Assessment of a Sensitive qPCR Assay Targeting a Multiple-Copy Gene to Detect Orientia tsutsugamushi DNA
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Orientia Genomic DNA
2.2. qPCR Condition for Single Copy 47 kDa Gene and Multiple Copy traD Gene
2.3. Comparison of Performance of Single-Copy Gene vs. Multiple-Copy Gene Using Genomic DNA
2.4. Demonstration of Specificity of traD Gene qPCR
2.5. Evaluation of traD qPCR Using DNA Extracted from Confirmed Scrub Typhus Positive and Negative Patient Blood
2.6. Statistical Analysis
3. Results
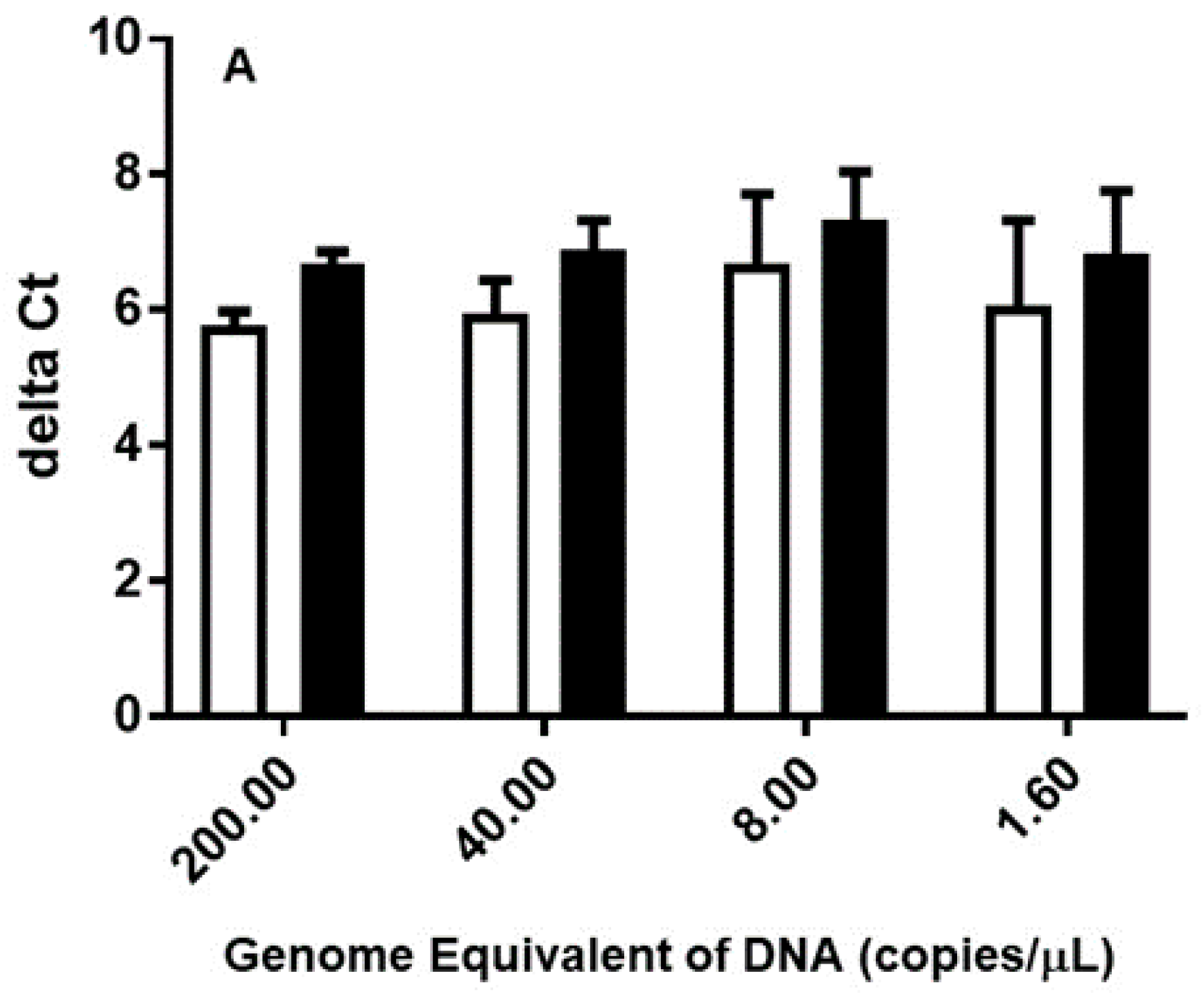
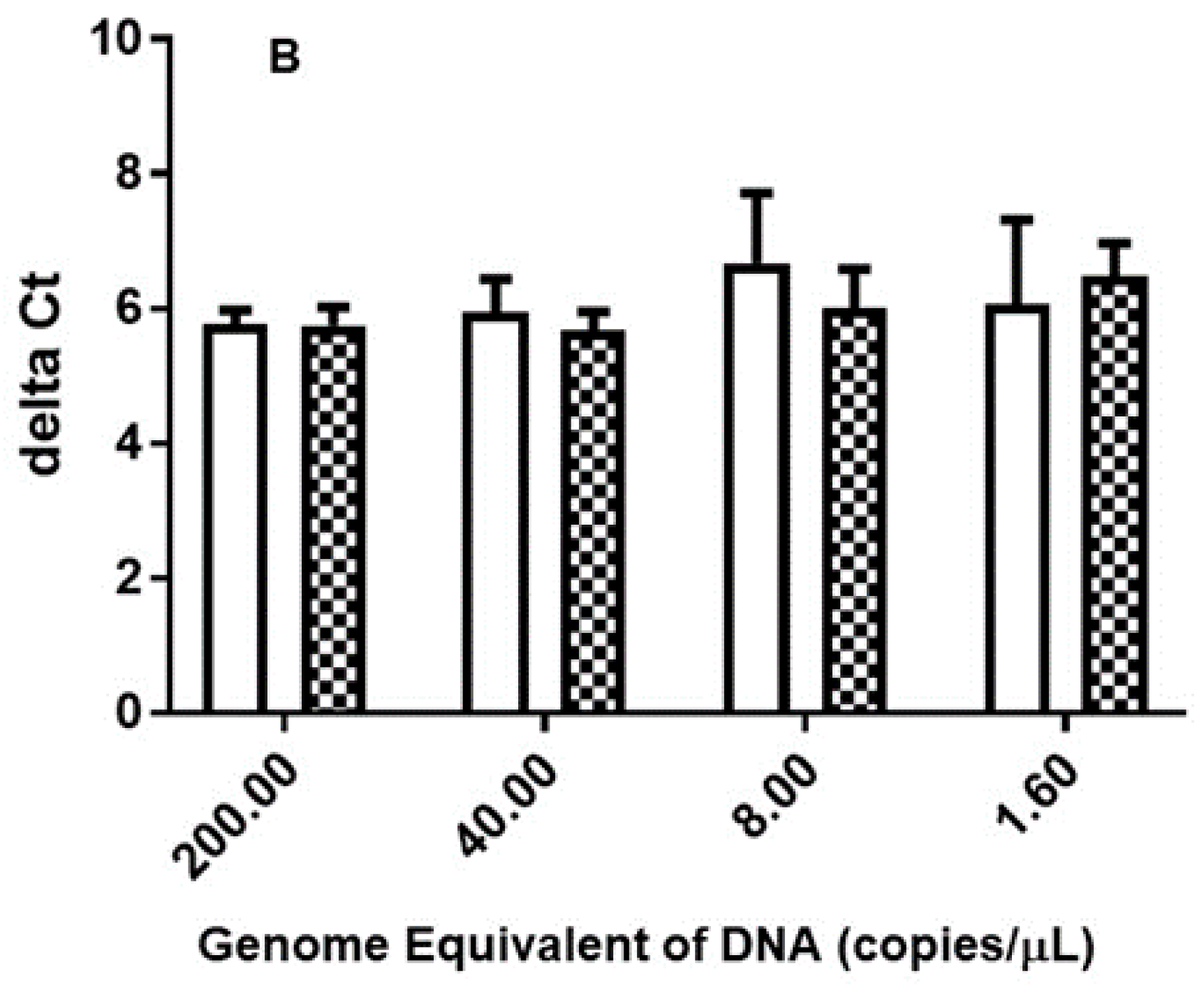
3.1. Multiple-Copy Gene qPCR Shows Lower Detection Limit in Comparison to Single-Copy Gene in DNA Extracted from Purified Organisms, Chigger-Mites and Chigger-Mite Infected Mouse Liver
3.2. traD Gene qPCR Is Specific in the Presence of Excessive Chigger-Mite or Mouse Liver DNA
3.3. traD Gene qPCR Is Sensitive and Specific in Clinically Confirmed Scrub Typhus Positive and Negative Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonell, A.; Lubell, Y.; Newton, P.N.; Crump, J.A.; Paris, D.H. Estimating the burden of scrub typhus: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005838. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Walker, D.H.; Jupiter, D.; Melby, P.C.; Arcari, C.M. A review of the global epidemiology of scrub typhus. PLoS Negl. Trop. Dis. 2017, 11, e0006062. [Google Scholar] [CrossRef] [PubMed]
- Suttinont, C.; Losuwanaluk, K.; Niwatayakul, K.; Hoontrakul, S.; Intaranongpai, W.; Silpasakorn, S.; Suwancharoen, D.; Panlar, P.; Saisongkorh, W.; Rolain, J.M.; et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: Results of a prospective observational study. Ann. Trop. Med. Parasitol. 2006, 100, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Phongmany, S.; Rolain, J.M.; Phetsouvanh, R.; Blacksell, S.D.; Soukkhaseum, V.; Rasachack, B.; Phiasakha, K.; Soukkhaseum, S.; Frichithavong, K.; Chu, V.; et al. Rickettsial infections and fever, Vientiane, Laos. Emerg. Infect. Dis. 2006, 12, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Kasper, M.R.; Blair, P.J.; Touch, S.; Sokhal, B.; Yasuda, C.Y.; Williams, M.; Richards, A.L.; Burgess, T.H.; Wierzba, T.F.; Putnam, S.D.; et