Helminthiasis among School-Age Children and Hygiene Conditions of Selected Schools in Lafia, Nasarawa State, Nigeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Ethical Approval
2.3. Selection of Schools
2.4. Surveys and Sample Collection
2.4.1. Collection and Examination of Stools Sample for Presence of STH
2.4.2. Collection of Soil Samples in Selected Locations around the School Environment
2.4.3. Questionnaire Administration
2.5. Statistical Analysis
3. Results
Summary of Infection Patterns
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Utzinger, J.; Becker, S.L.; Knopp, S.; Blum, J.; Neumayr, A.L.; Keiser, J.; Hatz, C.F. Neglected tropical diseases: Diagnosis, clinical management, treatment and control. Swiss Med Wkly. 2012, 142, 13727. [Google Scholar] [CrossRef] [PubMed]
- Pullan, R.L.; Smith, J.L.; Jasrasaria, R.; Brooker, S.J. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites Vectors 2014, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Concha, R.; Harrington, W., Jr.; Rogers, A.I. Intestinal strongyloidiasis: Recognition, management, and determinants of outcome. J. Clin. Gastroenterol. 2005, 39, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Montresor, A.; Crompton, D.W.T.; Hall, A.; Bundy, D.A.P.; Savioli, L. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level. A Guide for Managers of Control Programmes; World Health Organization: Geneva, Switzerland, 1998; pp. 1–45. [Google Scholar]
- Albonico, M.; Crompton, D.W.; Savioli, L. Control strategies for human intestinal nematode infections. Adv. Parasitol. 1999, 42, 277–341. [Google Scholar]
- WHO. A Roadmap for Implementation: Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases; World Health Organization: Geneva, Switzerland, 2012; Available online: http://www.who.int/neglected_diseases/NTD_RoadMap_2012_Fullversion.pdf http://apps.who.int/iris/bitstream/10665/70809/1/WHO_HTM_NTD_2012.1_eng.pdf (accessed on 11 September 2017).
