Further Evidence of Inadequate Quality in Lateral Flow Devices Commercially Offered for the Diagnosis of Rabies
Abstract
1. Introduction
2. Materials and Methods
2.1. Commercial LFD Test Kits for Rabies Diagnosis in Brain Material
2.2. Participating Laboratories
2.3. Sensitivity Analyses
2.4. Identification of the Binding Target of Antibodies Used in LFDs
3. Results
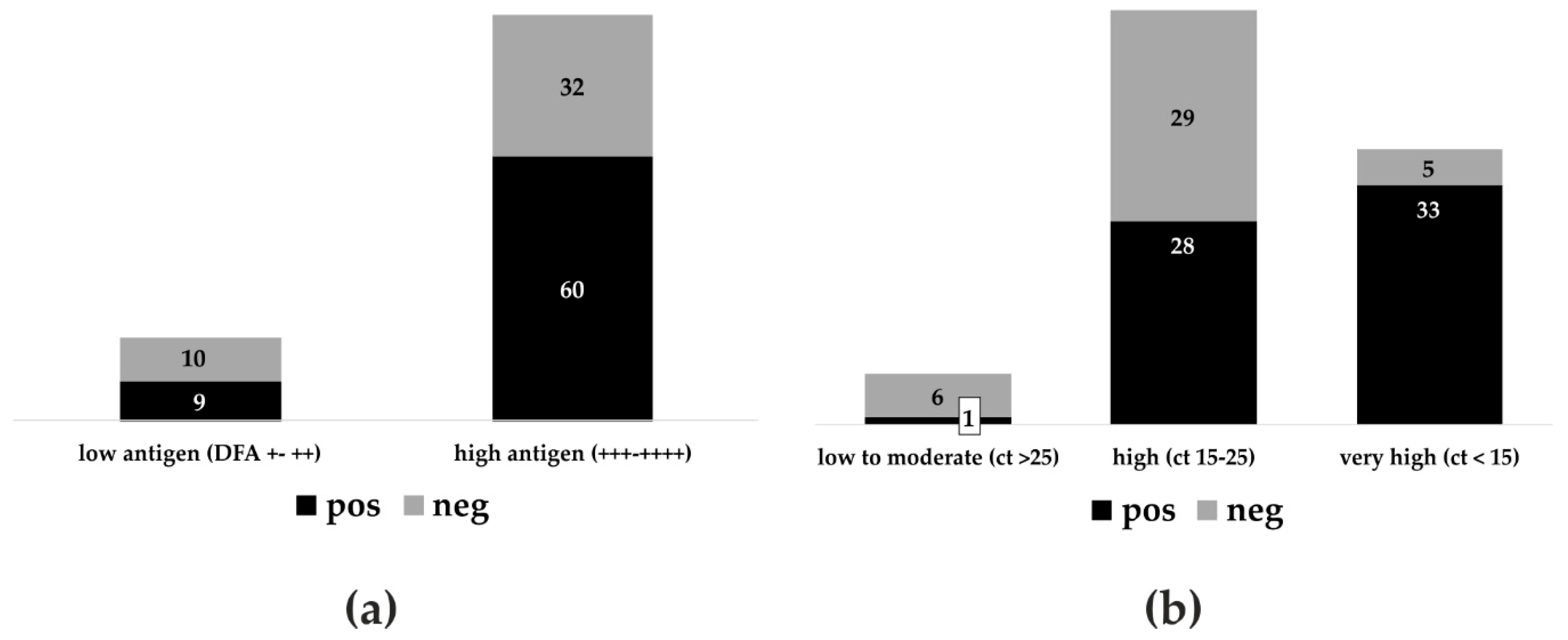
3.1. Diagnostic Sensitivity and Specificity of Five LFD Test Kits
3.2. Anigen/Bionote in Interlaboratory Comparison and Agreement with DFA and RT-qPCR
3.3. Identification of the Binding Target of Antibodies Used in LFDs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hampson, K.; Coudeville, L.; Lembo, T.; Sambo, M.; Kieffer, A.; Attlan, M.; Barrat, J.; Blanton, J.D.; Briggs, D.J.; Cleaveland, S.; et al. Estimating the global burden of endemic canine rabies. PLOS Negl. Trop. Dis. 2015, 9, e0003709. [Google Scholar] [CrossRef]
- World Health Organization. WHO expert consultation on rabies, third report. World Health Organ. Tech. Rep. Ser. 2018, 1012, 195. [Google Scholar]
- Fooks, A.R.; Cliquet, F.; Finke, S.; Freuling, C.; Hemachudha, T.; Mani, R.S.; Müller, T.; Nadin-Davis, S.; Picard-Meyer, E.; Wilde, H.; et al. Rabies. Nat. Rev. Dis. Primers. 2017, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, G.K.; Arechiga Ceballos, N.G.; Banyard, A.C.; Basler, C.F.; Bavari, S.; Bennett, A.J.; Blasdell, K.R.; Briese, T.; Bukreyev, A.; Cai, Y.; et al. Taxonomy of the order Mononegavirales: Update 2018. Arch. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Finke, S.; Conzelmann, K.K. Replication strategies of rabies virus. Virus Res. 2005, 111, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Abela-Ridder, B. Rabies elimination: Protecting vulnerable communities through their dogs-Authors‘ reply. Lancet Glob. Health 2017, 5, e142. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations; World Organisation for Animal Health. Global Alliance for Rabies Control. Zero by 30: The Global Strategic Plan to Prevent Human Deaths from Dog-Transmitted Rabies by 2030 In Executive Summary; World Organisation for Animal Health (OIE): Paris, France, 2018; Available online: http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Rabies_portal/EN_executiveSummary.pdf (accessed on 20 July 2018).
