Advances in Antiwolbachial Drug Discovery for Treatment of Parasitic Filarial Worm Infections
Abstract
1. Introduction
2. Filarial Worm Infection and the Antiwolbachial Approach
2.1. Filarial Worm Infection Biology—An Overview
2.2. Approved Therapies and Accompanying Challenges
- Results in death or permanent sterilization of adult onchocerciasis worms (O. volvulus).
- After one course, death or permanent sterilization of adult worms is achieved (minimum in 70%; ideally in 100%).
- Oral dose, once daily, up to 7 days or a single, intra-muscular injection.
- Target Population: at minimum, all individuals ≥ 5 who are infected, excluding pregnant women; ideally all individuals at risk for onchocerciasis.
- Safe for use in patients co-infected with L. loa (i.e., no rapid microfilaricidal activity).
- No significant drug–drug interactions.
- No monitoring for adverse events or monitoring manageable at local healthcare posts.
- Able to be delivered by health care facility or ideally by an appropriately trained community volunteer.
- Stable for ≥ 3 years in Zone 4B (30 ± 2 °C, 75 ± 5% relative humidity).
- At minimum, ≤ $2.00 per course of therapy; ideally ≤ $0.30.
2.3. Wolbachia, an Attractive Anti-Macrofilarial Target
3. Antiwolbachial Drug Discovery
3.1. Phenotypic Antiwolbachial High-Throughput Screening
3.2. Validation of Antiwolbachial Compounds Identified in Vitro Screens
4. Promising Antiwolbachial Candidates
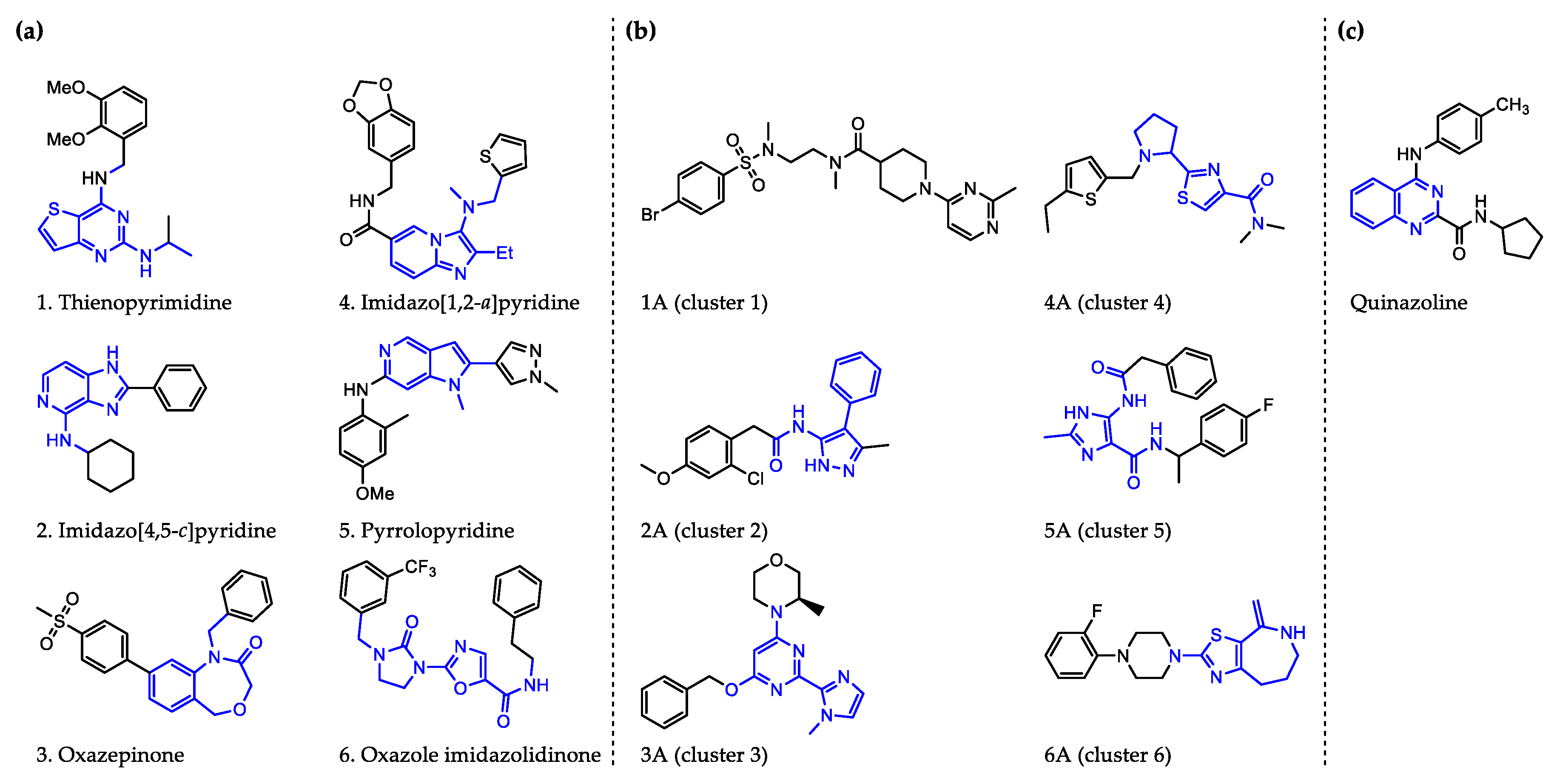
4.1. Novel Chemical Series
4.1.1. AWZ1066S
4.1.2. Quinazolines CBR417 and CBR490
4.2. Repurposing of Known Drugs and Alternative Dosing Regimens
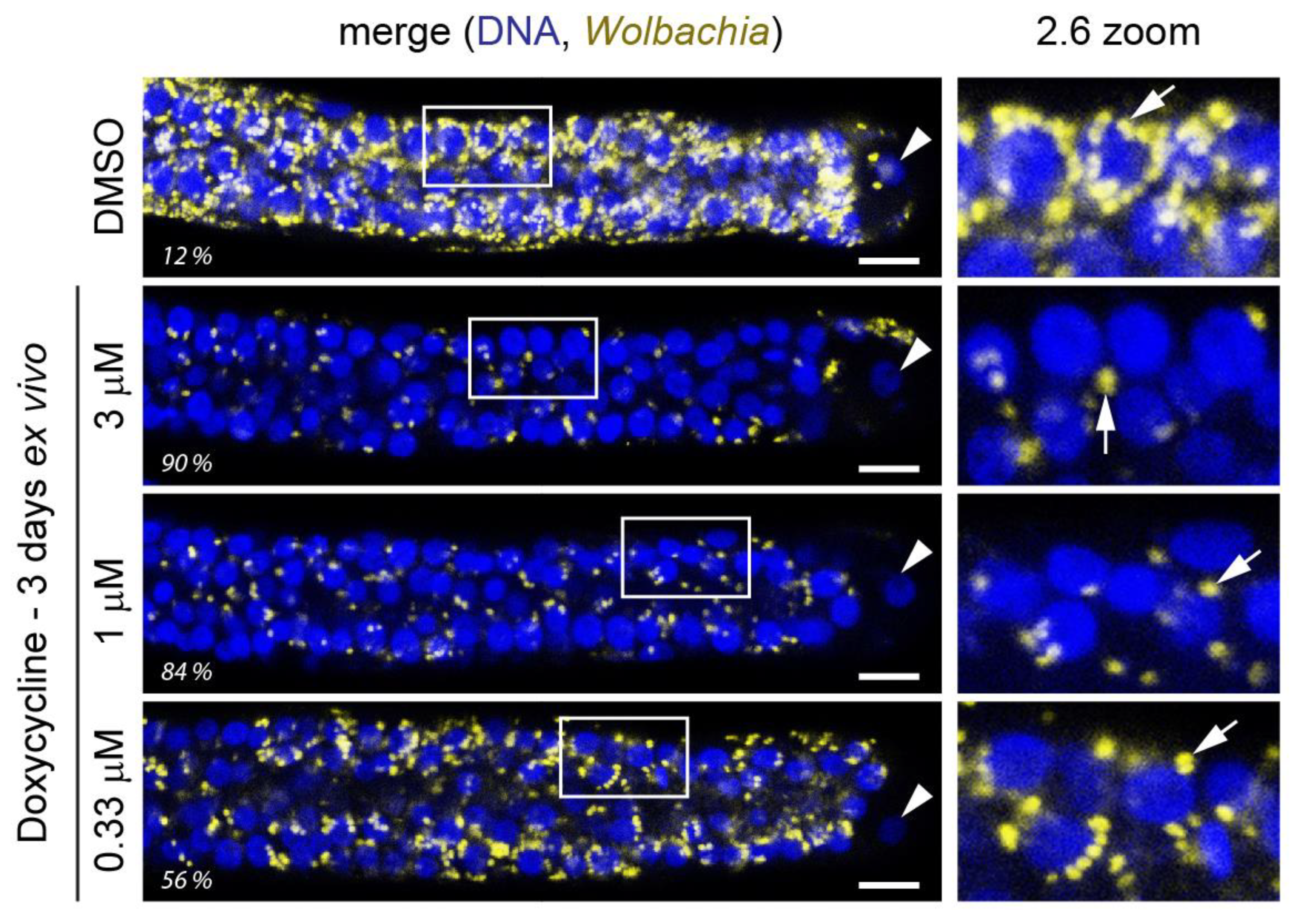
4.2.1. Minocycline
4.2.2. High Dose Rifampicin
4.2.3. Corallopyronin A
4.2.4. DNA Gyrase Inhibitors: Fluoroquinolones and Aminocoumarins
4.2.5. Kirromycins
4.3. Chemically-Optimized Antibiotics
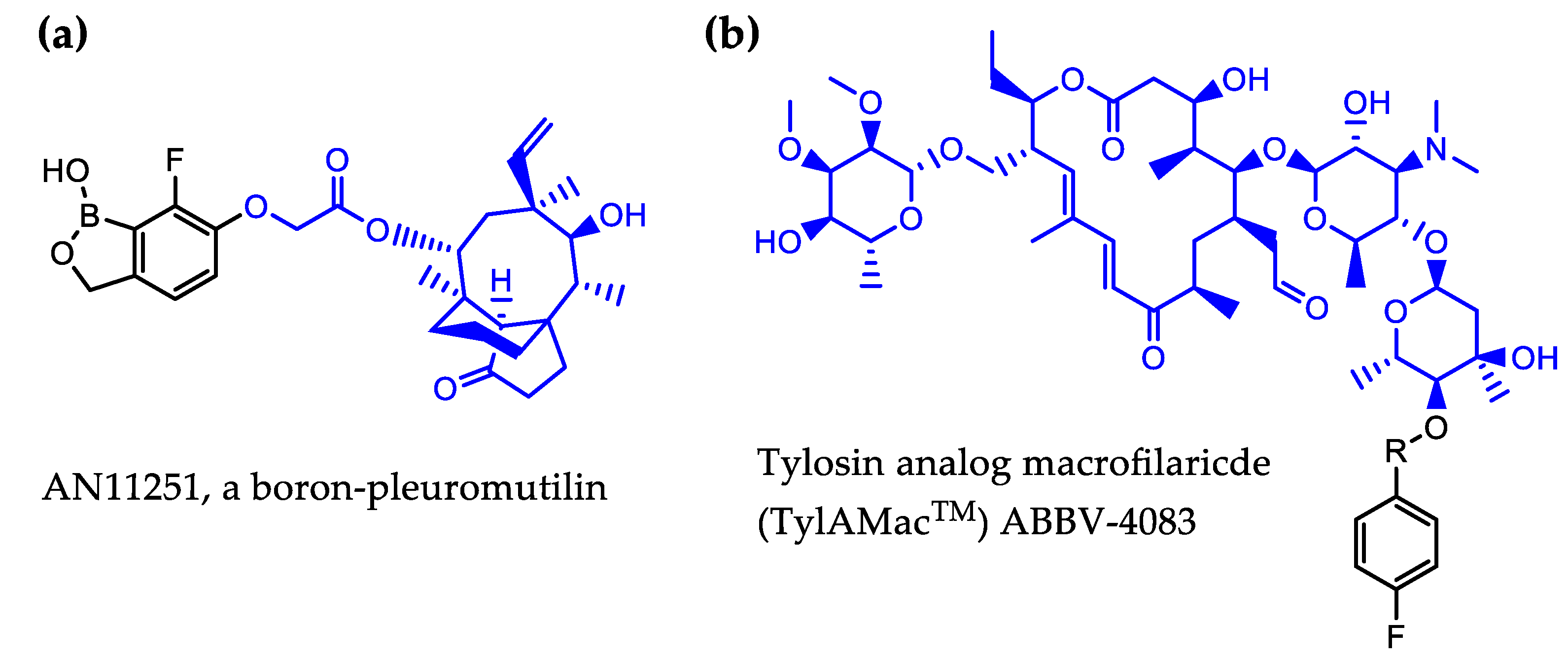
4.3.1. Boron-Pleuromutilin, AN11251
4.3.2. Tylosin Analog ABBV-4083
4.4. Combination Therapies and Target-Based Screening
5. Perspectives for the Future of Antiwolbachial Drug Discovery
Funding
Acknowledgments
Conflicts of Interest
References
- Taylor, M.J.; Hoerauf, A.; Bockarie, M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. [Google Scholar] [CrossRef]
- Hotterbeekx, A.; Namale Ssonko, V.; Oyet, W.; Lakwo, T.; Idro, R. Neurological manifestations in Onchocerca volvulus infection: A review. Brain Res. Bull. 2019, 145, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Makepeace, B.L.; Tanya, V.N. 25 Years of the Onchocerca ochengi Model. Trends Parasitol. 2016, 32, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Halliday, A.; Guimaraes, A.F.; Tyrer, H.E.; Metuge, H.M.; Patrick, C.N.; Arnaud, K.O.; Kwenti, T.D.; Forsbrook, G.; Steven, A.; Cook, D.; et al. A murine macrofilaricide pre-clinical screening model for onchocerciasis and lymphatic filariasis. Parasites Vectors 2014, 7, 472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- WHO. Global programme to eliminate lymphatic filariasis: Progress report, 2015. Wkly. Epidemiol. Rec. 2016, 91, 441–455. [Google Scholar]
- Muslim, A.; Fong, M.Y.; Mahmud, R.; Sivanandam, S. Vector and reservoir host of a case of human Brugia pahangi infection in Selangor, peninsular Malaysia. Trop. Biomed. 2013, 30, 727–730. [Google Scholar] [PubMed]
- Tan, L.H.; Fong, M.Y.; Mahmud, R.; Muslim, A.; Lau, Y.L.; Kamarulzaman, A. Zoonotic Brugia pahangi filariasis in a suburbia of Kuala Lumpur City, Malaysia. Parasitol. Int. 2011, 60, 111–113. [Google Scholar] [CrossRef] [PubMed]
- McNulty, S.N.; Mitreva, M.; Weil, G.J.; Fischer, P.U. Inter and intra-specific diversity of parasites that cause lymphatic filariasis. Infect. Genet. Evol. 2013, 14, 137–146. [Google Scholar] [CrossRef]
- Nutman, T.B. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat. Res. Biol. 2013, 11, 144–148. [Google Scholar] [CrossRef]
- Hertig, M.; Wolbach, S.B. Studies on Rickettsia-Like Micro-Organisms in Insects. J. Med. Res. 1924, 44, 329. [Google Scholar]
