Evaluation of Rapid Extraction Methods Coupled with a Recombinase Polymerase Amplification Assay for Point-of-Need Diagnosis of Post-Kala-Azar Dermal Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Participants
2.2. DNA Extraction from Clinical Specimen
2.3. DNA Purity and Concentration
2.4. Molecular Detection of LD-DNA
2.5. Reagent Cost and Time Analysis
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Participants’ Indices
3.2. Extraction Method-Based Performance of RPA/qPCR Assay
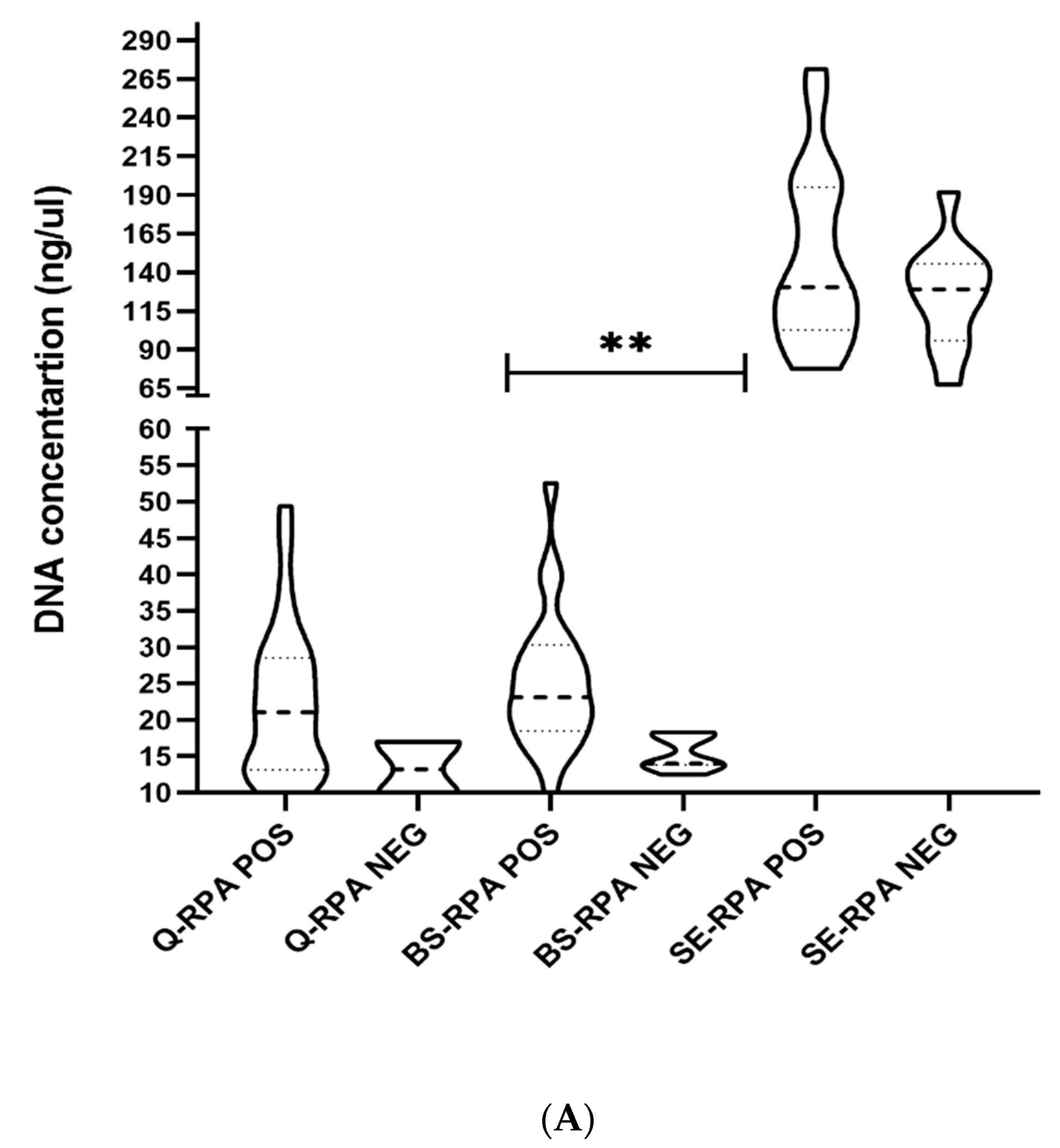
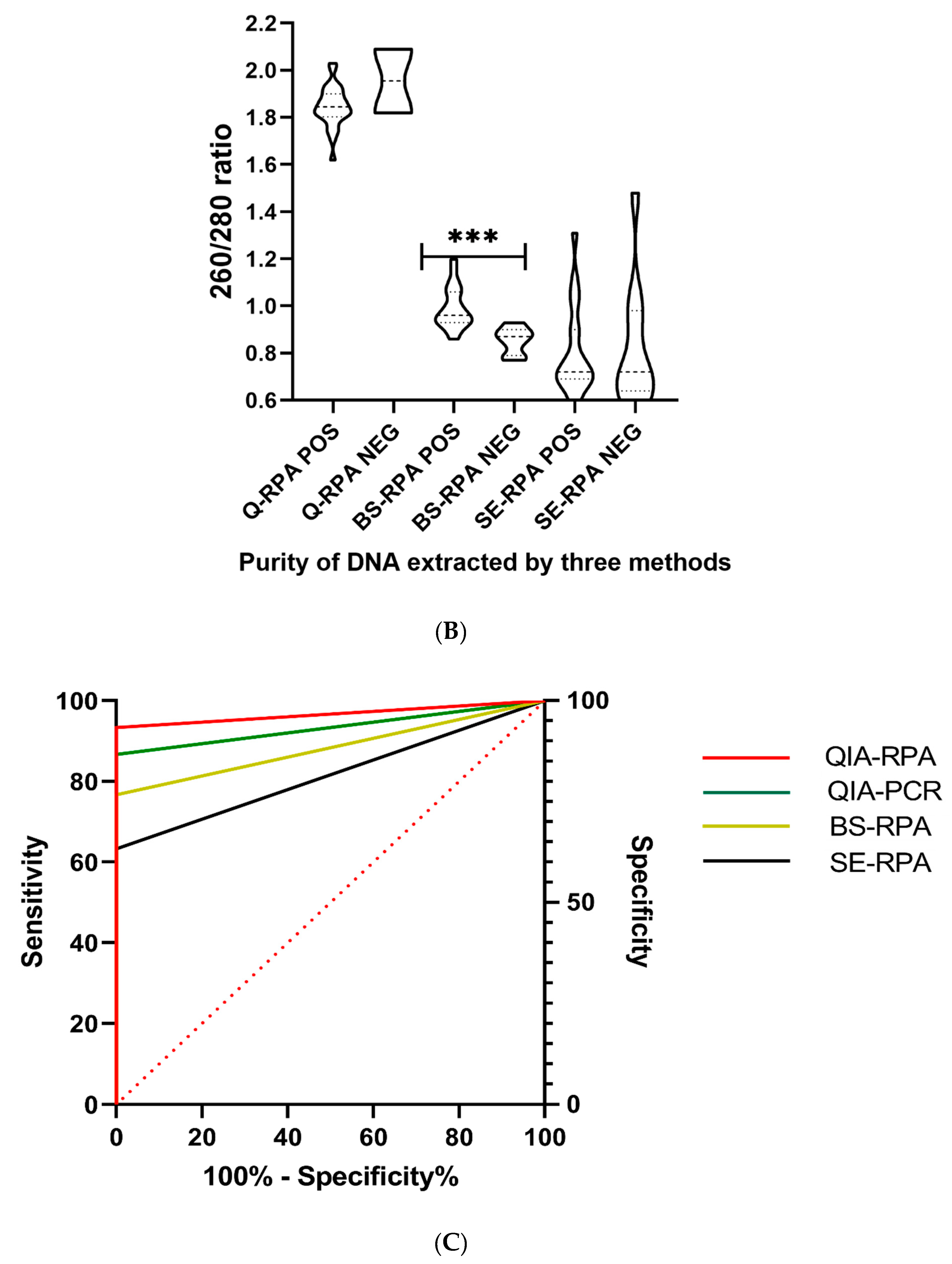
3.3. Efficiency of DNA Extraction Methods
3.4. Comparison of Assay Time and Cost-Effectiveness
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Bhandari, V.; Avishek, K.; Ramesh, V.; Salotra, P. Reliable diagnosis of post-kala-azar dermal leishmaniasis (PKDL) using slit aspirate specimen to avoid invasive sampling procedures. Trop. Med. Int. Heal. 2012, 18, 268–275. [Google Scholar] [CrossRef]
- Mondal, D.; Khan, G.M. Recent advances in post-kala-azar dermal leishmaniasis. Curr. Opin. Infect. Dis. 2011, 24, 418–422. [Google Scholar] [CrossRef]
- Zijlstra, E.; Musa, A.; Khalil, E.; El Hassan, I.; El-Hassan, A. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 2003, 3, 87–98. [Google Scholar] [CrossRef]
- Rahman, K.M.; Islam, S.; Rahman, M.W.; Kenah, E.; Galive, C.M.; Zahid, M.M.; Maguire, J.; Rahman, M.; Haque, R.; Luby, S.; et al. Increasing Incidence of Post–Kala-Azar Dermal Leishmaniasis in a Population-Based Study in Bangladesh. Clin. Infect. Dis. 2010, 50, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Saha, P.; Chatterjee, M.; Roy, S.; Ghosh, T.K.; Guha, S.K.; Kundu, P.K.; Bera, D.K.; Basu, N.; Maji, A.K. PKDL—A Silent Parasite Pool for Transmission of Leishmaniasis in Kala-azar Endemic Areas of Malda District, West Bengal, India. PLoS Neglected Trop. Dis. 2015, 9, e0004138. [Google Scholar] [CrossRef]