al. Infectious etiologies of acute febrile illness among patients seeking health care in south-central Cambodia. Am. J. Trop. Med. Hyg. 2012, 86, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.J.; Richards, A.L.; Temenak, J.; Strickman, D.; Dasch, G.A. The past and present threat of Rickettsial diseases to military medicine and international publich health. Clin. Infect. Dis. 2002, 34 (Suppl. S4), S145–S169. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.J.; Paris, D.H.; Newton, P.N. A Systematic Review of Mortality from Untreated Scrub Typhus (Orientia tsutsugamushi). PLoS Negl. Trop. Dis. 2015, 9, e0003971. [Google Scholar] [CrossRef]
- Varghese, G.M.; Abraham, O.C.; Mathai, D.; Thomas, K.; Aaron, R.; Kavitha, M.L.; Mathai, E. Scrub typhus among hospitalised patients with febrile illness in South India: Magnitude and clinical predictors. J. Infect. 2006, 52, 56–60. [Google Scholar] [CrossRef]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.; et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef]
- Balcells, M.E.; Rabagliati, R.; Garcia, P.; Poggi, H.; Oddo, D.; Concha, M.; Abarca, K.; Jiang, J.; Kelly, D.J.; Richards, A.L.; et al. Endemic scrub typhus-like illness, Chile. Emerg. Infect. Dis. 2011, 17, 1659–1663. [Google Scholar] [CrossRef]
- Weitzel, T.; Dittrich, S.; Lopez, J.; Phuklia, W.; Martinez-Valdebenito, C.; Velasquez, K.; Blacksell, S.D.; Paris, D.H.; Abarca, K. Endemic scrub typhus in South America. N. Engl. J. Med. 2016, 375, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Ghrobani, R.P.; Ghorbani, A.J.; Jain, M.K.; Walker, D.H. A Case of Scrub Typhus Probably Acquired in Africa. Clin. Infect. Dis. 1997, 25, 1473–1474. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chan, T.C.; Temenak, J.; Dasch, G.; Ching, W.M.; Richards, A.L. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am. J. Trop. Med. Hyg. 2004, 70, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Silpasakorn, S.; Srisamut, N.; Ekpo, P.; Zhang, Z.; Chao, C.C.; Ching, W.M.; Suputtamongkol, Y. Development of new, broadly reactive, rapid IgG and IgM lateral flow assays for diagnosis of scrub typhus. Am. J. Trop. Med. Hyg. 2012, 87, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Sonthayanon, P.; Chierakul, W.; Wuthiekanun, V.; Phimda, K.; Pukrittayakamee, S.; Day, N.P.; Peacock, S.J. Association of high Orientia tsutsugamushi DNA loads with disease of greater severity in adults with scrub typhus. J. Clin. Microbiol. 2009, 47, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Paris, D.H.; Aukkanit, N.; Jenjaroen, K.; Blacksell, S.D.; Day, N.P. A highly sensitive quantitative real-time PCR assay based on the groEL gene of contemporary Thai strains of Orientia tsutsugamushi. Clin. Microbiol. Infect. 2009, 15, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.M.; Adcox, H.E.; VieBrock, L.; Green, R.S.; Luce-Fedrow, A.; Chattopadhyay, S.; Jiang, J.; Marconi, R.T.; Paris, D.; Richards, A.L.; et al. Outer membrane protein a conservation among orientia tsutsugamushi isolates suggests its potential as a protective antigen and diagnostic target. Trop. Med. Infect. Dis. 2018, 3, 63. [Google Scholar] [CrossRef] [PubMed]
- Huber, E.; Ji, D.; Howell, L.; Zhang, Z.; Chen, H.C.; Ching, W.M.; Chao, C.C. Loop-Mediated Isothermal Amplification Assay Targeting the 47-Kda Gene of Orientia tsutsugamushi: A rapid and sensitive alternative to real-time PCR. J. Med. Microbiol. Diagn. 2012, 1, 112. [Google Scholar] [CrossRef]
- Blacksell, S.D.; Paris, D.H.; Chierakul, W.; Wuthiekanun, V.; Teeratakul, A.; Kantipong, P.; Day, N.P. Prospective evaluation of commercial antibody-based rapid tests in combination with a loop-mediated isothermal amplification PCR assay for detection of Orientia tsutsugamushi during the acute phase of scrub typhus infection. Clin. Vaccine Immunol. 2012, 19, 391–395. [Google Scholar] [CrossRef]
- Chao, C.C.; Belinskaya, T.; Zhang, Z.; Ching, W.M. Development of recombinase polymerase amplification assays for detection of Orientia tsutsugamushi or rickettsia typhi. PLoS Negl. Trop. Dis. 2015, 9, e0003884. [Google Scholar] [CrossRef]
- Keller, C.A.; Hauptmann, M.; Kolbaum, J.; Gharaibeh, M.; Neumann, M.; Glatzel, M.; Fleischer, B. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl. Trop. Dis. 2014, 8, e3064. [Google Scholar] [CrossRef] [PubMed]