- Lima, N.F.; Veggiani Aybar, C.A.; Dantur Juri, M.J.; Ferreira, M.U. Mansonella ozzardi: A neglected New World filarial nematode. Pathog. Glob. Health 2016, 110, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, M.; Favia, G.; Cancrini, G.; Bartoloni, A.; Bandi, C. Molecular identification of Wolbachia from the filarial nematode Mansonella ozzardi. Parasitol. Res. 2001, 87, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Ta-Tang, T.H.; Crainey, J.L.; Post, R.J.; Luz, S.L.; Rubio, J.M. Mansonellosis: Current perspectives. Res. Rep. Trop. Med. 2018, 9, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Keiser, P.B.; Coulibaly, Y.; Kubofcik, J.; Diallo, A.A.; Klion, A.D.; Traore, S.F.; Nutman, T.B. Molecular identification of Wolbachia from the filarial nematode Mansonella perstans. Mol. Biochem. Parasitol. 2008, 160, 123–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- King, D.F. Some developments in techniques for the study of the rodent filarial parasite Litomosoides carinii. II. A quantitative method for the culture of the mite Ornithonyssus bacoti and for the routine transmission of Litomosoides carinii to Praomys (Mastomys) natalensis. Ann. Trop. Med. Parasitol. 1968, 62, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Pringle, G.; King, D.F. Some developments in techniques for the study of the rodent filarial parasite Litomosoides carinii. I. A preliminary comparison of the host effciency of the multimammate rat, Praomys (Mastomys) natalensis, with that of the cotton rat, Sigmodon hispidus. Ann. Trop. Med. Parasitol. 1968, 62, 462–468. [Google Scholar] [CrossRef]
- Morris, C.P.; Evans, H.; Larsen, S.E.; Mitre, E. A comprehensive, model-based review of vaccine and repeat infection trials for filariasis. Clin. Microbiol. Rev. 2013, 26, 381–421. [Google Scholar] [CrossRef]
- Hawking, F.; Sewell, P. The maintenance of a filarial infection (Litomosoides carinii) for chemotherapeutic investigations. Br. J. Pharmacol. Chemother. 1948, 3, 285–296. [Google Scholar] [CrossRef]
- Turner, J.D.; Mand, S.; Debrah, A.Y.; Muehlfeld, J.; Pfarr, K.; McGarry, H.F.; Adjei, O.; Taylor, M.J.; Hoerauf, A. A randomized, double-blind clinical trial of a 3-week course of doxycycline plus albendazole and ivermectin for the treatment of Wuchereria bancrofti infection. Clin. Infect. Dis. 2006, 42, 1081–1089. [Google Scholar] [CrossRef]
- Simon, F.; Siles-Lucas, M.; Morchon, R.; Gonzalez-Miguel, J.; Mellado, I.; Carreton, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef]
- Alho, A.M.; Marcelino, I.; Colella, V.; Flanagan, C.; Silva, N.; Correia, J.J.; Latrofa, M.S.; Otranto, D.; Madeira de Carvalho, L. Dirofilaria immitis in pinnipeds and a new host record. Parasites Vectors 2017, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Genchi, C.; Kramer, L. Subcutaneous dirofilariosis (Dirofilaria repens): An infection spreading throughout the old world. Parasites Vectors 2017, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.; Walker, M.; Pion, S.D.S.; Chesnais, C.B.; Boussinesq, M.; Basanez, M.G. The Population Biology and Transmission Dynamics of Loa loa. Trends Parasitol. 2018, 34, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Remme, J.H.F.; Feenstra, P.; Lever, P.R.; Medici, A.C.; Morel, C.M.; Noma, M.; Ramaiah, K.D.; Richards, F.; Seketeli, A.; Schmunis, G.; et al. Tropical Diseases Targeted for Elimination: Chagas Disease, Lymphatic Filariasis, Onchocerciasis, and Leprosy. In Disease Control Priorities in Developing Countries, 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; World Bank and Oxford University Press: Washington, DC, USA, 2006. [Google Scholar]
- Gems, D. Longevity and ageing in parasitic and free-living nematodes. Biogerontology 2000, 1, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Mawson, A.R.; WaKabongo, M. Onchocerciasis-associated morbidity: Hypothesis. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 541–542. [Google Scholar] [CrossRef]
- Shenoy, R.K. Clinical and pathological aspects of filarial lymphedema and its management. Korean J. Parasitol. 2008, 46, 119–125. [Google Scholar] [CrossRef]
- Kar, S.K.; Dwibedi, B.; Das, B.K.; Agrawala, B.K.; Ramachandran, C.P.; Horton, J. Lymphatic pathology in asymptomatic and symptomatic children with Wuchereria bancrofti infection in children from Odisha, India and its reversal with DEC and albendazole treatment. PLoS Negl. Trop. Dis. 2017, 11, e0005631. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.K.; Dwibedi, B.; Kerketa, A.S.; Maharana, A.; Panda, S.S.; Mohanty, P.C.; Horton, J.; Ramachandran, C.P. A randomized controlled trial of increased dose and frequency of albendazole with standard dose DEC for treatment of Wuchereria bancrofti microfilaremics in Odisha, India. PLoS Negl. Trop. Dis. 2015, 9, e0003583. [Google Scholar] [CrossRef] [PubMed]
