(+)-Spectaline and Iso-6-Spectaline Induce a Possible Cross-Talk between Autophagy and Apoptosis in Trypanosoma brucei rhodesiense
Abstract
1. Introduction
2. Materials and Methods
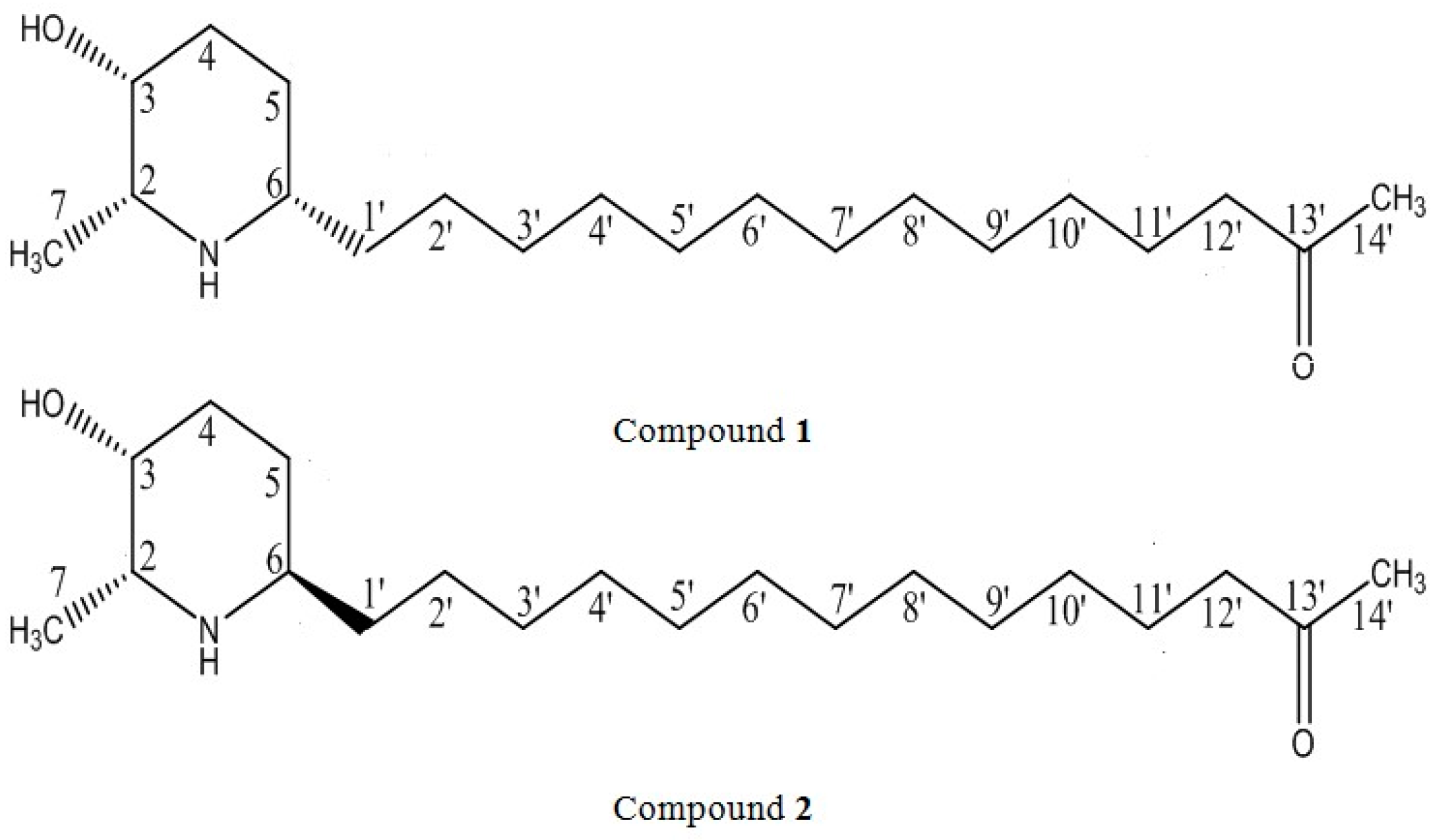
2.1. General Experimental Procedures
2.2. Measurement of Mitochondrial Membrane Potential
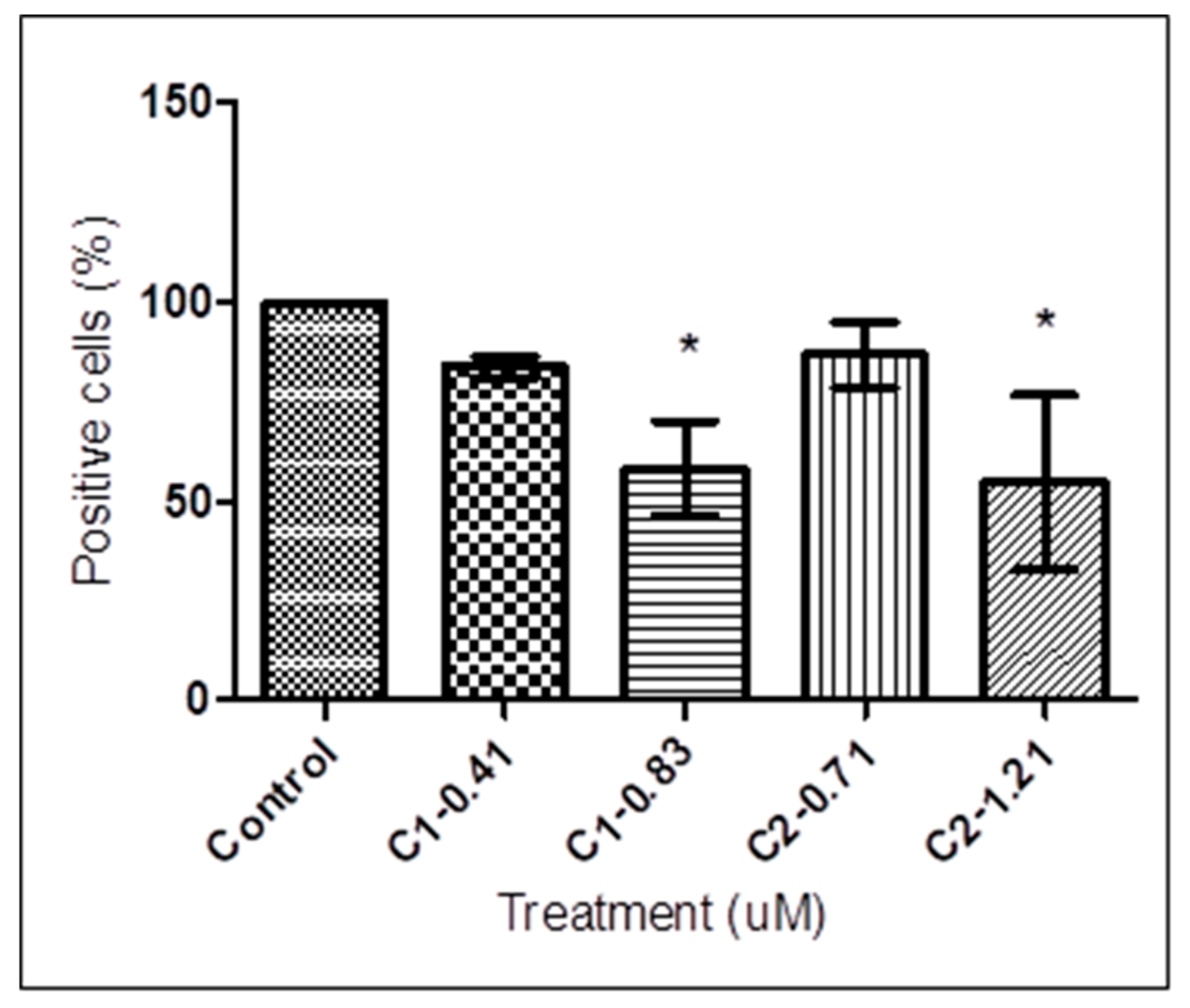
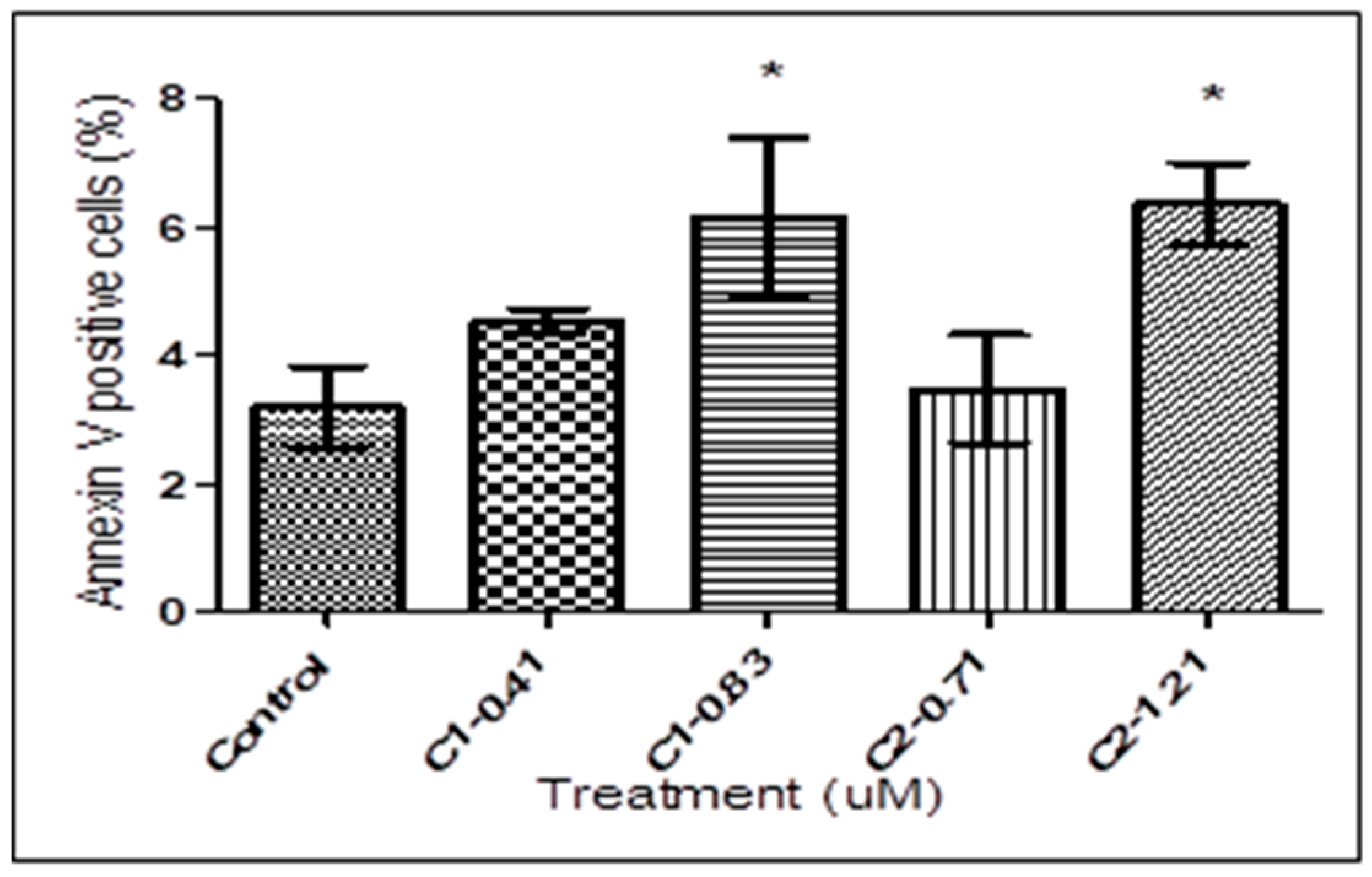
2.3. Detection of PS Exposure
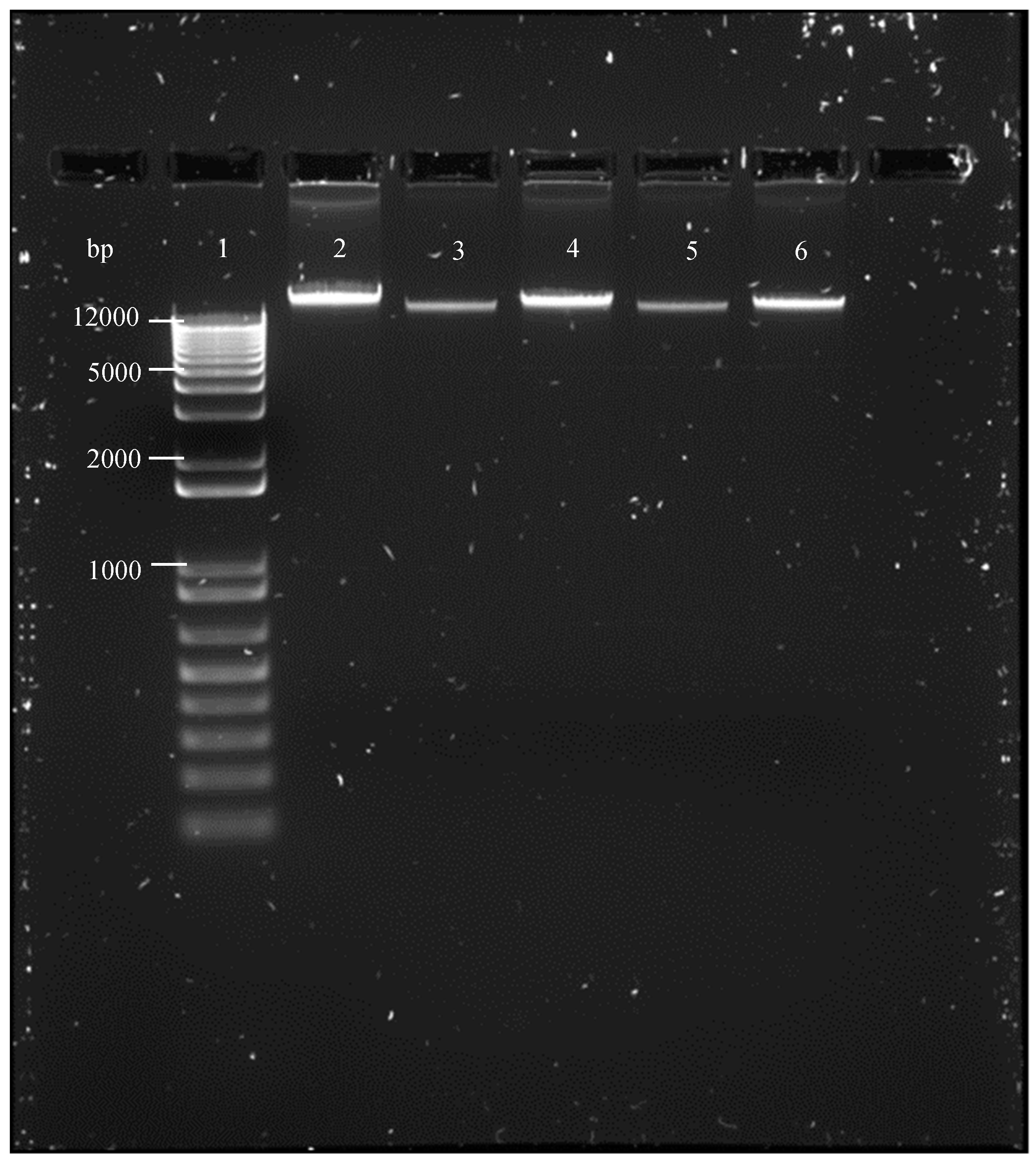
2.4. DNA Fragmentation Analysis
2.5. Determination of Caspase-Like Protease Activity
2.6. Autophagy Assay
3. Results and Discussion
3.1. Measurement of Mitochondrial Membrane Potential