- Islam, S.; Kenah, E.; Bhuiyan, M.U.; Rahman, K.M.; Goodhew, B.; Ghalib, C.M.; Zahid, M.M.; Ozaki, M.; Rahman, M.W.; Haque, R.; et al. Clinical and Immunological Aspects of Post–Kala-Azar Dermal Leishmaniasis in Bangladesh. Am. J. Trop. Med. Hyg. 2013, 89, 345–353. [Google Scholar] [CrossRef]
- Mondal, D.; Hasnain, M.G.; Hossain, M.S.; Ghosh, D.; Ghosh, P.; Hossain, H.; Baker, J.; Nath, R.; Haque, R.; Matlashewski, G.; et al. Study on the safety and efficacy of miltefosine for the treatment of children and adolescents with post-kala-azar dermal leishmaniasis in Bangladesh, and an association of serum vitamin E and exposure to arsenic with post-kala-azar dermal leishmaniasis: An open clinical trial and case–control study protocol. BMJ Open 2016, 6, e010050. [Google Scholar] [CrossRef][Green Version]
- Garapati, P.; Pal, B.; Siddiqui, N.A.; Bimal, S.; Das, P.; Murti, K.; Pandey, K. Knowledge, stigma, health seeking behaviour and its determinants among patients with post kalaazar dermal leishmaniasis, Bihar, India. PLoS ONE 2018, 13, e0203407. [Google Scholar] [CrossRef]
- Molina, R.; Ghosh, D.; Carrillo, E.; Monnerat, S.; Bern, C.; Mondal, D.; Alvar, J. Infectivity of Post-Kala-azar Dermal Leishmaniasis Patients to Sand Flies: Revisiting a Proof of Concept in the Context of the Kala-azar Elimination Program in the Indian Subcontinent. Clin. Infect. Dis. 2017, 65, 150–153. [Google Scholar] [CrossRef]
- Mondal, D.; Bern, C.; Ghosh, D.; Rashid, M.; Molina, R.; Chowdhury, R.; Nath, R.; Ghosh, P.; Chapman, L.A.C.; Alim, A.; et al. Quantifying the Infectiousness of Post-Kala-Azar Dermal Leishmaniasis Toward Sand Flies. Clin. Infect. Dis. 2019, 69, 251–258. [Google Scholar] [CrossRef]
- Zijlstra, E.E.; Alves, F.; Rijal, S.; Arana, B.; Alvar, J. Post-kala-azar dermal leishmaniasis in the Indian subcontinent: A threat to the South-East Asia Region Kala-azar Elimination Programme. PLoS Neglected Trop. Dis. 2017, 11, e0005877. [Google Scholar] [CrossRef]