- Program Coordinating, C.; Staff, O. Guide to detecting a potential recrudescence of onchocerciasis during the posttreatment surveillance period: The American paradigm. Res. Rep. Trop. Med. 2012, 3, 21–33. [Google Scholar] [CrossRef]
- Sauerbrey, M. The Onchocerciasis Elimination Program for the Americas (OEPA). Ann. Trop. Med. Parasitol. 2008, 102 (Suppl. 1), 25–29. [Google Scholar] [CrossRef]
- Dadzie, Y.; Neira, M.; Hopkins, D. Final report of the Conference on the eradicability of Onchocerciasis. Filaria J. 2003, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Kuesel, A.C. Research for new drugs for elimination of onchocerciasis in Africa. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Boussinesq, M.; Gardon, J.; Gardon-Wendel, N.; Chippaux, J.P. Clinical picture, epidemiology and outcome of Loa-associated serious adverse events related to mass ivermectin treatment of onchocerciasis in Cameroon. Filaria J. 2003, 2 (Suppl. 1). [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, C.L.; Budhathoki, S.S.; Johnson, S.; Richardson, M.; Garner, P. Albendazole alone or in combination with microfilaricidal drugs for lymphatic filariasis. Cochrane Database Syst. Rev. 2019, 1, CD003753. [Google Scholar] [CrossRef] [PubMed]
- McLaren, D.J.; Worms, M.J.; Laurence, B.R.; Simpson, M.G. Micro-organisms in filarial larvae (Nematoda). Trans. R. Soc. Trop. Med. Hyg. 1975, 69, 509–514. [Google Scholar] [CrossRef]
- Kozek, W.J. Transovarially-transmitted intracellular microorganisms in adult and larval stages of Brugia malayi. J. Parasitol. 1977, 63, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Kozek, W.J.; Marroquin, H.F. Intracytoplasmic bacteria in Onchocerca volvulus. Am. J. Trop. Med. Hyg. 1977, 26, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Niang, E.H.A.; Bassene, H.; Fenollar, F.; Mediannikov, O. Biological Control of Mosquito-Borne Diseases: The Potential of Wolbachia-Based Interventions in an IVM Framework. J. Trop. Med. 2018, 2018, 1470459. [Google Scholar] [CrossRef]
- Townson, S.; Tagboto, S.; McGarry, H.F.; Egerton, G.L.; Taylor, M.J. Onchocerca parasites and Wolbachia endosymbionts: Evaluation of a spectrum of antibiotic types for activity against Onchocerca gutturosa in vitro. Filaria J. 2006, 5, 4. [Google Scholar] [CrossRef]
- Townson, S. The development of a laboratory model for onchocerciasis using Onchocerca gutturosa: In vitro culture, collagenase effects, drug studies and cryopreservation. Trop. Med. Parasitol. 1988, 39 (Suppl. 4), 475–479. [Google Scholar]
- Godel, C.; Kumar, S.; Koutsovoulos, G.; Ludin, P.; Nilsson, D.; Comandatore, F.; Wrobel, N.; Thompson, M.; Schmid, C.D.; Goto, S.; et al. The genome of the heartworm, Dirofilaria immitis, reveals drug and vaccine targets. FASEB J. 2012, 26, 4650–4661. [Google Scholar] [CrossRef]
- Allen, J.E.; Adjei, O.; Bain, O.; Hoerauf, A.; Hoffmann, W.H.; Makepeace, B.L.; Schulz-Key, H.; Tanya, V.N.; Trees, A.J.; Wanji, S.; et al. Of mice, cattle, and humans: The immunology and treatment of river blindness. PLoS Negl. Trop. Dis. 2008, 2, e217. [Google Scholar] [CrossRef]
- Fulton, A.; Babayan, S.A.; Taylor, M.D. Use of the Litomosoides sigmodontis Infection Model of Filariasis to Study Type 2 Immunity. Methods Mol. Biol. 2018, 1799, 11–26. [Google Scholar] [CrossRef]
- Hubner, M.P.; Torrero, M.N.; McCall, J.W.; Mitre, E. Litomosoides sigmodontis: A simple method to infect mice with L3 larvae obtained from the pleural space of recently infected jirds (Meriones unguiculatus). Exp. Parasitol. 2009, 123, 95–98. [Google Scholar] [CrossRef]
- Pionnier, N.P.; Sjoberg, H.; Chunda, V.C.; Fombad, F.F.; Chounna, P.W.; Njouendou, A.J.; Metuge, H.M.; Ndzeshang, B.L.; Gandjui, N.V.; Akumtoh, D.N.; et al. Mouse models of Loa loa. Nat. Commun. 2019, 10, 1429. [Google Scholar] [CrossRef]
- Cotton, J.A.; Bennuru, S.; Grote, A.; Harsha, B.; Tracey, A.; Beech, R.; Doyle, S.R.; Dunn, M.; Hotopp, J.C.; Holroyd, N.; et al. The genome of Onchocerca volvulus, agent of river blindness. Nat. Microbiol. 2016, 2, 16216. [Google Scholar] [CrossRef]
- Darby, A.C.; Armstrong, S.D.; Bah, G.S.; Kaur, G.; Hughes, M.A.; Kay, S.M.; Koldkjaer, P.; Rainbow, L.; Radford, A.D.; Blaxter, M.L.; et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012, 22, 2467–2477. [Google Scholar] [CrossRef]