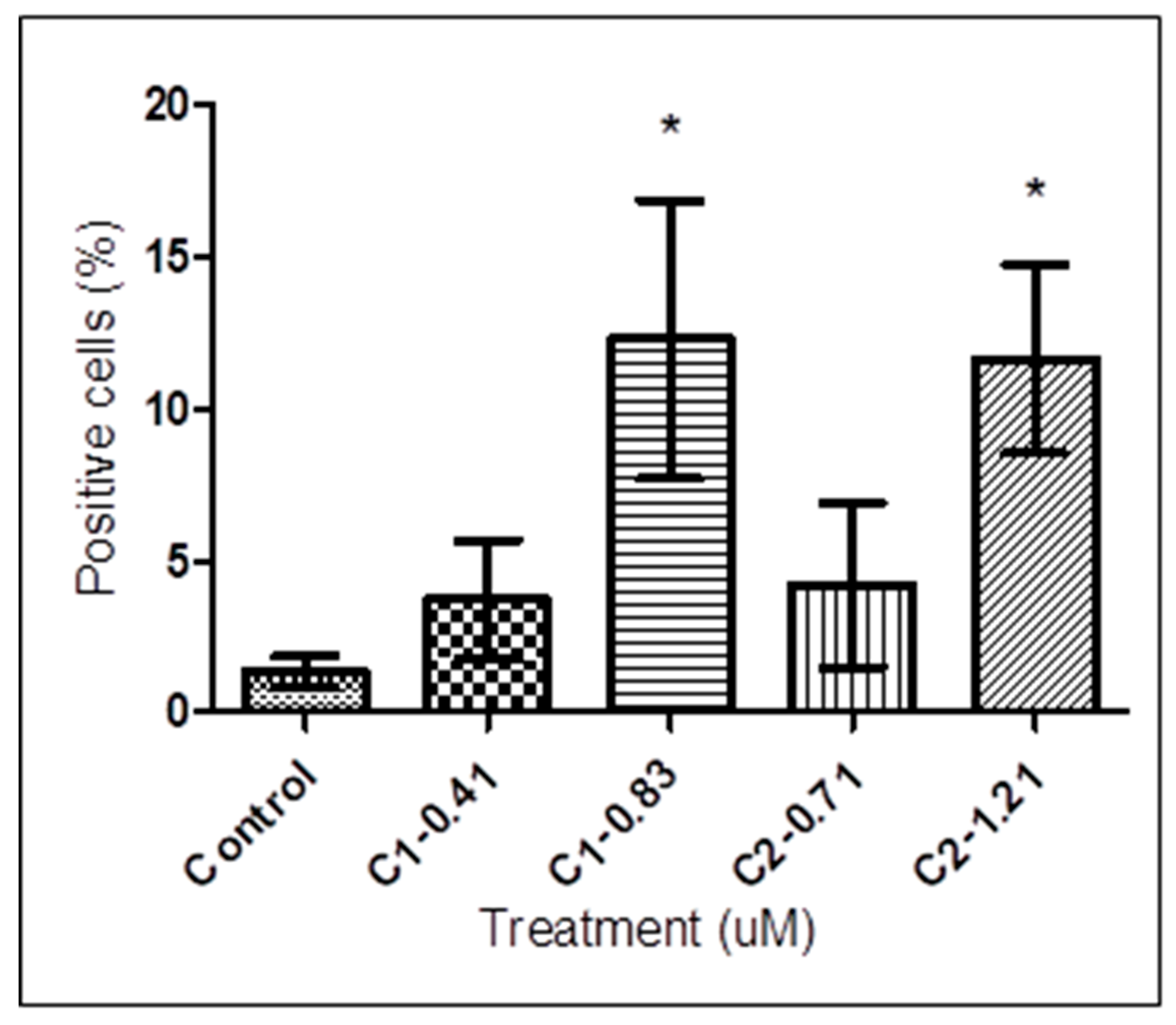
3.2. Detection of PS Exposure
3.3. DNA Fragmentation Analysis
3.4. Determination of Caspase-Like Protease Activity
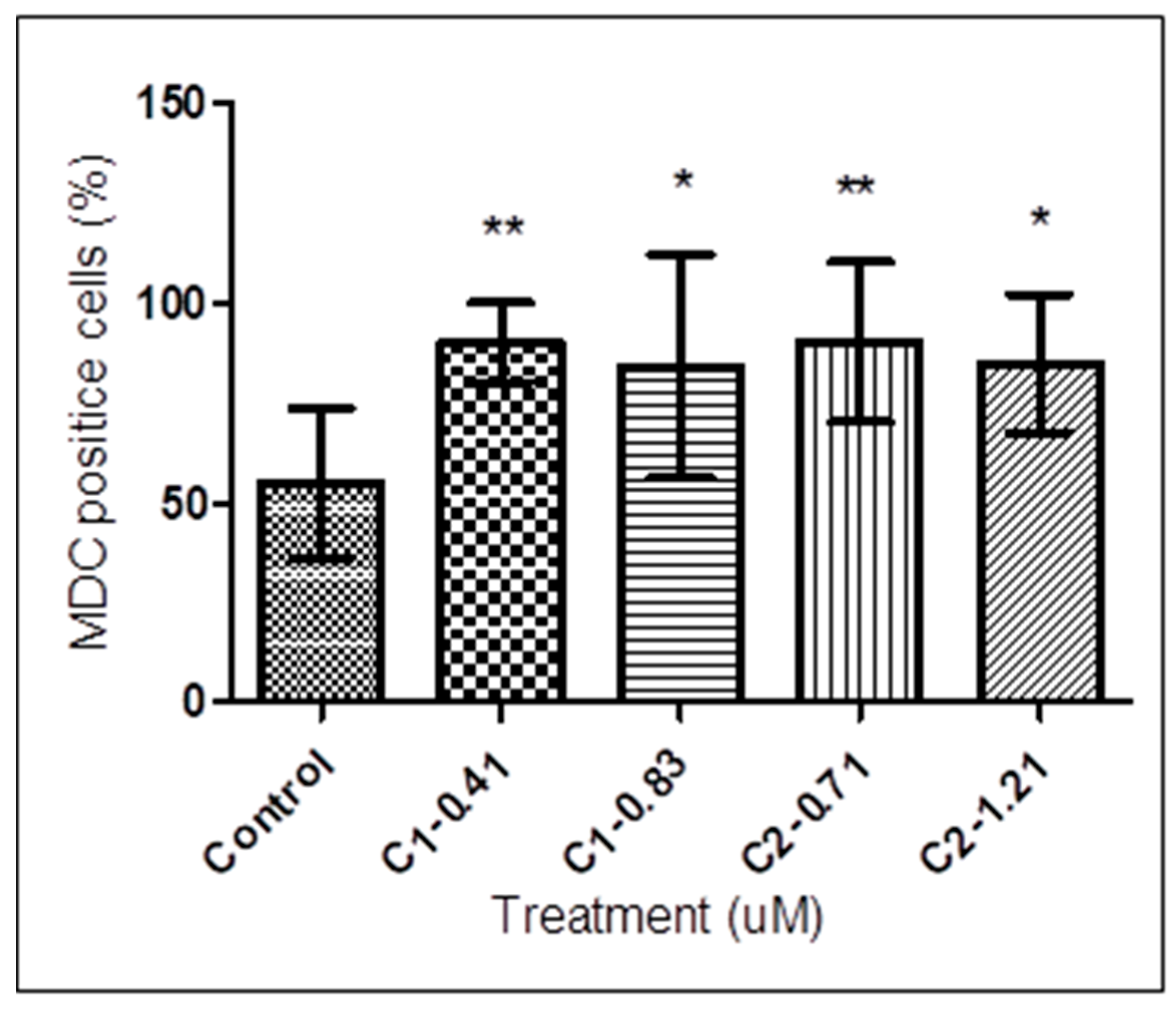
3.5. Autophagy Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.A.; Sexton, D.W.; Steverding, D. Bitter melon extract inhibits proliferation of Trypanosoma brucei bloodstream forms in vitro. Exp. Parasitol. 2013, 133, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Steverding, D. The development of drugs for treatment of sleeping sickness: A historical review. Parasit. Vectors 2010, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J. Chemotherapy of human African trypanosomiasis. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, S.; Efferth, T. Development of drug resistance in Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense. Treatment of human African trypanosomiasis with natural products (Review). Int. J. Mol. Med. 2008, 22, 411–419. [Google Scholar] [PubMed]
- Martyn, D.C.; Jones, D.C.; Fairlamb, A.H.; Clardy, J. High-throughput screening affords novel and selective trypanothione reductase inhibitors with anti-trypanosomal activity. Bioorg. Med. Chem. Lett. 2007, 17, 1280–1283. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.T.; Amanah, A.; Chear, N.J.; Zahari, Z.; Zainuddin, Z.; Adenan, M.I. Inhibitory effects of (+)-spectaline and iso-6-spectaline from Senna spectabilis on the growth and ultrastructure of human-infective species Trypanosoma brucei rhodesiense bloodstream form. Exp. Parasitol. 2018, 184, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, V.; Paredes, R.; Sosa, M.A.; Galanti, N. Natural programmed cell death in T. cruzi epimastigotes maintained in axenic cultures. J. Cell Biochem. 2008, 105, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, V.; Wink, M. Alkaloids Induce Programmed Cell Death in Bloodstream Forms of Trypanosomes (Trypanosoma b. brucei). Molecules 2008, 13, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Helms, M.J.; Ambit, A.; Appleton, P.; Tetley, L.; Coombs, G.H.; Mottram, J.C. Bloodstream form Trypanosoma brucei depend upon multiple metacaspases associated with RAB11-positive endosomes. J. Cell Sci. 2006, 119, 1105–1117. [Google Scholar] [CrossRef][Green Version]
- Casanova, M.; Gonzalez, I.J.; Sprissler, C.; Zalila, H.; Dacher, M.; Basmaciyan, L.; Späth, G.F.; Azas, N.; Fasel, N. Implication of different domains of the Leishmania major metacaspase in cell death and autophagy. Cell Death Dis. 2015, 6, e1933. [Google Scholar] [CrossRef]
- Menna-Barreto, R.F.S.; Salomão, K.; Dantas, A.P.; Santa-Rita, R.M.; Soares, M.J.; Barbosa, H.S.; de Castro, S.L. Different cell death