- Hirve, S.; Boelaert, M.; Matlashewski, G.; Mondal, D.; Arana, B.; Kroeger, A.; Olliaro, P. Transmission Dynamics of Visceral Leishmaniasis in the Indian Subcontinent—A Systematic Literature Review. PLoS Neglected Trop. Dis. 2016, 10, e0004896. [Google Scholar] [CrossRef]
- Alemayehu, B. Leishmaniasis: A Review on Parasite, Vector and Reservoir Host. Heal. Sci. J. 2017, 11, 1. [Google Scholar] [CrossRef]
- WHO. Regional Strategic Framework for Elimination of Kala-Azar from the South-East Asia Region (2011–2015); WHO Regional Office for South-East Asia: New Delhi, India, 2012; p. 24. [Google Scholar]
- Moulik, S.; Chaudhuri, S.J.; Sardar, B.; Ghosh, M.; Saha, B.; Das, N.K.; Chatterjee, M. Monitoring of Parasite Kinetics in Indian Post–Kala-azar Dermal Leishmaniasis. Clin. Infect. Dis. 2017, 66, 404–410. [Google Scholar] [CrossRef]
- Salotra, P.; Kaushal, H.; Ramesh, V. Containing Post Kala-Azar Dermal Leishmaniasis (PKDL): Pre-requisite for Sustainable Elimination of Visceral Leishmaniasis (VL) from South Asia. Kala Azar in South Asia; Springer: Cham, Switzerland, 2016; pp. 7–22. [Google Scholar]
- Adams, E.R.; Versteeg, I.; Leeflang, M. Systematic Review into Diagnostics for Post-Kala-Azar Dermal Leishmaniasis (PKDL). J. Trop. Med. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Huda, M.M.; Hirve, S.; Siddiqui, N.A.; Malaviya, P.; Banjara, M.R.; Das, P.; Kansal, S.; Gurung, C.K.; Naznin, E.; Rijal, S.; et al. Active case detection in national visceral leishmaniasis elimination programs in Bangladesh, India, and Nepal: Feasibility, performance and costs. BMC Public Heal. 2012, 12, 1001. [Google Scholar] [CrossRef]