- Chung, M.; Small, S.T.; Serre, D.; Zimmerman, P.A.; Dunning Hotopp, J.C. Draft genome sequence of the Wolbachia endosymbiont of Wuchereria bancrofti wWb. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef][Green Version]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef]
- Lau, Y.L.; Lee, W.C.; Xia, J.; Zhang, G.; Razali, R.; Anwar, A.; Fong, M.Y. Draft genome of Brugia pahangi: High similarity between B. pahangi and B. malayi. Parasites Vectors 2015, 8, 451. [Google Scholar] [CrossRef]
- Pfarr, K.; Foster, J.; Slatko, B.; Hoerauf, A.; Eisen, J.A. On the taxonomic status of the intracellular bacterium Wolbachia pipientis: Should this species name include the intracellular bacteria of filarial nematodes? Int. J. Syst. Evol Microbiol. 2007, 57, 1677–1678. [Google Scholar] [CrossRef]
- Lo, N.; Paraskevopoulos, C.; Bourtzis, K.; O’Neill, S.L.; Werren, J.H.; Bordenstein, S.R.; Bandi, C. Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int. J. Syst. Evol. Microbiol. 2007, 57, 654–657. [Google Scholar] [CrossRef]
- Klasson, L.; Walker, T.; Sebaihia, M.; Sanders, M.J.; Quail, M.A.; Lord, A.; Sanders, S.; Earl, J.; O’Neill, S.L.; Thomson, N.; et al. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol. Biol. Evol. 2008, 25, 1877–1887. [Google Scholar] [CrossRef]
- Nunes, A.; Gomes, J.P. Evolution, phylogeny, and molecular epidemiology of Chlamydia. Infect. Genet. Evol. 2014, 23, 49–64. [Google Scholar] [CrossRef]
- Renesto, P.; Ogata, H.; Audic, S.; Claverie, J.M.; Raoult, D. Some lessons from Rickettsia genomics. FEMS Microbiol. Rev. 2005, 29, 99–117. [Google Scholar] [CrossRef]
- Katinka, M.D.; Duprat, S.; Cornillot, E.; Metenier, G.; Thomarat, F.; Prensier, G.; Barbe, V.; Peyretaillade, E.; Brottier, P.; Wincker, P.; et al. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature 2001, 414, 450–453. [Google Scholar] [CrossRef]
- Bosshardt, S.C.; McCall, J.W.; Coleman, S.U.; Jones, K.L.; Petit, T.A.; Klei, T.R. Prophylactic activity of tetracycline against Brugia pahangi infection in jirds (Meriones unguiculatus). J. Parasitol. 1993, 79, 775–777. [Google Scholar] [CrossRef]
- Hoerauf, A.; Nissen-Pahle, K.; Schmetz, C.; Henkle-Duhrsen, K.; Blaxter, M.L.; Buttner, D.W.; Gallin, M.Y.; Al-Qaoud, K.M.; Lucius, R.; Fleischer, B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Investig. 1999, 103, 11–18. [Google Scholar] [CrossRef]
- Landmann, F.; Voronin, D.; Sullivan, W.; Taylor, M.J. Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes. PLoS Pathog. 2011, 7, e1002351. [Google Scholar] [CrossRef]
- Foray, V.; Perez-Jimenez, M.M.; Fattouh, N.; Landmann, F. Wolbachia Control Stem Cell Behavior and Stimulate Germline Proliferation in Filarial Nematodes. Dev. Cell 2018, 45, 198–211. [Google Scholar] [CrossRef]
- Slatko, B.E.; Luck, A.N.; Dobson, S.L.; Foster, J.M. Wolbachia endosymbionts and human disease control. Mol. Biochem. Parasitol. 2014, 195, 88–95. [Google Scholar] [CrossRef]
- Hoerauf, A.; Specht, S.; Buttner, M.; Pfarr, K.; Mand, S.; Fimmers, R.; Marfo-Debrekyei, Y.; Konadu, P.; Debrah, A.Y.; Bandi, C.; et al. Wolbachia endobacteria depletion by doxycycline as antifilarial therapy has macrofilaricidal activity in onchocerciasis: A randomized placebo-controlled study. Med. Microbiol. Immunol. 2008, 197, 295–311. [Google Scholar] [CrossRef]
- Turner, J.D.; Tendongfor, N.; Esum, M.; Johnston, K.L.; Langley, R.S.; Ford, L.; Faragher, B.; Specht, S.; Mand, S.; Hoerauf, A.; et al. Macrofilaricidal activity after doxycycline only treatment of Onchocerca volvulus in an area of Loa loa co-endemicity: A randomized controlled trial. PLoS Negl. Trop. Dis. 2010, 4, e660. [Google Scholar] [CrossRef]
- Debrah, A.Y.; Mand, S.; Marfo-Debrekyei, Y.; Batsa, L.; Albers, A.; Specht, S.; Klarmann, U.; Pfarr, K.; Adjei, O.; Hoerauf, A. Macrofilaricidal Activity in Wuchereria bancrofti after 2 Weeks Treatment with a Combination of Rifampicin plus Doxycycline. J. Parasitol. Res. 2011, 2011, 201617. [Google Scholar] [CrossRef]
- Debrah, A.Y.; Mand, S.; Marfo-Debrekyei, Y.; Batsa, L.; Pfarr, K.; Buttner, M.; Adjei, O.; Buttner, D.; Hoerauf, A. Macrofilaricidal effect of 4 weeks of treatment with doxycycline on Wuchereria bancrofti. Trop. Med. Int. Health 2007, 12, 1433–1441. [Google Scholar] [CrossRef]
- Taylor, M.J.; Makunde, W.H.; McGarry, H.F.; Turner, J.D.; Mand, S.; Hoerauf, A. Macrofilaricidal activity after doxycycline treatment of Wuchereria bancrofti: A double-blind, randomised placebo-controlled trial. Lancet 2005, 365, 2116–2121. [Google Scholar] [CrossRef]