- Tantibhedhyangkul, W.; Wongsawat, E.; Silpasakorn, S.; Waywa, D.; Saenyasiri, N.; Suesuay, J.; Thipmontree, W.; Suputtamongkol, Y. Use of multiplex real-time PCR to diagnose scrub typhus. J. Clin. Microbiol. 2017, 55, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Ching, W.M.; Lurchachaiwong, W.; Zhang, Z.; Awoyomi, T.; Chao, C.C.; Schuster, A. Evaluation of a recombinant vaccine candidate r56Lc-1 in a chigger challenge mouse model. J. Vaccines Vaccin 2014, 5. [Google Scholar] [CrossRef]
- Chao, C.C. Rapid and sensitive detection of Orientia tsutsugamushi DNA using loop mediated isothermal amplification (LAMP). In Proceedings of the JITMM 2010 and IMC 2010. Centara Grand and Bangkok Convention Centre at Central World, Bangkok, Thailand, 1–3 December 2010. [Google Scholar]
| Orientia Isolate | Country of Origin | ΔCt * |
|---|---|---|
| AFC-27 | Thailand | 8.29 ± 0.72 |
| 18-030641 | Malaysia | 8.14 ± 0.34 |
| 18-032029 | Malaysia | 8.70 ± 1.24 |
| AFC-12 | Thailand | 7.14 ± 1.40 |
| Gilliam | Burma | 5.09 ± 0.32 |
| MAK 119 | Taiwan | 4.60 ± 0.35 |
| MAK 243 | Taiwan | 9.17 ± 1.03 |
| Karp | New Guinea | 5.10 ± 0.26 |
| AFPL12 | Thailand | 7.54 ± 0.49 |
| TA763 | Thailand | 7.26 ± 1.19 |
| AFC-1 | Thailand | 8.30 ± 0.34 |
| Citrano | Australia | 7.68 ± 1.63 |
| Garton | Australia | 11.74 ± 1.59 |
| Boryong | South Korea | 5.40 ± 0.13 |
| Kato | Japan | 7.41 ± 1.17 |
| Sample 1 | Sample 2 | |
|---|---|---|
| Original DNA preparation (copy/μL in 200 μL NHS) | 0.4 | 10 |
| Extracted DNA (copy/μL in 50 μL water) | 1.6 | 40 |
| expected copy # in a qPCR reaction a | 3.2 (4.7, 5.9) | 80 (31.3 ± 15.4) |
| % 47 kDa qPCR positive (range of Ct) b | 28.6% (33.7, 34.1) | 100% (30.3–33.3) |
| % traD qPCR positive (range of Ct) | 100% (30.2–30.5) | 100% (25.2–26.1) |
| ΔCt | 3.62 c | 5.66 ± 0.65 |
| Sample | Based on Melting Curve | Ct | PCR/IFA Result of Acute Sample |
|---|---|---|---|
| NHB1 | Neg. | 36.5 | PCR(−) IFA(−) |
| NHB2 | Neg. | Undetermined | PCR(−) IFA(−) |
| NHB3 | Neg. | 36.5 | PCR(−) IFA(−) |
| NHB4 | Neg. | 38.0 | PCR(−) IFA(−) |
| NHB5 | Neg. | 38.5 | PCR(−) IFA(−) |