- Basher, A.; Nath, P.; Nabi, S.G.; Selim, S.; Rahman, F.; Sutradhar, S.R.; Faiz, A.; Bhuiyan, M.R.; Ahmed, B.-N.; Rahman, R. A Study on Health Seeking Behaviors of Patients of Post-Kala-Azar Dermal Leishmaniasis. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Ghosh, P.; Hasnain, G.; Hossain, F.; Khan, A.A.; Chowdhury, R.; Faisal, K.; Mural, M.A.; Baker, J.; Nath, R.; Ghosh, D.; et al. Evaluation of Real-time PCR for Diagnosis of Post-Kala-azar Dermal Leishmaniasis in Endemic Foci of Bangladesh. Open Forum Infect. Dis. 2018, 5, ofy234. [Google Scholar] [CrossRef]
- Salotra, P.; Singh, R. Challenges in the diagnosis of post kala-azar dermal leishmaniasis. Indian J. Med Res. 2006, 123, 295. [Google Scholar]
- Verma, N.; Singh, D.; Pandey, K.; Das, V.N.R.; Lal, C.S.; Verma, R.B.; Sinha, P.K.; Das, P. Comparative Evaluation of PCR and Imprint Smear Microscopy Analyses of Skin Biopsy Specimens in Diagnosis of Macular, Papular, and Mixed Papulo-Nodular Lesions of Post-Kala-Azar Dermal Leishmaniasis. J. Clin. Microbiol. 2013, 51, 4217–4219. [Google Scholar] [CrossRef]
- Chappuis, F.; Rijal, S.; Soto, A.; Menten, J.; Boelaert, M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ 2006, 333, 723. [Google Scholar] [CrossRef]
- Hossain, F.; Ghosh, P.; Khan, A.A.; Duthie, M.S.; Vallur, A.C.; Picone, A.; Howard, R.F.; Reed, S.G.; Mondal, D. Real-time PCR in detection and quantitation of Leishmania donovani for the diagnosis of Visceral Leishmaniasis patients and the monitoring of their response to treatment. PLoS ONE 2017, 12, e0185606. [Google Scholar] [CrossRef]
- Srivastava, P.; Mehrotra, S.; Tiwary, P.; Chakravarty, J.; Sundar, S. Diagnosis of Indian Visceral Leishmaniasis by Nucleic Acid Detection Using PCR. PLoS ONE 2011, 6, e19304. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Faye, O.; Soropogui, B.; Patel, P.; El Wahed, A.A.; Loucoubar, C.; Fall, G.; Kiory, D.; Magassouba, N.; Keita, S.; et al. Development and deployment of a rapid recombinase polymerase amplification Ebola virus detection assay in Guinea in 2015. Eurosurveillance 2015, 20, 30053. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA Detection Using Recombination Proteins. PLoS Boil. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Ghosh, P.; Khan, A.A.; Hossain, F.; Böhlken-Fascher, S.; Matlashewski, G.; Kroeger, A.; Olliaro, P.; El Wahed, A.A. Mobile suitcase laboratory for rapid detection of Leishmania donovani using recombinase polymerase amplification assay. Parasites Vectors 2016, 9, 281. [Google Scholar] [CrossRef]
- Mukhtar, M.; Ali, S.S.; Boshara, S.A.; Albertini, A.; Monnerat, S.; Bessell, P.; Mori, Y.; Kubota, Y.; Ndung’U, J.M.; Cruz, I. Sensitive and less invasive confirmatory diagnosis of visceral leishmaniasis in Sudan using loop-mediated isothermal amplification (LAMP). PLoS Neglected Trop. Dis. 2018, 12, e0006264. [Google Scholar] [CrossRef]
- Hopkins, H.; González, I.J.; Polley, S.D.; Angutoko, P.; Ategeka, J.; Asiimwe, C.; Agaba, B.; Kyabayinze, D.J.; Sutherland, C.J.; Perkins, M.D.; et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: Performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J. Infect. Dis. 2013, 208, 645–652. [Google Scholar] [CrossRef]
- Desjardins, P.; Conklin, D. NanoDrop Microvolume Quantitation of Nucleic Acids. J. Vis. Exp. 2010, 45, 2565. [Google Scholar] [CrossRef]
- Vallur, A.C.; Duthie, M.S.; Reinhart, C.; Tutterrow, Y.; Hamano, S.; Bhaskar, K.; Coler, R.N.; Mondal, D.; Reed, S.G. Biomarkers for intracellular pathogens: Establishing tools as vaccine and therapeutic endpoints for visceral leishmaniasis. Clin. Microbiol. Infect. 2013, 20, O374–O383. [Google Scholar] [CrossRef]
- Burke, R.; McKenna, J.; Cox, C.; Coyle, P.V.; Shields, M.D.; Fairley, D. A comparison of different pre-lysis methods and extraction kits for recovery of Streptococcus agalacticae (Lancefield group B Streptococcus) DNA from whole blood. J. Microbiol. Methods 2016, 129, 103–108. [Google Scholar] [CrossRef]