- Hoerauf, A.; Mand, S.; Fischer, K.; Kruppa, T.; Marfo-Debrekyei, Y.; Debrah, A.Y.; Pfarr, K.M.; Adjei, O.; Buttner, D.W. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med. Microbiol. Immunol. 2003, 192, 211–216. [Google Scholar] [CrossRef]
- Debrah, A.Y.; Specht, S.; Klarmann-Schulz, U.; Batsa, L.; Mand, S.; Marfo-Debrekyei, Y.; Fimmers, R.; Dubben, B.; Kwarteng, A.; Osei-Atweneboana, M.; et al. Doxycycline Leads to Sterility and Enhanced Killing of Female Onchocerca volvulus Worms in an Area With Persistent Microfilaridermia After Repeated Ivermectin Treatment: A Randomized, Placebo-Controlled, Double-Blind Trial. Clin. Infect. Dis. 2015, 61, 517–526. [Google Scholar] [CrossRef]
- Walker, M.; Specht, S.; Churcher, T.S.; Hoerauf, A.; Taylor, M.J.; Basanez, M.G. Therapeutic efficacy and macrofilaricidal activity of doxycycline for the treatment of river blindness. Clin. Infect. Dis. 2015, 60, 1199–1207. [Google Scholar] [CrossRef]
- Serbus, L.R.; Landmann, F.; Bray, W.M.; White, P.M.; Ruybal, J.; Lokey, R.S.; Debec, A.; Sullivan, W. A cell-based screen reveals that the albendazole metabolite, albendazole sulfone, targets Wolbachia. PLoS Pathog. 2012, 8, e1002922. [Google Scholar] [CrossRef]
- White, P.M.; Pietri, J.E.; Debec, A.; Russell, S.; Patel, B.; Sullivan, W. Mechanisms of Horizontal Cell-to-Cell Transfer of Wolbachia spp. in Drosophila melanogaster. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Karpova, N.; Bobinnec, Y.; Fouix, S.; Huitorel, P.; Debec, A. Jupiter, a new Drosophila protein associated with microtubules. Cell Motil. Cytoskeleton 2006, 63, 301–312. [Google Scholar] [CrossRef]
- Emery, G.; Hutterer, A.; Berdnik, D.; Mayer, B.; Wirtz-Peitz, F.; Gaitan, M.G.; Knoblich, J.A. Asymmetric Rab 11 endosomes regulate delta recycling and specify cell fate in the Drosophila nervous system. Cell 2005, 122, 763–773. [Google Scholar] [CrossRef]
- White, P.M.; Serbus, L.R.; Debec, A.; Codina, A.; Bray, W.; Guichet, A.; Lokey, R.S.; Sullivan, W. Reliance of Wolbachia on High Rates of Host Proteolysis Revealed by a Genome-Wide RNAi Screen of Drosophila Cells. Genetics 2017, 205, 1473–1488. [Google Scholar] [CrossRef]
- Grobler, Y.; Yun, C.Y.; Kahler, D.J.; Bergman, C.M.; Lee, H.; Oliver, B.; Lehmann, R. Whole genome screen reveals a novel relationship between Wolbachia levels and Drosophila host translation. PLoS Pathog. 2018, 14, e1007445. [Google Scholar] [CrossRef]
- Fenollar, F.; Maurin, M.; Raoult, D. Wolbachia pipientis growth kinetics and susceptibilities to 13 antibiotics determined by immunofluorescence staining and real-time PCR. Antimicrob. Agents Chemother. 2003, 47, 1665–1671. [Google Scholar] [CrossRef]
- Sinha, A.; Li, Z.; Sun, L.; Carlow, C.K.S. Complete Genome Sequence of the Wolbachia wAlbB Endosymbiont of Aedes albopictus. Genome Biol. Evol. 2019. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Pettigrew, M.M.; Sinkins, S.P.; Braig, H.R.; Andreadis, T.G.; Tesh, R.B. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect. Mol. Biol. 1997, 6, 33–39. [Google Scholar] [CrossRef]
- Hermans, P.G.; Hart, C.A.; Trees, A.J. In vitro activity of antimicrobial agents against the endosymbiont Wolbachia pipientis. J. Antimicrob. Chemother. 2001, 47, 659–663. [Google Scholar] [CrossRef]
- Fenollar, F.; La Scola, B.; Inokuma, H.; Dumler, J.S.; Taylor, M.J.; Raoult, D. Culture and phenotypic characterization of a Wolbachia pipientis isolate. J. Clin. Microbiol. 2003, 41, 5434–5441. [Google Scholar] [CrossRef]
- Igarashi, A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J. Gen. Virol. 1978, 40, 531–544. [Google Scholar] [CrossRef]
- Turner, J.D.; Langley, R.S.; Johnston, K.L.; Egerton, G.; Wanji, S.; Taylor, M.J. Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J. Immunol. 2006, 177, 1240–1249. [Google Scholar] [CrossRef]
- Johnston, K.L.; Wu, B.; Guimaraes, A.; Ford, L.; Slatko, B.E.; Taylor, M.J. Lipoprotein biosynthesis as a target for anti-Wolbachia treatment of filarial nematodes. Parasites Vectors 2010, 3, 99. [Google Scholar] [CrossRef]
- Jacobs, R.T.; Lunde, C.S.; Freund, Y.R.; Hernandez, V.; Li, X.; Xia, Y.; Carter, D.S.; Berry, P.; Halladay, J.; Rock, F.; et al. Boron-Pleuromutilins as Anti-Wolbachia Agents with Potential for Treatment of Onchocerciasis and Lymphatic Filariasis. J. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Hong, W.D.; Benayoud, F.; Nixon, G.L.; Ford, L.; Johnston, K.L.; Clare, R.H.; Cassidy, A.; Cook, D.A.N.; Siu, A.; Shiotani, M.; et al. AWZ1066S, a highly specific anti-Wolbachia drug candidate for a short-course treatment of filariasis. Proc. Natl. Acad. Sci. USA 2019, 116, 1414–1419. [Google Scholar] [CrossRef]