- Office International des Epizooties (OIE). Chapter 2.1.17. Rabies (Infection with Rabies virus and other Lyssaviruses). In OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; OIE: Paris, France, 2018; Available online: http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.17_RABIES.pdf (accessed on 8 June 2019).
- WHO. Chapter 11. The direct florescent antibody test (DFAT). In Laboratory Techniques in Rabies, 5th ed.; Rupprech, C.E., Fooks, A.R., Abela-Ridder, B., Eds.; World Health Organization: Geneva, Switzerland, 2018; pp. 74–84. [Google Scholar]
- Lembo, T.; Niezgoda, M.; Velasco-Villa, A.; Cleaveland, S.; Ernest, E.; Rupprecht, C.E. Evaluation of a direct, rapid immunohistochemical test for rabies diagnosis. Emerg. Infect. Dis. 2006, 12, 310–313. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chapter 12. The direct rapid immunohistochemistry test (DRIT) for the detection of lyssavirus antigens. In Laboratory Techniques in Rabies, 5th ed.; Rupprech, C.E., Fooks, A.R., Abela-Ridder, B., Eds.; World Health Organization: Geneva, Switzerland, 2018; pp. 130–135. [Google Scholar]
- World Health Organization. Chapter 9. Virus isolation in cell culture: The rabies tissue culture infection test (RTCIT). In Laboratory Techniques in Rabies, 5th ed.; Rupprech, C.E., Fooks, A.R., Abela-Ridder, B., Eds.; World Health Organization: Geneva, Switzerland, 2018; pp. 85–95. [Google Scholar]
- World Health Organization. Chapter 8. Virus isolation in animals: The mouse inoculation test (MIT). In Laboratory Techniques in Rabies, 5th ed.; Rupprech, C.E., Fooks, A.R., Abela-Ridder, B., Eds.; World Health Organization: Geneva, Switzerland, 2018; pp. 74–84. [Google Scholar]
- Banyard, A.C.; Horton, D.; Freuling, C.; Müller, T.; Fooks, A.R. Control and prevention of canine rabies: The need for building laboratory-based surveillance capacity. Antivir. Res. 2013, 98, 357–364. [Google Scholar] [CrossRef]
- Cleaveland, S.; Beyer, H.; Hampson, K.; Haydon, D.; Lankester, F.; Lembo, T.; Meslin, F.X.; Morters, M.; Mtema, Z.; Sambo, M.; et al. The changing landscape of rabies epidemiology and control. Onderstepoort J. Vet. Res. 2014, 81, E1–E8. [Google Scholar] [CrossRef]
- Bourhy, H.; Dautry-Varsat, A.; Hotez, P.J.; Salomon, J. Rabies, Still Neglected after 125 Years of Vaccination. PLOS Negl. Trop. Dis. 2010, 4, e839. [Google Scholar] [CrossRef]
- O’Farrell, B. Lateral Flow Technology for Field-Based Applications-Basics and Advanced Developments. Top. Companion Anim. Med. 2015, 30, 139–147. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Chapter 17. Rapid immunochromatographic tests for the detection of rabies virus antigens in brain material. In Laboratory Techniques in Rabies, 5th ed.; Rupprech, C.E., Fooks, A.R., Abela-Ridder, B., Eds.; World Health Organization: Geneva, Switzerland, 2018; pp. 176–182. [Google Scholar]
- Léchenne, M.; Naïssengar, K.; Lepelletier, A.; Alfaroukh, I.O.; Bourhy, H.; Zinsstag, J.; Dacheux, L. Validation of a Rapid Rabies Diagnostic Tool for Field Surveillance in Developing Countries. PLOS Negl. Trop. Dis. 2016, 10, e0005010. [Google Scholar] [CrossRef] [PubMed]
- Servat, A.; Robardet, E.; Cliquet, F. An inter-laboratory comparison to evaluate the technical performance of rabies diagnosis lateral flow assays. J. Virol. Methods 2019, 272, 113702. [Google Scholar] [CrossRef] [PubMed]