- De Assis, T.S.M.; Azeredo-Da-Silva, A.L.F.; Werneck, G.L.; Rabello, A. Cost-effectiveness analysis of diagnostic tests for human visceral leishmaniasis in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 464–471. [Google Scholar] [CrossRef]
- Xing, W.; Yu, X.; Feng, J.; Sun, K.; Fu, W.; Wang, Y.; Zou, M.; Xia, W.; Luo, Z.; He, H.; et al. Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in Hunan province of China. BMC Infect. Dis. 2017, 17, 164. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, O.P.; Chakravarty, J. Visceral leishmaniasis elimination targets in India, strategies for preventing resurgence. Expert Rev. Anti-infective Ther. 2018, 16, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.P.; Hasker, E.; Boelaert, M.; Sundar, S. Elimination of visceral leishmaniasis on the Indian subcontinent. Lancet Infect. Dis. 2016, 16, e304–e309. [Google Scholar] [CrossRef]
- Hirve, S.; Kroeger, A.; Matlashewski, G.; Mondal, D.; Banjara, M.R.; Das, P.; Be-Nazir, A.; Arana, B.; Olliaro, P. Towards elimination of visceral leishmaniasis in the Indian subcontinent—Translating research to practice to public health. PLoS Neglected Trop. Dis. 2017, 11, e0005889. [Google Scholar] [CrossRef] [PubMed]
- Gunaratna, G.; Manamperi, A.; Böhlken-Fascher, S.; Wickremasinghe, R.; Gunawardena, K.; Yapa, B.; Pathirana, N.; Pathirana, H.; De Silva, N.R.; Sooriyaarachchi, M.; et al. Evaluation of rapid extraction and isothermal amplification techniques for the detection of Leishmania donovani DNA from skin lesions of suspected cases at the point of need in Sri Lanka. Parasites Vectors 2018, 11, 665. [Google Scholar] [CrossRef]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, O.D.; Abbasi, I.; Horwitz, B.Z.; Skrip, L.; Hailu, A.; Jaffe, C.; Lin, L.L.; Prow, T.W.; Warburg, A. Minimally invasive microbiopsies: A novel sampling method for identifying asymptomatic, potentially infectious carriers of Leishmania donovani. Int. J. Parasitol. 2017, 47, 609–616. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Majumdar, K.; Gangopadhyay, M.; Koley, S. Fine-needle sampling provides appreciable diagnostic yield in lesions of post kala azar dermal leishmaniasis: Analysis of four cases from North Eastern India. Diagn. Cytopathol. 2013, 42, 525–529. [Google Scholar] [CrossRef]
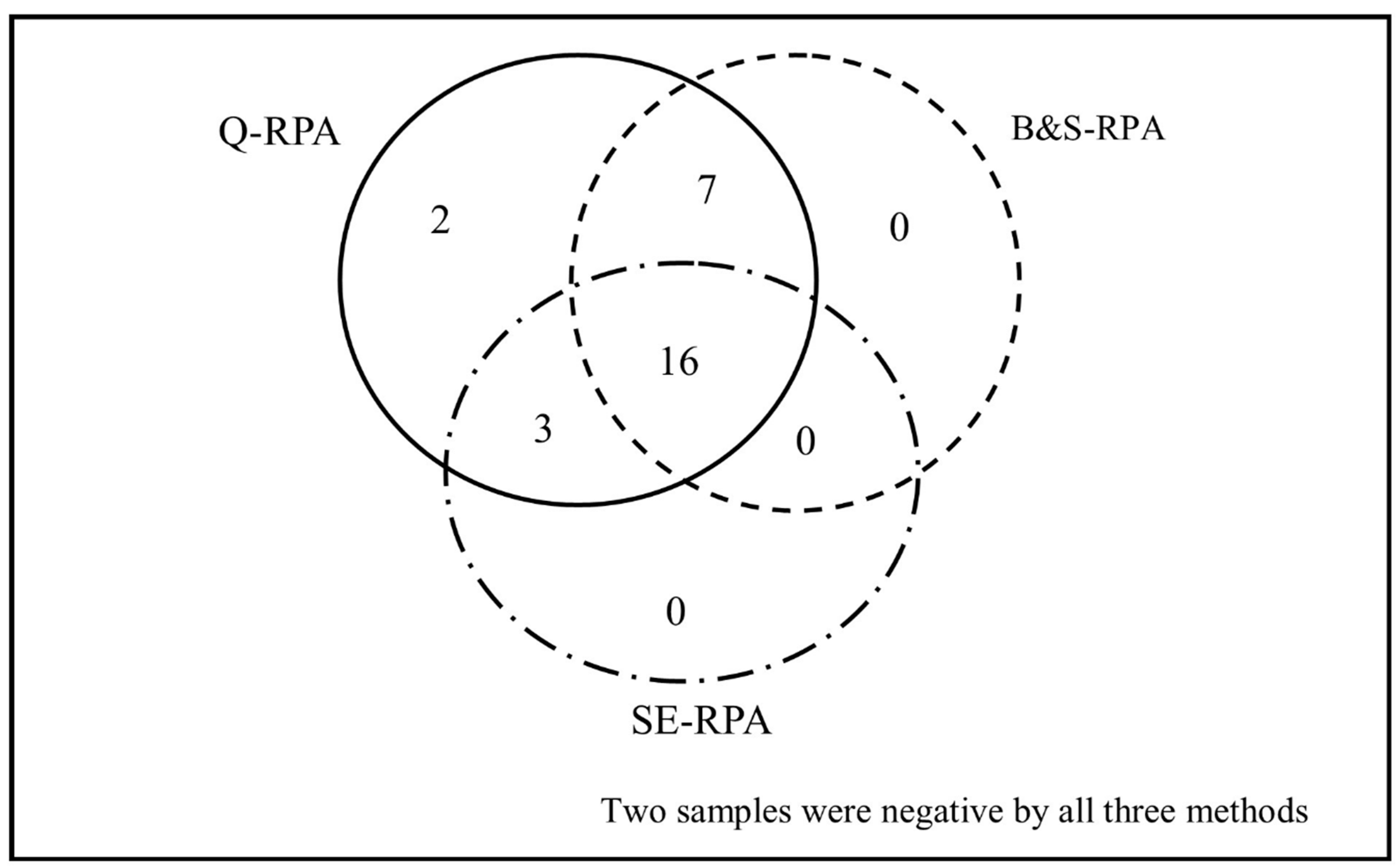
| Variable | Value | |
|---|---|---|
| Male, n (%) | 18/30 (60%) | |
| Age in years, mean ± SD | 26.47 ± 12.41 | |
| Children (<17 years), n (%) | 9/30 (30%) | |
| Adults (>17 years), n (%) | 21/30 (70%) | |
| Past history of VL, n (%) | 28/30 (93.3%) | |
| Past history of PKDL, n (%) | 7/30 (23.3%) | |
| Type of Rash, n (%) | Macular | 22/30 (73.3%) |
| Nodular | 2/30 (6.7%) | |
| Mixed | 6/30 (20%) | |
| DNA Extraction Methods | Mean DNA conc. (ng/µL) [95% CI] N = 30 | Mean OD 260/280 ratio [95% CI] N = 30 | Sensitivity of qPCR [95% CI] (Pos/Neg) | Sensitivity of RPA [95% CI] (Pos/Neg) | Specificity of Q-qPCR and RPA [95% CI] (Pos/Neg) | App. Time (per Sample) | App. Cost (in US$ per Sample) | ||
|---|---|---|---|---|---|---|---|---|---|
| qPCR | RPA | qPCR | RPA | ||||||
| Spin-column method (Qiagen) | 21.6 ± 10.4 [17.71–25.49] | 1.85 ± 0.09 [1.81–1.88] | 86.67% [69.28–96.24%] (26/4) | 93.33% [77.93–99.18%] (28/2) | 100.00% [88.43–100.00%] (0/30) | 17 h [30] | 15 h [20] min [26] | 16.5 [27] | 7.5 [26,33] |
| Boil & Spin (B&S) | 22.9 ± 9.3 [19.47–26.44] | 0.91 ± 0.11 [0.87-0.95] | N/A | 76.67% [57.72–90.07%] (23/7) | 15 h [27,30] | 13 h [27] | 14.0 [31] | 5.0 [31] | |
| SpeedXtract (SE) | 140.7 ± 50.5 [121.89–159.63] | 0.77 ± 0.22 [0.68–0.85] | N/A | 63.33% [43.86–80.07%] (19/11) | 2 h 20 min [26] | 40 min [26] | 15.5 [33] | 6.5 [26,33] | |
| Methods Comparison | Kappa (k) | Agreement | McNemar (p Value) |
|---|---|---|---|
| Q-RPA vs. B&S-RPA | 0.831 | Excellent | 0.06 |
| Q-RPA vs. SE-RPA | 0.692 | Good | 0.004 |