- Johnston, K.L.; Cook, D.A.N.; Berry, N.G.; David Hong, W.; Clare, R.H.; Goddard, M.; Ford, L.; Nixon, G.L.; O’Neill, P.M.; Ward, S.A.; et al. Identification and prioritization of novel anti-Wolbachia chemotypes from screening a 10,000-compound diversity library. Sci. Adv. 2017, 3, eaao1551. [Google Scholar] [CrossRef]
- Johnston, K.L.; Ford, L.; Umareddy, I.; Townson, S.; Specht, S.; Pfarr, K.; Hoerauf, A.; Altmeyer, R.; Taylor, M.J. Repurposing of approved drugs from the human pharmacopoeia to target Wolbachia endosymbionts of onchocerciasis and lymphatic filariasis. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 278–286. [Google Scholar] [CrossRef]
- Clare, R.H.; Cook, D.A.; Johnston, K.L.; Ford, L.; Ward, S.A.; Taylor, M.J. Development and validation of a high-throughput anti-Wolbachia whole-cell screen: A route to macrofilaricidal drugs against onchocerciasis and lymphatic filariasis. J. Biomol. Screen. 2015, 20, 64–69. [Google Scholar] [CrossRef]
- Clare, R.H.; Bardelle, C.; Harper, P.; Hong, W.D.; Borjesson, U.; Johnston, K.L.; Collier, M.; Myhill, L.; Cassidy, A.; Plant, D.; et al. Industrial scale high-throughput screening delivers multiple fast acting macrofilaricides. Nat. Commun. 2019, 10, 11. [Google Scholar] [CrossRef]
- Wu, M.; Sun, L.V.; Vamathevan, J.; Riegler, M.; Deboy, R.; Brownlie, J.C.; McGraw, E.A.; Martin, W.; Esser, C.; Ahmadinejad, N.; et al. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004, 2, e69. [Google Scholar] [CrossRef]
- Bakowski, M.A.; Shiroodi, R.K.; Liu, R.; Olejniczak, J.; Yang, B.; Gagaring, K.; Guo, H.; White, P.M.; Chappell, L.; Debec, A.; et al. Discovery of short-course antiwolbachial quinazolines for elimination of filarial worm infections. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Xu, Z.; Fang, S.M.; Bakowski, M.A.; Rateb, M.E.; Yang, D.; Zhu, X.; Huang, Y.; Zhao, L.X.; Jiang, Y.; Duan, Y.; et al. Discovery of Kirromycins with Anti-Wolbachia Activity from Streptomyces sp. CB00686. ACS Chem. Biol. 2019. [Google Scholar] [CrossRef]
- Casper-Lindley, C.; Kimura, S.; Saxton, D.S.; Essaw, Y.; Simpson, I.; Tan, V.; Sullivan, W. Rapid fluorescence-based screening for Wolbachia endosymbionts in Drosophila germ line and somatic tissues. Appl. Environ. Microbiol. 2011, 77, 4788–4794. [Google Scholar] [CrossRef]
- Ferree, P.M.; Frydman, H.M.; Li, J.M.; Cao, J.; Wieschaus, E.; Sullivan, W. Wolbachia utilizes host microtubules and Dynein for anterior localization in the Drosophila oocyte. PLoS Pathog. 2005, 1, e14. [Google Scholar] [CrossRef]
- Kramer, L.H.; Passeri, B.; Corona, S.; Simoncini, L.; Casiraghi, M. Immunohistochemical/immunogold detection and distribution of the endosymbiont Wolbachia of Dirofilaria immitis and Brugia pahangi using a polyclonal antiserum raised against WSP (Wolbachia surface protein). Parasitol. Res. 2003, 89, 381–386. [Google Scholar] [CrossRef]
- Venard, C.M.; Crain, P.R.; Dobson, S.L. SYTO11 staining vs FISH staining: A comparison of two methods to stain Wolbachia pipientis in cell cultures. Lett. Appl. Microbiol. 2011, 52, 168–176. [Google Scholar] [CrossRef]
- Fischer, K.; Beatty, W.L.; Jiang, D.; Weil, G.J.; Fischer, P.U. Tissue and stage-specific distribution of Wolbachia in Brugia malayi. PLoS Negl. Trop. Dis. 2011, 5, e1174. [Google Scholar] [CrossRef]
- Sharma, R.; Al Jayoussi, G.; Tyrer, H.E.; Gamble, J.; Hayward, L.; Guimaraes, A.F.; Davies, J.; Waterhouse, D.; Cook, D.A.; Myhill, L.J.; et al. Minocycline as a re-purposed anti-Wolbachia macrofilaricide: Superiority compared with doxycycline regimens in a murine infection model of human lymphatic filariasis. Sci. Rep. 2016, 6, 23458. [Google Scholar] [CrossRef]
- Mutafchiev, Y.; Bain, O.; Williams, Z.; McCall, J.W.; Michalski, M.L. Intraperitoneal development of the filarial nematode Brugia malayi in the Mongolian jird (Meriones unguiculatus). Parasitol. Res. 2014, 113, 1827–1835. [Google Scholar] [CrossRef][Green Version]
- Voronin, D.; Tricoche, N.; Jawahar, S.; Shlossman, M.; Bulman, C.A.; Fischer, C.; Suderman, M.T.; Sakanari, J.A.; Lustigman, S. Development of a preliminary in vitro drug screening assay based on a newly established culturing system for pre-adult fifth-stage Onchocerca volvulus worms. PLoS Negl. Trop. Dis. 2019, 13, e0007108. [Google Scholar] [CrossRef]