pathways induced by drugs in Trypanosoma cruzi: An ultrastructural study. Micron 2009, 40, 157–168. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.T.; Yeoh, C.Y.; Zainuddin, Z.; Ilham Adenan, M. (+)-Spectaline and Iso-6-Spectaline Induce a Possible Cross-Talk between Autophagy and Apoptosis in Trypanosoma brucei rhodesiense. Trop. Med. Infect. Dis. 2019, 4, 98. https://doi.org/10.3390/tropicalmed4030098
Lim KT, Yeoh CY, Zainuddin Z, Ilham Adenan M. (+)-Spectaline and Iso-6-Spectaline Induce a Possible Cross-Talk between Autophagy and Apoptosis in Trypanosoma brucei rhodesiense. Tropical Medicine and Infectious Disease. 2019; 4(3):98. https://doi.org/10.3390/tropicalmed4030098
Chicago/Turabian StyleLim, Kah Tee, Chiann Ying Yeoh, Zafarina Zainuddin, and Mohd. Ilham Adenan. 2019. "(+)-Spectaline and Iso-6-Spectaline Induce a Possible Cross-Talk between Autophagy and Apoptosis in Trypanosoma brucei rhodesiense" Tropical Medicine and Infectious Disease 4, no. 3: 98. https://doi.org/10.3390/tropicalmed4030098
APA StyleLim, K. T., Yeoh, C. Y., Zainuddin, Z., & Ilham Adenan, M. (2019). (+)-Spectaline and Iso-6-Spectaline Induce a Possible Cross-Talk between Autophagy and Apoptosis in Trypanosoma brucei rhodesiense. Tropical Medicine and Infectious Disease, 4(3), 98. https://doi.org/10.3390/tropicalmed4030098



