil Science; Springer, Dordrecht: Berlin, Germany, 2017; pp. 1232–1236. [Google Scholar]
- Winter, B.; Oliveira, F.A.; Wilcke, T.; Heukelbach, J.; Feldmeier, H. Tungiasis-related knowledge and treatment practices in two endemic communities in northeast Brazil. J. Infect. Dev. Ctries. 2009, 3, 458–466. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Hay, S.; Tatem, A.; Graham, A.; Goetz, S.; Rogers, D. Global environmental data for mapping infectious disease distribution. Adv. Parasitol. 2006, 62, 37–77. [Google Scholar]
- Bavia, M.E.; Malone, J.; Hale, L.; Dantas, A.; Marroni, L.; Reis, R. Use of thermal and vegetation index data from earth observing satellites to evaluate the risk of schistosomiasis in Bahia, Brazil. Acta Trop. 2001, 79, 79–85. [Google Scholar] [CrossRef]
- Danson, F.; Armitage, R.; Marston, C. Spatial and temporal modelling for parasite transmission studies and risk assessment. Parasite 2008, 15, 463–468. [Google Scholar] [CrossRef][Green Version]
- Pilger, D.; Schwalfenberg, S.; Heukelbach, J.; Witt, L.; Mencke, N.; Khakban, A.; Feldmeier, H. Controlling tungiasis in an impoverished community: An intervention study. PLoS Negl. Trop. Dis. 2008, 2, e324. [Google Scholar] [CrossRef]
- Mutebi, F.; Krücken, J.; Feldmeier, H.; Waiswa, C.; Mencke, N.; von Samson-Himmelstjerna, G. Tungiasis-associated morbidity in pigs and dogs in endemic villages of Uganda. Parasit. Vectors 2016, 9, 44. [Google Scholar] [CrossRef]
- Feitelson, E.; Chenoweth, J. Water poverty: Towards a meaningful indicator. Water Policy 2002, 4, 263–281. [Google Scholar] [CrossRef]
- Manandhar, S.; Pandey, V.P.; Kazama, F. Application of water poverty index (WPI) in Nepalese context: A case study of Kali Gandaki River Basin (KGRB). Water Resour. Manag. 2012, 26, 89–107. [Google Scholar] [CrossRef]
- Koirala, S.; Fang, Y.; Dahal, N.M.; Zhang, C.; Pandey, B.; Shrestha, S. Application of Water Poverty Index (WPI) in Spatial Analysis of Water Stress in Koshi River Basin, Nepal. Sustainability 2020, 12, 727. [Google Scholar] [CrossRef]
- Pruss-Ustun, A. World Health Organization Safer Water, Better Health: Costs, Benefits and Sustainability of Interventions to Protect and Promote Health; World Health Organization: Geneva, Switzerland, 2008; ISBN 92-4-159643-0. [Google Scholar]
- Samy, A.M.; Alkishe, A.A.; Thomas, S.M.; Wang, L.; Zhang, W. Mapping the potential distributions of etiological agent, vectors, and reservoirs of Japanese Encephalitis in Asia and Australia. Acta Trop. 2018, 188, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Okely, M.; Anan, R.; Gad-Allah, S.; Samy, A.M. Mapping the environmental suitability of etiological agent and tick vectors of Crimean-Congo hemorrhagic fever. Acta Trop. 2020, 203, 105319. [Google Scholar] [CrossRef]
- Qiao, H.; Peterson, A.T.; Campbell, L.P.; Soberón, J.; Ji, L.; Escobar, L.E. NicheA: Creating virtual species and ecological niches in multivariate environmental scenarios. Ecography 2016, 39, 805–813. [Google Scholar] [CrossRef]
- Johnson, E.E.; Escobar, L.E.; Zambrana-Torrelio, C. An ecological framework for modeling the geography of disease transmission. Trends Ecol. Evol. 2019, 34, 655–668. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Eberhard, F.E.; Cunze, S.; Kochmann, J.; Klimpel, S. Modelling the climatic suitability of Chagas disease vectors on a global scale. Elife 2020, 9, e52072. [Google Scholar] [CrossRef]
- Guisan, A.; Edwards, T.C., Jr.; Hastie, T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002, 157, 89–100. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.; Boosted Regression Trees for Ecological Modeling. R Documentation. Available online: https://cran.r-project.org/web/packages/dismo/vignettes/brt.pdf (accessed on 12 June 2011).