- Bah, G.S.; Ward, E.L.; Srivastava, A.; Trees, A.J.; Tanya, V.N.; Makepeace, B.L. Efficacy of three-week oxytetracycline or rifampin monotherapy compared with a combination regimen against the filarial nematode Onchocerca ochengi. Antimicrob. Agents Chemother. 2014, 58, 801–810. [Google Scholar] [CrossRef]
- Landmann, F.; Bain, O.; Martin, C.; Uni, S.; Taylor, M.J.; Sullivan, W. Both asymmetric mitotic segregation and cell-to-cell invasion are required for stable germline transmission of Wolbachia in filarial nematodes. Biol. Open 2012, 1, 536–547. [Google Scholar] [CrossRef]
- Klarmann-Schulz, U.; Specht, S.; Debrah, A.Y.; Batsa, L.; Ayisi-Boateng, N.K.; Osei-Mensah, J.; Mubarik, Y.; Konadu, P.; Ricchiuto, A.; Fimmers, R.; et al. Comparison of Doxycycline, Minocycline, Doxycycline plus Albendazole and Albendazole Alone in Their Efficacy against Onchocerciasis in a Randomized, Open-Label, Pilot Trial. PLoS Negl. Trop. Dis. 2017, 11, e0005156. [Google Scholar] [CrossRef]
- Agwuh, K.N.; MacGowan, A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 2006, 58, 256–265. [Google Scholar] [CrossRef]
- Specht, S.; Pfarr, K.M.; Arriens, S.; Hubner, M.P.; Klarmann-Schulz, U.; Koschel, M.; Sternberg, S.; Martin, C.; Ford, L.; Taylor, M.J.; et al. Combinations of registered drugs reduce treatment times required to deplete Wolbachia in the Litomosoides sigmodontis mouse model. PLoS Negl. Trop. Dis. 2018, 12, e0006116. [Google Scholar] [CrossRef]
- Specht, S.; Mand, S.; Marfo-Debrekyei, Y.; Debrah, A.Y.; Konadu, P.; Adjei, O.; Buttner, D.W.; Hoerauf, A. Efficacy of 2- and 4-week rifampicin treatment on the Wolbachia of Onchocerca volvulus. Parasitol. Res. 2008, 103, 1303–1309. [Google Scholar] [CrossRef]
- Aljayyoussi, G.; Tyrer, H.E.; Ford, L.; Sjoberg, H.; Pionnier, N.; Waterhouse, D.; Davies, J.; Gamble, J.; Metuge, H.; Cook, D.A.N.; et al. Short-Course, High-Dose Rifampicin Achieves Wolbachia Depletion Predictive of Curative Outcomes in Preclinical Models of Lymphatic Filariasis and Onchocerciasis. Sci. Rep. 2017, 7, 210. [Google Scholar] [CrossRef]
- Schiefer, A.; Schmitz, A.; Schaberle, T.F.; Specht, S.; Lammer, C.; Johnston, K.L.; Vassylyev, D.G.; Konig, G.M.; Hoerauf, A.; Pfarr, K. Corallopyronin A specifically targets and depletes essential obligate Wolbachia endobacteria from filarial nematodes in vivo. J. Infect. Dis. 2012, 206, 249–257. [Google Scholar] [CrossRef]
- Schaberle, T.F.; Schiefer, A.; Schmitz, A.; Konig, G.M.; Hoerauf, A.; Pfarr, K. Corallopyronin A - a promising antibiotic for treatment of filariasis. Int. J. Med. Microbiol. 2014, 304, 72–78. [Google Scholar] [CrossRef]
- Hoerauf, A.; Volkmann, L.; Nissen-Paehle, K.; Schmetz, C.; Autenrieth, I.; Buttner, D.W.; Fleischer, B. Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: Comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop. Med. Int. Health 2000, 5, 275–279. [Google Scholar] [CrossRef]
- Prezioso, S.M.; Brown, N.E.; Goldberg, J.B. Elfamycins: Inhibitors of elongation factor-Tu. Mol. Microbiol. 2017, 106, 22–34. [Google Scholar] [CrossRef]
- Hansen, J.L.; Ippolito, J.A.; Ban, N.; Nissen, P.; Moore, P.B.; Steitz, T.A. The structures of four macrolide antibiotics bound to the large ribosomal subunit. Mol. Cell 2002, 10, 117–128. [Google Scholar] [CrossRef]
- von Geldern, T.W.; Morton, H.E.; Clark, R.F.; Brown, B.S.; Johnston, K.L.; Ford, L.; Specht, S.; Carr, R.A.; Stolarik, D.F.; Ma, J.; et al. Discovery of ABBV-4083, a novel analog of Tylosin A that has potent anti-Wolbachia and anti-filarial activity. PLoS Negl. Trop. Dis. 2019, 13, e0007159. [Google Scholar] [CrossRef]
- Taylor, M.J.; von Geldern, T.W.; Ford, L.; Hubner, M.P.; Marsh, K.; Johnston, K.L.; Sjoberg, H.T.; Specht, S.; Pionnier, N.; Tyrer, H.E.; et al. Preclinical development of an oral anti-Wolbachia macrolide drug for the treatment of lymphatic filariasis and onchocerciasis. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Turner, J.D.; Sharma, R.; Al Jayoussi, G.; Tyrer, H.E.; Gamble, J.; Hayward, L.; Priestley, R.S.; Murphy, E.A.; Davies, J.; Waterhouse, D.; et al. Albendazole and antibiotics synergize to deliver short-course anti-Wolbachia curative treatments in preclinical models of filariasis. Proc. Natl. Acad. Sci. USA 2017, 114, E9712–E9721. [Google Scholar] [CrossRef]