| B&S-RPA vs. SE-RPA | 0.635 | Good | 0.34 |
| Q-RPA vs. Q-qPCR | 0.933 | Excellent | 0.50 |
| B&S-RPA vs. Q-qPCR | 0.828 | Excellent | 0.38 |
| SE-RPA vs. Q-qPCR | 0.755 | Good | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chowdhury, R.; Ghosh, P.; Khan, M.A.A.; Hossain, F.; Faisal, K.; Nath, R.; Baker, J.; Wahed, A.A.E.; Maruf, S.; Nath, P.; et al. Evaluation of Rapid Extraction Methods Coupled with a Recombinase Polymerase Amplification Assay for Point-of-Need Diagnosis of Post-Kala-Azar Dermal Leishmaniasis. Trop. Med. Infect. Dis. 2020, 5, 95. https://doi.org/10.3390/tropicalmed5020095
Chowdhury R, Ghosh P, Khan MAA, Hossain F, Faisal K, Nath R, Baker J, Wahed AAE, Maruf S, Nath P, et al. Evaluation of Rapid Extraction Methods Coupled with a Recombinase Polymerase Amplification Assay for Point-of-Need Diagnosis of Post-Kala-Azar Dermal Leishmaniasis. Tropical Medicine and Infectious Disease. 2020; 5(2):95. https://doi.org/10.3390/tropicalmed5020095
Chicago/Turabian StyleChowdhury, Rajashree, Prakash Ghosh, Md. Anik Ashfaq Khan, Faria Hossain, Khaledul Faisal, Rupen Nath, James Baker, Ahmed Abd El Wahed, Shomik Maruf, Proggananda Nath, and et al. 2020. "Evaluation of Rapid Extraction Methods Coupled with a Recombinase Polymerase Amplification Assay for Point-of-Need Diagnosis of Post-Kala-Azar Dermal Leishmaniasis" Tropical Medicine and Infectious Disease 5, no. 2: 95. https://doi.org/10.3390/tropicalmed5020095
APA StyleChowdhury, R., Ghosh, P., Khan, M. A. A., Hossain, F., Faisal, K., Nath, R., Baker, J., Wahed, A. A. E., Maruf, S., Nath, P., Ghosh, D., Masud-Ur-Rashid, M., Utba Bin Rashid, M., Duthie, M. S., & Mondal, D. (2020). Evaluation of Rapid Extraction Methods Coupled with a Recombinase Polymerase Amplification Assay for Point-of-Need Diagnosis of Post-Kala-Azar Dermal Leishmaniasis. Tropical Medicine and Infectious Disease, 5(2), 95. https://doi.org/10.3390/tropicalmed5020095