- WHO. Water Sanitation and Hygiene for Accelerating and Sustaining Progress on Neglected Tropical Diseases a Global Strategy 2015–2020; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Tosson, M.; Farrang, A.M.; Sabry, A.H.; Salama, M.M.; Arafa, M.A. Ectoparasites and endoparasites in two primary schools in Qualyob city, Egypt. J. Egypt Soc. Parasitol. 1991, 21, 391–401. [Google Scholar] [PubMed]
- Savioli, L.; Bundy, D.; Tomkins, A. Intestinal parasitic infections: A soluble public health problem. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 353–354. [Google Scholar] [CrossRef]
- Oluwole, A.S.; Ekpo, U.F.; Karagiannis-Voules, D.; Abe, E.M.; Olamiju, F.O.; Isiyaku, S.; Okoronkwo, C.; Saka, Y.; Nebe, O.J.; Braide, E.I.; et al. Bayesian geostatistical model-based estimates of soil-transmitted helminth infection in Nigeria, including annual deworming requirements. PLoS Negl. Trop. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Wagbasoma, V.A.; Aisien, M.S.O. Helminthiasis in selected children seen at the University of Benin Teaching hospital, Benin City. Nig. Postgrad. Med. J. 2005, 12, 25–27. [Google Scholar]
- Escobendo, A.A.; Canete, R.; Nunezi, F.A. Prevalence, risk and clinical features associated with intestinal parasitic infection in children from San Juan Martinez, Pinar de Rio. Cuba. West Indian Med. J. 2008, 57, 378–382. [Google Scholar]
- Eke, S.S.; Omalu, I.C.J.; Otuu, C.A.; Salihu, I.M.; Udeogu, V.O.; Hassan, S.C.; Idris, A.R.; Abubakar, N.E.; Auta, Y.I. Prevalence of geohelminth in soil and primary school children in Panda Development Area, Karu Local Government Area, Nasarawa State, Nigeria. Niger. J. Parasitol. 2015, 36, 91–95. [Google Scholar]
- Eke, S.S.; Omalu, I.C.J.; Otuu, C.A.; Hassan, S.C.; Ibrahim, S.; Boyi, A.A. Hookworm infection among humans in Panda, Panda development area, Karu LGA of Nasarawa state, Nigeria. Int. J. Appl. Biol. Res. 2014, 6, 66–73. [Google Scholar]
- Bundy, D.A.P.; Guyatt, H.L. Schools for health: Focus on health, education, and the school age-child. Parasitol. Today 1996, 12, 1–14. [Google Scholar] [CrossRef]