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 24, 123–140. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A language and environment for statistical computing. Open J. Stat. 2013, 6, 3. [Google Scholar]
- Thuiller, W.; Georges, D.; Engler, R.; Breiner, F.; Georges, M.D.; Thuiller, C.W. Package’ biomod2.’ Ensemble Platform for Species Distribution Modeling. 2016. Available online: https://cran.r-project.org/web/packages/biomod2/biomod2.pdf (accessed on 20 July 2020).
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Gama, M.; Crespo, D.; Dolbeth, M.; Anastácio, P. Predicting global habitat suitability for Corbicula fluminea using species distribution models: The importance of different environmental datasets. Ecol. Model. 2016, 319, 163–169. [Google Scholar] [CrossRef]
- Alkire, S.; Roche, J.M.; Santos, M.E.; Seth, S. Multidimensional Poverty Index 2011: Brief Methodological Note; Oxford Poverty & Human Development Initiative: Oxford, UK, 2011. [Google Scholar]
- Balk, D.L.; Deichmann, U.; Yetman, G.; Pozzi, F.; Hay, S.I.; Nelson, A. Determining global population distribution: Methods, applications and data. Adv. Parasitol. 2006, 62, 119–156. [Google Scholar]
- Köppen, W. Die Wärmezonen der Erde, nach der Dauer der heissen, gemässigten und kalten Zeit und nach der Wirkung der Wärme auf die organische Welt betrachtet. Meteorol. Z. 1884, 1, 5–226. [Google Scholar]
- World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2010; ISBN 92-4-156409-1.
- Engels, D.; Zhou, X.-N. Neglected tropical diseases: An effective global response to local poverty-related disease priorities. Infect. Dis. Poverty 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rood, E.J.; Goris, M.G.; Pijnacker, R.; Bakker, M.I.; Hartskeerl, R.A. Environmental risk of leptospirosis infections in the Netherlands: Spatial modelling of environmental risk factors of leptospirosis in the Netherlands. PLoS ONE 2017, 12, e0186987. [Google Scholar] [CrossRef]
- Hotez, P.J. Forgotten People, Forgotten Diseases: The Neglected Tropical Diseases and Their Impact on Global Health and Development; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 1-68367-348-4. [Google Scholar]
- Amzat, J.; Razum, O. Towards a Sociology of Health Discourse in Africa; Springer: Berlin, Germany, 2017; ISBN 3-319-61672-2. [Google Scholar]
- Riedel, N.; Vounatsou, P.; Miller, J.M.; Gosoniu, L.; Chizema-Kawesha, E.; Mukonka, V.; Steketee, R.W. Geographical patterns and predictors of malaria risk in Zambia: Bayesian geostatistical modelling of the 2006 Zambia national malaria indicator survey (ZMIS). Malar. J. 2010, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Schur, N.; Hürlimanftn, E.; Garba, A.; Traoré, M.S.; Ndir, O.; Ratard, R.C.; Tchuenté, L.-A.T.; Kristensen, T.K.; Utzinger, J.; Vounatsou, P. Geostatistical model-based estimates of schistosomiasis prevalence among individuals aged≤ 20 years in West Africa. PLoS Negl. Trop. Dis. 2011, 5, e1194. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Beasley, M.; Ndinaromtan, M.; Madjiouroum, E.M.; Baboguel, M.; Djenguinabe, E.; Hay, S.I.; Bundy, D.A. Use of remote sensing and a geographical information system in a national helminth control programme in Chad. Bull. World Health Organ. 2002, 80, 783–789. [Google Scholar] [PubMed]
- Khoury, M.J.; Iademarco, M.F.; Riley, W.T. Precision public health for the era of precision medicine. Am. J. Prev. Med. 2016, 50, 398. [Google Scholar] [CrossRef]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef]