- Markotter, W.; York, D.; Sabeta, C.T.; Shumba, W.; Zulu, G.; Roux Le, K.; Nel, L.H. Evaluation of a rapid immunodiagnostic test kit for detection of African lyssaviruses from brain material. Onderstepoort J. Vet. Res. 2009, 76, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Reta, T.; Teshale, S.; Deresa, A.; Ali, A.; Getahun, G.; Baumann, M.P.O.; Müller, T.; Freuling, C.M. Evaluation of Rapid Immunodiagnostic Test for Rabies Diagnosis Using Clinical Brain Samples in Ethiopia. J. Vet. Sci. Med. Diagn. 2013, 2. [Google Scholar] [CrossRef]
- Servat, A.; Picard-Meyer, E.; Robardet, E.; Muzniece, Z.; Must, K.; Cliquet, F. Evaluation of a Rapid Immunochromatographic Diagnostic Test for the detection of rabies from brain material of European mammals. Biologicals 2012, 40, 61–66. [Google Scholar] [CrossRef]
- Voehl, K.M.; Saturday, G.A. Evaluation of a rapid immunodiagnostic rabies field surveillance test on samples collected from military operations in Africa, Europe, and the Middle East. US Army Med. Dep. J. 2014, 27–32. [Google Scholar]
- Pranoti, S.; Singh, C.K.; Deepti, N. Comparison of immunochromatographic diagnostic test with heminested reverse transcriptase polymerase chain reaction for detection of rabies virus from brain samples of various species. Vet. World 2015, 8, 135–138. [Google Scholar] [CrossRef]
- Yang, D.K.; Shin, E.K.; Oh, Y.I.; Lee, K.W.; Lee, C.S.; Kim, S.Y.; Lee, J.A.; Song, J.Y. Comparison of four diagnostic methods for detecting rabies viruses circulating in Korea. J. Vet. Sci. 2012, 13, 43–48. [Google Scholar] [CrossRef]
- Certoma, A.; Lunt, R.A.; Vosloo, W.; Smith, I.; Colling, A.; Williams, D.T.; Tran, T.; Blacksell, S.D. Assessment of a Rabies Virus Rapid Diagnostic Test for the Detection of Australian Bat Lyssavirus. Trop. Med. Infect. Dis. 2018, 3, 109. [Google Scholar] [CrossRef]
- Kang, B.; Oh, J.; Lee, C.; Park, B.K.; Park, Y.; Hong, K.; Lee, K.; Cho, B.; Song, D. Evaluation of a rapid immunodiagnostic test kit for rabies virus. J. Virol. Methods 2007, 145, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Nishizono, A.; Khawplod, P.; Ahmed, K.; Goto, K.; Shiota, S.; Mifune, K.; Yasui, T.; Takayama, K.; Kobayashi, Y.; Mannen, K.; et al. A simple and rapid immunochromatographic test kit for rabies diagnosis. Microbiol. Immunol. 2008, 52, 243–249. [Google Scholar] [CrossRef]
- Eggerbauer, E.; de Benedictis, P.; Hoffmann, B.; Mettenleiter, T.C.; Schlottau, K.; Ngoepe, E.C.; Sabeta, C.T.; Freuling, C.M.; Müller, T. Evaluation of Six Commercially Available Rapid Immunochromatographic Tests for the Diagnosis of Rabies in Brain Material. PLOS Negl. Trop. Dis. 2016, 10, e0004776. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.G.; Morgan, A.P. Licensing veterinary diagnostic test kits in the United States. Clin. Immunol. Newslett. 1993, 13, 142–145. [Google Scholar] [CrossRef]
- Anonym. Animal Health Act (TierGesG). In Federal Law Gazette; Bundesanzeiger Verlagsgesellschaft mbH: Berlin, Germany, 2013; p. 1324. [Google Scholar]
- Clopper, C.J.; Pearson, E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Conzelmann, K.H.; Cox, J.H.; Schneider, L.G.; Thiel, H.J. Molecular Cloning and Complete Nucleotide Sequence of the Attenuated Rabies Virus SAD B19. Virology 1990, 175, 485–499. [Google Scholar] [CrossRef]