- WHO. Prevention and Control of Schistosomiasis and Soil Transmitted Helminthiasis; WHO Technical Report Services No 912; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- WHO. Bench Aids for Diagnosis of Intestinal Parasites; World Health Organization: Geneva, Switzerland, 1994. [Google Scholar]
- Chiodini, P.L.; Moody, A.H.; Manser, D.W. Atlas of Medical Helminthology and Protozoology, 4th ed.; Churchill Livingstone: London, UK, 2001; pp. 4–10. [Google Scholar]
- Aniwada, E.C.; Uleanya, N.D.; Igbokwe, L.N.; Onwasigwe, C. Soil Transmitted Helminths; Prevalence, Perception and Determinants among Primary School Children in Rural Enugu State, Nigeria. Int. J. Trop. Dis. Health 2016, 15, 1–12. Available online: www.sciencedomain.org (accessed on 16 May 2016). [CrossRef]
- Pullan, R.L.; Gething, P.W.; Smith, J.L.; Mwandawiro, C.S.; Sturrock, H.J.W.; Gitonga, C.W.; Hay, S.I.; Brooker, S. Spatial modelling of soiltransmitted helminth infections in Kenya: A disease control planning tool. PLoS Negl. Trop. Dis. 2011, 5, e958. [Google Scholar] [CrossRef] [PubMed]
- Chammartin, F.; Scholte, R.G.C.; Malone, J.B.; Bavia, M.E.; Nieto, P.; Utzinger, J.; Vounatsou, P. Modelling the geographical distribution of soil transmitted helminth infections in Bolivia. Parasites Vectors 2013, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Zhou, X.N.; Utzinger, J.; Vounatsou, P. Bayesian geostatistical modelling of soil-transmitted helminth survey data in the People’s Republic of China. Parasites Vectors 2013, 6, 359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schule, S.A.; Clowes, P.; Kroidl, I.; Kowuor, D.O.; Nsojo, A.; Mangu, C.; Riess, H.; Geldmacher, C.; Laubender, R.P.; Mhina, S.; et al. Ascaris lumbricoides infection and Its relation to environmental factors in the Mbeya region of Tanzania, a cross-sectional, population-based study. PLoS ONE 2014, 9, e92032. [Google Scholar] [CrossRef] [PubMed]