- Feldmeier, H. Personal Observation; Charité University Medicine: Berlin, Germany, 2015. [Google Scholar]
| Covariates | Spatial Resolution | Data Source | Units | Average |
|---|---|---|---|---|
| Set1 | ||||
| Annual Potential Evapotranspiration (PET) | ~5 km | ENVIREM | mm/year | 1625.14 |
| Thornthwaite Aridity Index | ~5 km | ENVIREM | 55.19 | |
| Climatic Moisture Index | ~5 km | ENVIREM | –0.13 | |
| Continentality | ~5 km | ENVIREM | °C | 3.55 |
| Emberger’s Q | ~5 km | ENVIREM | 352.04 | |
| Growing Degree Days Greater than 0 °C | ~5 km | ENVIREM | 97,927.53 | |
| Growing Degree Days Greater than 5 °C | ~5 km | ENVIREM | 98,456.92 | |
| Max Temp Coldest Month | ~5 km | ENVIREM | °C × 10 | 26.4 |
| Min Temp Warmest Month | ~5 km | ENVIREM | °C × 10 | 18.3 |
| Month Count with Temp Greater than 10 °C | ~5 km | ENVIREM | months | 12 |
| PET Coldest Quarter | ~5 km | ENVIREM | mm/month | 120.57 |
| PET Driest Quarter | ~5 km | ENVIREM | mm/month | 133.28 |
| PET Seasonality | ~5 km | ENVIREM | mm/month | 1428.88 |
| PET Warmest Quarter | ~5 km | ENVIREM | mm/month | 150.87 |
| PET Wettest Quarter | ~5 km | ENVIREM | mm/month | 133.78 |
| Thermicity Index | ~5 km | ENVIREM | °C | 584.62 |
| Set2 | ||||
| Sand (0–5 cm) | ~1 km | ISRIC | g/100 (w%) | 49.13 |
| Silt (0–5 cm) | ~1 km | ISRIC | g/100 (w%) | 18.27 |
| Clay (0–5 cm) | ~1 km | ISRIC | g/100 (w%) | 33.20 |
| Soil pH (0–5 cm) | ~1 km | ISRIC | g/100 (w%) | 5.7 |
| Set 3 | ||||
| Enhanced Vegetation Index (EVI) 16-day composites (2001–2012) | ~1 km | NASA | 0. 36 | |
| Cropland (2013) | ~1 km | GLC-Share | % | 29.76 |
| Herbaceous (2013) | ~1 km | GLC-Share | % | 1.95 |
| Grassland (2013) | ~1 km | GLC-Share | % | 12.66 |
| Shrubland (2013) | ~1 km | GLC-Share | % | 13.3 |
| Tree Covered Area (2013) | ~1 km | GLC-Share | % | 22 |
| Set 4 | ||||
| Goats Density (2014) | ~1 km | FAO | head/km2 | 32.05 |
| Pig Density (2014) | ~1 km | FAO | head/km2 | 8.48 |
| Chicken Density (2014) | ~1 km | FAO | head/km2 | 585.49 |
| Set 5 | ||||
| Distance to Water (2014) | 250 meters | ESRI | km | 64.25 |
| Rural Poverty in SSA (2010) | ~5 km | CIESIN | person/km2 | 65.26 |
| WHO GBD Region | Total Population | % of Total | Urban (>1000) | Nonurban (<1000) |
|---|---|---|---|---|
| West | 263,954,435 | 40% | 148,452,658 | 115,475,777 |
| East | 304,529,659 | 46% | 86,753,145 | 217,725,514 |
| Central | 61,693,453 | 9% | 22,428,932 | 39,261,521 |
| South | 37,945,399 | 6% | 17,213,237 | 20,728,162 |
| Total | 668,122,946 | 274,847,972 | 393,190,974 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, M.A. Mapping the Geographic Distribution of Tungiasis in Sub-Saharan Africa. Trop. Med. Infect. Dis. 2020, 5, 122. https://doi.org/10.3390/tropicalmed5030122
Deka MA. Mapping the Geographic Distribution of Tungiasis in Sub-Saharan Africa. Tropical Medicine and Infectious Disease. 2020; 5(3):122. https://doi.org/10.3390/tropicalmed5030122
Chicago/Turabian StyleDeka, Mark A. 2020. "Mapping the Geographic Distribution of Tungiasis in Sub-Saharan Africa" Tropical Medicine and Infectious Disease 5, no. 3: 122. https://doi.org/10.3390/tropicalmed5030122
APA StyleDeka, M. A. (2020). Mapping the Geographic Distribution of Tungiasis in Sub-Saharan Africa. Tropical Medicine and Infectious Disease, 5(3), 122. https://doi.org/10.3390/tropicalmed5030122