- Finke, S.; Granzow, H.; Hurst, J.; Pollin, R.; Mettenleiter, T.C. Intergenotypic replacement of lyssavirus matrix proteins demonstrates the role of lyssavirus M proteins in intracellular virus accumulation. J. Virol. 2010, 84, 1816–1827. [Google Scholar] [CrossRef]
- Fischer, S.; Freuling, C.M.; Muller, T.; Pfaff, F.; Bodenhofer, U.; Hoper, D.; Fischer, M.; Marston, D.A.; Fooks, A.R.; Mettenleiter, T.C.; et al. Defining objective clusters for rabies virus sequences using affinity propagation clustering. PLOS Negl. Trop. Dis. 2018, 12, e0006182. [Google Scholar] [CrossRef]
- Greiner, M.; Gardner, I.A. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev. Vet. Med. 2000, 45, 3–22. [Google Scholar] [CrossRef]
- Office International des Epizooties (OIE). Chapter 1.1.6 Principles and methods of validation of diagnostic assays for infectious diseases. In OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 8th ed.; OIE: Paris, France, 2018; pp. 1–16. [Google Scholar]
- Hanlon, C. Rabies in Terrestrial Animals. In Rabies: Scientific Basis of the Disease and Its Management, 3rd ed.; Jackson, A.C., Ed.; Academic Press: New York, NY, USA, 2013; Volume 3, pp. 179–213. [Google Scholar]

| Manufacturer | RABV Pos | RABV Neg | Sensitivity | 95% CI | ||
|---|---|---|---|---|---|---|
| LFD Pos | LFD Neg | LFD Pos | LFD Neg | |||
| Span Biotec (China) | 0 | 105 | 0 | 12 | 0% | 0% to 3% |
| Lillidale (UK) | 1 | 103 | 0 | 12 | 1% | 0.2% to 5% |
| Intermedical (Italy) | 2 | 66 | 0 | 11 | 3% | 0.3% to 10% |
| Elabscience (China/USA) | 19 | 74 | 0 | 12 | 20% | 12.8% to 30.1% |
| Anigen/Bionote (Korea) | 69 | 43 | 0 | 16 | 62% | 51.9% to 70.6% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, A.; Fahrion, A.; Finke, S.; Eyngor, M.; Novak, S.; Yakobson, B.; Ngoepe, E.; Phahladira, B.; Sabeta, C.; De Benedictis, P.; et al. Further Evidence of Inadequate Quality in Lateral Flow Devices Commercially Offered for the Diagnosis of Rabies. Trop. Med. Infect. Dis. 2020, 5, 13. https://doi.org/10.3390/tropicalmed5010013
Klein A, Fahrion A, Finke S, Eyngor M, Novak S, Yakobson B, Ngoepe E, Phahladira B, Sabeta C, De Benedictis P, et al. Further Evidence of Inadequate Quality in Lateral Flow Devices Commercially Offered for the Diagnosis of Rabies. Tropical Medicine and Infectious Disease. 2020; 5(1):13. https://doi.org/10.3390/tropicalmed5010013
Chicago/Turabian StyleKlein, Antonia, Anna Fahrion, Stefan Finke, Marina Eyngor, Shiri Novak, Boris Yakobson, Ernest Ngoepe, Baby Phahladira, Claude Sabeta, Paola De Benedictis, and et al. 2020. "Further Evidence of Inadequate Quality in Lateral Flow Devices Commercially Offered for the Diagnosis of Rabies" Tropical Medicine and Infectious Disease 5, no. 1: 13. https://doi.org/10.3390/tropicalmed5010013
APA StyleKlein, A., Fahrion, A., Finke, S., Eyngor, M., Novak, S., Yakobson, B., Ngoepe, E., Phahladira, B., Sabeta, C., De Benedictis, P., Gourlaouen, M., Orciari, L. A., Yager, P. A., Gigante, C. M., Knowles, M. K., Fehlner-Gardiner, C., Servat, A., Cliquet, F., Marston, D., ... Freuling, C. M. (2020). Further Evidence of Inadequate Quality in Lateral Flow Devices Commercially Offered for the Diagnosis of Rabies. Tropical Medicine and Infectious Disease, 5(1), 13. https://doi.org/10.3390/tropicalmed5010013