- Nduka, F.O.; Nwaugo, V.O.; Nwachukwu, N.C. Intestinal parasite infections in Ishiagu, Abia State. Anim. Res. Int. 2006, 3, 505–507. [Google Scholar]
- Modjarrad, K.; Zulu, I.; Redden, D.T.; Njobvu, L.; Freedman, D.O.; Vermund, S.H. Prevalence and Predictors of intestinal helminth infections among human immunodeficiency virus type-1 infected adults in an urban African setting. Am. J. Trop. Med. Hyg. 2005, 73, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Nmorsi, O.P.G.; Isaac, C.; Aashikpelokhai, I.S.; Ukwandu, N.C.D. Geohelminthiasis among Nigerian preschool age children. Int. J. Med. Med. Sci. 2009, 1, 407–411. [Google Scholar]
- Oluwole, A.S.; Adeniran, A.A.; Mogaji, H.O.; Olabinke, D.B.; Abe, E.M.; Bankole, S.O.; Sam-Wobo, S.O.; Ekpo, U.F. Prevalence, intensity and spatial co-distribution of schistosomiasis and soil transmitted helminths infections in Ogun state, Nigeria. Parasitol. Open 2018, 4, e8. [Google Scholar] [CrossRef]
- Salawu, S.A.; Ughele, V.A. Prevalence of soil-transmitted helminths among school-age children in Ife East Local Government Area, Osun State, Nigeria. FUTA J. Res. Sci. 2013, 11, 139–151. [Google Scholar]
- Adedayo, O.; Akinlabi, A. Intestinal parasitic infections among primary school children in a rural community South-West Nigeria. Nig. J. Parasitol. 2002, 23, 11–18. [Google Scholar]
- Uneke, C.J.; Nnachi, M.I.; Arua, U. Assessment of polyparasitism with intestinal parasite infections and urinary Schistosomiasis among school children in a semi-urban area of South-eastern Nigeria. Int. J. Health 2009, 9, 1–7. [Google Scholar]
- Albonico, M.; Stoltzfus, R.J.; Savioli, L.; Tielsch, J.M.; Chwaya, H.M.; Ercole, E.; Cancrini, G. Epidemiological evidence for a differential effect of hookworm species, Ancylostoma duodenale or Necator americanus on iron status of children. Int. J. Epidemiol. 1998, 27, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Naish, S.; McCarth, J.; Williams, G.M. Prevalence, Intensity and Risk factors for Soil transmitted helminths infections in a South India Fishing Village. Acta Trop. 2004, 19, 177–187. [Google Scholar] [CrossRef]