| Other Disease 1 | Neg. | Undetermined | PCR(−) IFA(−) |
| Other Disease 2 | Neg. | Undetermined | PCR(−) IFA(−) |
| Other Disease 3 | Neg. | 38.4 | PCR(−) IFA(−) |
| Other Disease 4 | Neg. | Undetermined | PCR(−) IFA(−) |
| Other Disease 5 | Neg. | 36.8 | PCR(−) IFA(−) |
| Other Disease 6 | Neg. | 35.5 | PCR(−) IFA(−) |
| Scrub typhus 1 | Pos. | 32.3 | PCR(+) IFA(+) |
| Scrub typhus 2 | Pos. | 25.8 | PCR(−) IFA(+) |
| Scrub typhus 3 | Pos. | 31.9 | PCR(−) IFA(+) |
| Scrub typhus 4 | Pos. | 32.4 | PCR(+) IFA(−) |
| Scrub typhus 5 a | Pos. | 38.3 | PCR(+) IFA(−) |
| Scrub typhus 6 | Pos. | 28.1 | PCR(+) IFA(+) |
| Scrub typhus 7 | Pos. | 30.2 | PCR(−) IFA(+) |
| Scrub typhus 8 | Pos. | 30.3 | PCR(+) IFA(+) |
| Scrub typhus 9 | Pos. | 24.7 | PCR(+) IFA(−) |
| Scrub typhus 10 b | Pos. | 22.4 | PCR(−) IFA(−) |
| Patients are ST Positive or Negative Based on the Results from | |||
|---|---|---|---|
| IFA | PCR | Clinical Diagnosis | |
| Sensitivity (%) | 100 | 100 | 100 |
| Specificity (%) | 73.3 a | 73.3 a | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, C.-C.; Belinskaya, T.; Zhang, Z.; Jiang, L.; Ching, W.-M. Assessment of a Sensitive qPCR Assay Targeting a Multiple-Copy Gene to Detect Orientia tsutsugamushi DNA. Trop. Med. Infect. Dis. 2019, 4, 113. https://doi.org/10.3390/tropicalmed4030113
Chao C-C, Belinskaya T, Zhang Z, Jiang L, Ching W-M. Assessment of a Sensitive qPCR Assay Targeting a Multiple-Copy Gene to Detect Orientia tsutsugamushi DNA. Tropical Medicine and Infectious Disease. 2019; 4(3):113. https://doi.org/10.3390/tropicalmed4030113
Chicago/Turabian StyleChao, Chien-Chung, Tatyana Belinskaya, Zhiwen Zhang, Le Jiang, and Wei-Mei Ching. 2019. "Assessment of a Sensitive qPCR Assay Targeting a Multiple-Copy Gene to Detect Orientia tsutsugamushi DNA" Tropical Medicine and Infectious Disease 4, no. 3: 113. https://doi.org/10.3390/tropicalmed4030113
APA StyleChao, C.-C., Belinskaya, T., Zhang, Z., Jiang, L., & Ching, W.-M. (2019). Assessment of a Sensitive qPCR Assay Targeting a Multiple-Copy Gene to Detect Orientia tsutsugamushi DNA. Tropical Medicine and Infectious Disease, 4(3), 113. https://doi.org/10.3390/tropicalmed4030113