- Grimes, J.E.; Croll, D.; Harrison, W.E.; Utzinger, J.; Freeman, M.C.; Templeton, M.R. The relationship between water, sanitation and schistosomiasis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2014, 8, e3296. [Google Scholar] [CrossRef]
- Stephenson, L.S. Impact of Helminthes Infection on Human Nutrition; Taylor and Francis: New York, NY, USA, 1987; pp. 252–260. [Google Scholar]
- Borghi, J.; Borghi, L.; Guiness, J.; Oudraogo, C.; Curtis, V. Is hygiene promotion cost-effective? A case study in Burkina Faso. Trop. Med. Int. Health 2002, 7, 960–969. [Google Scholar] [CrossRef]
- Taiwo, A.K.; Agbolade, O.M. Intestinal helminthiasis among schoolchildren in Oru, Ogun State. Niger. J. Sci. 2000, 34, 283–286. [Google Scholar]
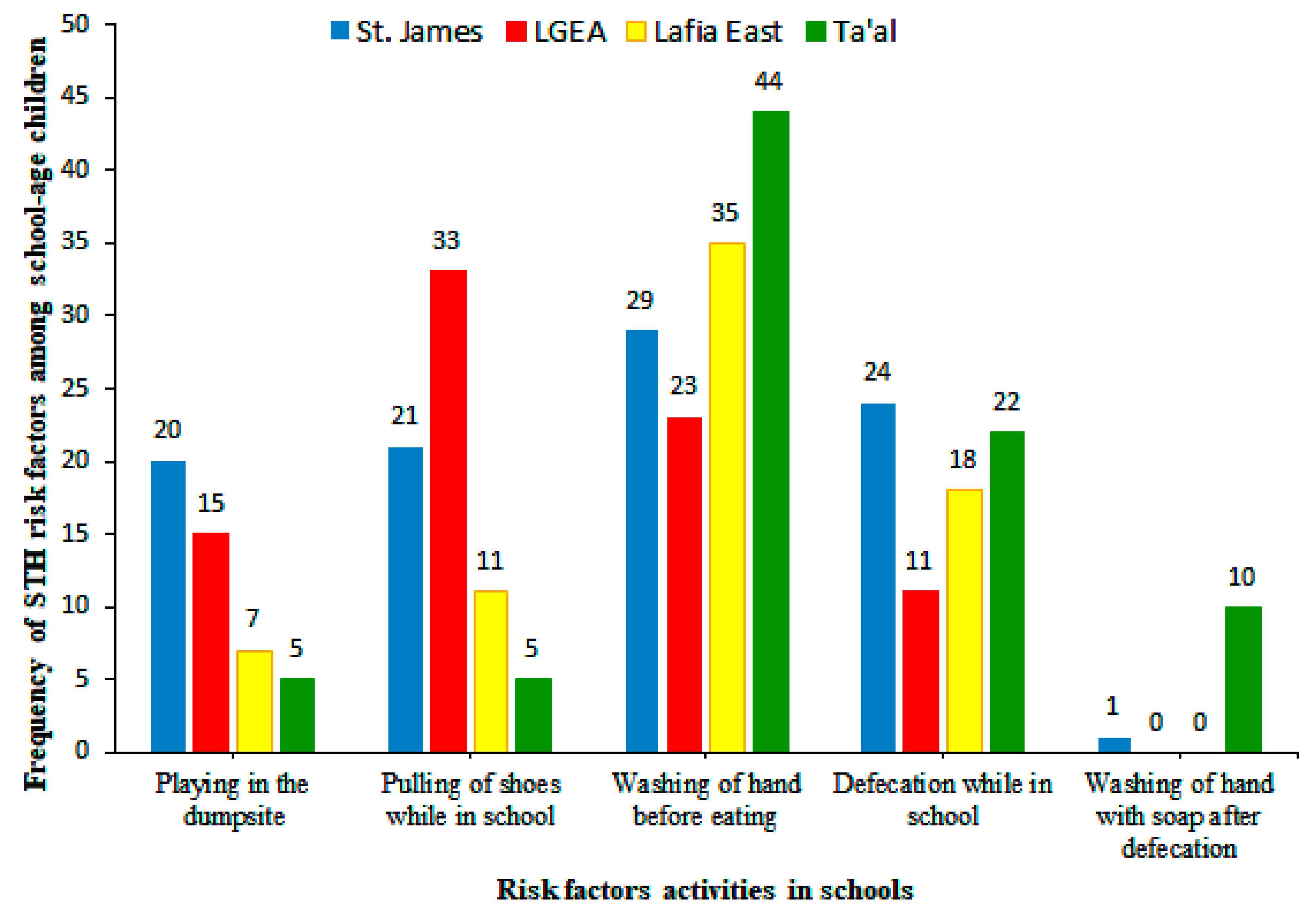
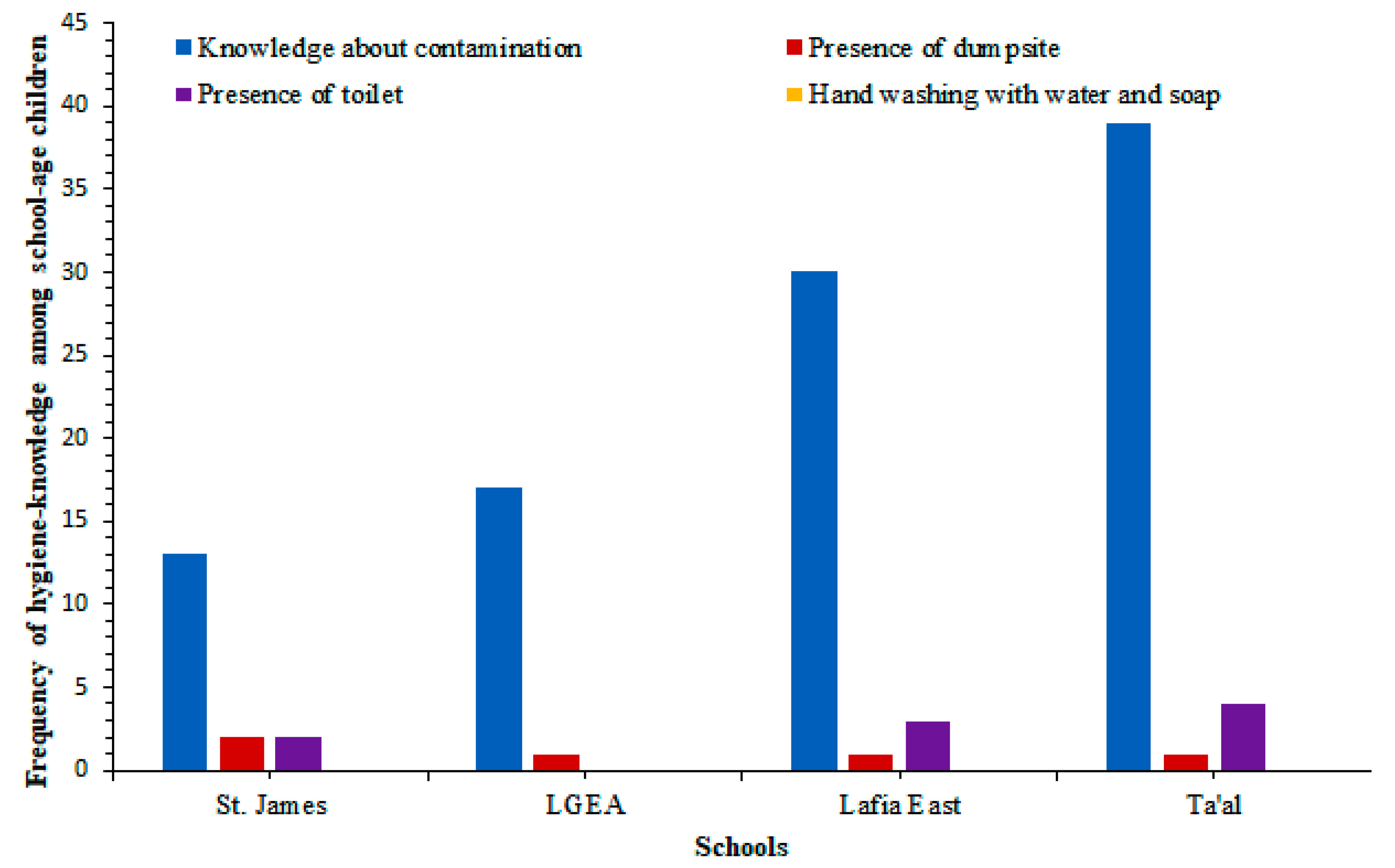
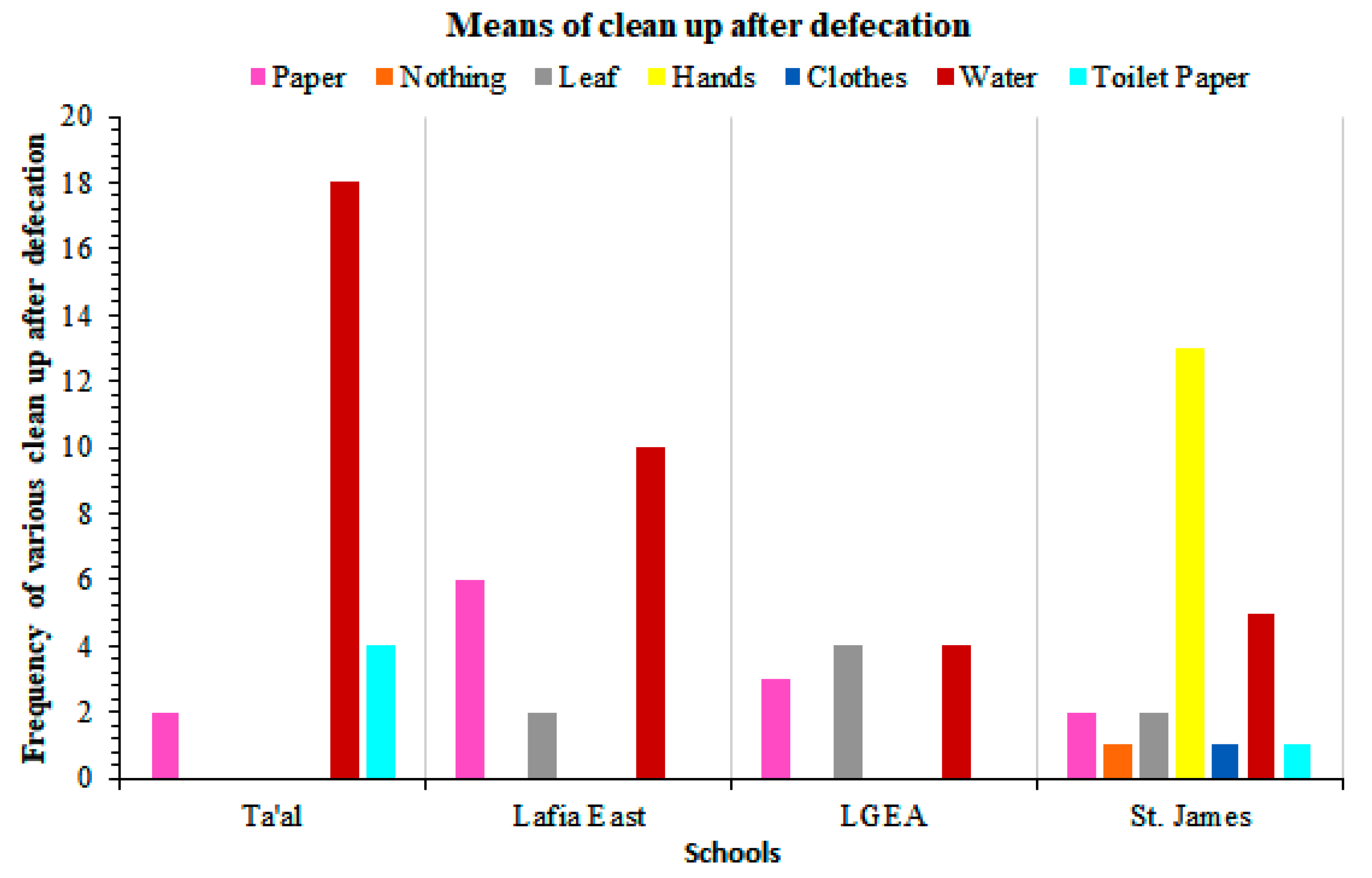

| Ta’al Model School Lafia | Lafia East Pilot Science Primary School | LGEA Mararaba | St. James Pilot Science Primary School | Total No. Examined | Total No. Infected (%) | χ2 | P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Examined | No Infected with STHs | (%) | No. Examined | No Infected with STHs | (%) | No. Examined | No Infected with STHs | (%) | No. Examined | No Infected with STHs | (%) | ||||||
| Age | 5–7 | 9 | 0 | 0 | 11 | 6 | 54.5 | 17 | 6 | 35.3 | 10 | 8 | 80.0 | 47 | 20 (42.6) | 4.9605 * | 0.1747 * |
| 8–10 | 17 | 5 | 29.4 | 14 | 4 | 28.6 | 14 | 3 | 21.4 | 17 | 6 | 35.3 | 62 | 18 (29.0) | |||
| 11–13 | 15 | 3 | 20 | 13 | 3 | 23.1 | 11 | 4 | 36.4 | 16 | 4 | 25.0 | 55 | 14 (25.5) | |||
| 14–16 | 9 | 7 | 20 | 13 | 3 | 23.1 | 11 | 4 | 36.4 | 16 | 4 | 25.0 | 55 | 14 (25.5) | |||
| Sex | Male | 20 | 6 | 30.0 | 18 | 7 | 38.9 | 20 | 6 | 30.0 | 22 | 10 | 45.5 | 80 | 29 (36.3) | 0.27027 ** | 0.6032 ** |
| Female | 30 | 9 | 30.0 | 32 | 8 | 25.0 | 30 | 9 | 30.0 | 28 | 12 | 42.9 | 120 | 38 (31.7) | |||
| Parasites | Ta’al Model School | Lafia East Pilot Science Primary School | LGEA Mararaba | St. James Pilot Science Primary School | Total No. Examined | Total No. Infected | % | χ2 | P Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. Examined | No. Infected | % | No. Examined | No. Infected | % | No. Examined | No. Infected | % | No. Examined | No. Infected | % | ||||||
| Any infection | 50 | 15 | 30 | 50 | 15 | 30 | 50 | 15 | 30 | 50 | 22 | 44 | 200 | 67 | 33.5 | 3.2993 | 0.3477 |
| A. lumbricoides | 50 | 7 | 14 | 50 | 6 | 12 | 50 | 6 | 12 | 50 | 7 | 14 | 200 | 26 | 13.0 | 0.17683 | 0.9812 |
| T. trichiuria | 50 | 3 | 6 | 50 | 3 | 6 | 50 | 4 | 8 | 50 | 5 | 10 | 200 | 15 | 7.5 | 0.79279 | 0.8512 |
| Hookworm | 50 | 4 | 8 | 50 | 4 | 8 | 50 | 3 | 6 | 50 | 5 | 10 | 200 | 16 | 8.0 | 0.54348 | 0.9092 |
| S. stercoralis | 50 | 1 | 2 | 50 | 0 | 0 | 50 | 1 | 2 | 50 | 3 | 6 | 200 | 5 | 2.5 | 3.8974 | 0.2728 |
| Multiple infection | 50 | 0 | 0 | 50 | 2 | 4 | 50 | 1 | 2 | 50 | 2 | 4 | 200 | 5 | 2.5 | 2.2564 | 0.5209 |
| Site of Collection | Ta’al Model School | Lafia East Pilot Science Primary School | LGEA Mararaba | St. James Pilot Science Primary School | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toilet Area | Refuse Bin | Playground | Toilet Area | Refuse Bin | Playground | Toilet Area | Refuse Bin | Playground | Toilet Area | Refuse Bin | Playground | |
| Grams of soil taken | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| Grams examined | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Nature | None | 12 T. trichiura eggs | None | 35 Ascaris lumbricoides eggs; 1 adult A. lumbricoides worms | 2 T. trichiura eggs | None | None | 10 Hookworm eggs | 5 T. trichiura eggs | 5 Ascaris lumbricoides eggs | 15 Ascaris lumbricoides eggs | 6 Ascaris lumbricoides eggs; Escherichia coli and Giardia lambdia eggs |
| Parasite found | ||||||||||||
| Indicators | Ta’al Model School Lafia | Lafia East Pilot Science Primary School | LGEA Mararaba | St. James Pilot Science Primary School |
|---|---|---|---|---|
| Condition of water supply | Not consistent | Not available | Not available | Not available |
| Type of toilet facility | Water cistern | Pit | None | Pit |
| Number of toilets | 3 | 2 | None | 2 |
| Adequacy of toilet facility | Not adequate | Not adequate | Not adequate | Not adequate |
| Condition of toilet | Clean | Dirty | Not applicable | Dirty |
| Usage of toilet | In use | Not always | No toilet | In use |
| Soap for hand washing after defecation | Absent | Absent | Absent | Absent |
| Garbage around school compound | Present | Present | Present | Present |
| Presence of bushes | No | Yes | Yes | Yes |
| Food vendors | Yes | Yes | Yes | Yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, E.M.; Echeta, O.C.; Ombugadu, A.; Ajah, L.; Aimankhu, P.O.; Oluwole, A.S. Helminthiasis among School-Age Children and Hygiene Conditions of Selected Schools in Lafia, Nasarawa State, Nigeria. Trop. Med. Infect. Dis. 2019, 4, 112. https://doi.org/10.3390/tropicalmed4030112
Abe EM, Echeta OC, Ombugadu A, Ajah L, Aimankhu PO, Oluwole AS. Helminthiasis among School-Age Children and Hygiene Conditions of Selected Schools in Lafia, Nasarawa State, Nigeria. Tropical Medicine and Infectious Disease. 2019; 4(3):112. https://doi.org/10.3390/tropicalmed4030112
Chicago/Turabian StyleAbe, Eniola M., Onyinye C. Echeta, Akwashiki Ombugadu, Linus Ajah, Peter O. Aimankhu, and Akinola S. Oluwole. 2019. "Helminthiasis among School-Age Children and Hygiene Conditions of Selected Schools in Lafia, Nasarawa State, Nigeria" Tropical Medicine and Infectious Disease 4, no. 3: 112. https://doi.org/10.3390/tropicalmed4030112
APA StyleAbe, E. M., Echeta, O. C., Ombugadu, A., Ajah, L., Aimankhu, P. O., & Oluwole, A. S. (2019). Helminthiasis among School-Age Children and Hygiene Conditions of Selected Schools in Lafia, Nasarawa State, Nigeria. Tropical Medicine and Infectious Disease, 4(3), 112. https://doi.org/10.3390/tropicalmed4030112