- Li, Z.; Garner, A.L.; Gloeckner, C.; Janda, K.D.; Carlow, C.K. Targeting the Wolbachia cell division protein FtsZ as a new approach for antifilarial therapy. PLoS Negl. Trop. Dis. 2011, 5, e1411. [Google Scholar] [CrossRef]
- Wu, B.; Novelli, J.; Foster, J.; Vaisvila, R.; Conway, L.; Ingram, J.; Ganatra, M.; Rao, A.U.; Hamza, I.; Slatko, B. The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Negl. Trop. Dis. 2009, 3, e475. [Google Scholar] [CrossRef]
- Lentz, C.S.; Halls, V.; Hannam, J.S.; Niebel, B.; Strubing, U.; Mayer, G.; Hoerauf, A.; Famulok, M.; Pfarr, K.M. A selective inhibitor of heme biosynthesis in endosymbiotic bacteria elicits antifilarial activity in vitro. Chem. Biol. 2013, 20, 177–187. [Google Scholar] [CrossRef][Green Version]
- Taylor, M.J.; Hoerauf, A.; Townson, S.; Slatko, B.E.; Ward, S.A. Anti-Wolbachia drug discovery and development: Safe macrofilaricides for onchocerciasis and lymphatic filariasis. Parasitology 2014, 141, 119–127. [Google Scholar] [CrossRef]
| Filarial Nematode | Common Host | Wolbachia | Vector | Disease | Location in Host | General Symptoms | Geographical Distribution | Ref. |
|---|---|---|---|---|---|---|---|---|
| Onchocerca volvulus | humans | yes | black flies, Simulium spp. | onchocerciasis, aka. river blindness | adults in subcutaneous nodules, mf migrate through skin and eyes | skin disease (onchodermatitis: itching, depigmentation), onchocercomata (nodules), blindness, neurological disease (nodding syndrome, Nakalanga syndrome, epilepsy) | sub-Saharan Africa, small foci in South America and Yemen | [1,2] |
| Onchocerca ochengi | cattle (experimental models: mice) | yes | black flies, Simulium spp. | onchocerciasis aka. onchocercosis | adults in intradermal nodules, occasionally in subcutis | intradermal nodules (noted due to damage to bovine hides); other clinical impact unknown | documented in Uganda and Cameroon; used as a model to study filarial infection | [3,4] |
| Wuchereria bancrofti | humans (~90% of LF cases) | yes | mosquitoes | lymphatic filariasis, aka. Bancroftian filariasis | adults in lymphatic vessels; mf in peripheral blood with varying periodicities | mostly asymptomatic but with time cause damage to lymphatic system and kidneys; chronic symptoms include lymphoedema, elephantiasis, hydroceles;acute symptoms include local inflammation, fevers, secondary bacterial infections, acute filarial lymphangitis, acute dermatolymphangioadenitis | tropics in Asia, Africa, Pacific, and Americas | [1,5,6,7,8,9,10] |
| Brugia malayi | humans (experimental models: mice, jirds) | yes | lymphatic filariasis, aka. Brugian filariasis | East and South Asia | ||||
| Brugia timori | humans | yes | Indonesia and Timor-Leste | |||||
| Brugia pahangi | cats, dogs, rarely humans (experimental models: jirds) | yes | Malaysia, Thailand, and Indonesia | |||||
| Mansonella ozzardi | humans | yes | biting midges (mostly Culicoides) and black flies, Simulium spp. | ozzardi mansonellosis | uncertain; adults potentially in subcutaneous tissues/thoracic and peritoneal cavity; mf in blood and skin | potential ocular lesions; mostly asymptomatic but also fever, headaches, itching, joint pain, rash, sensation of coldness in the legs, foot and face edema, keratitis | Caribbean, the Amazon, border between Bolivia and Argentina | [11,12] |
| Mansonella perstans | humans and primates | yes (potentially strain dependent) | biting midges (Culicoides) | mansonellosis | adults in serous body cavities, may also appear subcutaneously; mf in blood | mostly asymptomatic; occasionally Calabar swellings, itching, pruritus, joint pain, enlarged lymph glands, neurological symptoms | western, eastern, central Africa; equatorial Brazil to Caribbean | [13,14] |
| Mansonella streptocerca | humans and primates | not reported | biting midges (Culicoides) | mansonellosis | adults in subcutaneous tissues; mf in skin | mostly asymptomatic; occasionally dermatitis, pruritus, rash, papular skin, inguinal adenopathy, dizziness | western, eastern, central Africa | [13] |
| Litomosoides sigmodontis (aka. Litomosoides carinii in older literature) | cotton rats Sigmodon hispidus (experimental models: rats, Mastomys, mice, jirds) | yes | rat mites (Ornithonyssus bacoti) | cotton-rat filariasis | adults in pleural cavity (less commonly in peritoneal cavity); mf in peripheral blood | can cause wasting and affect survival; pathological changes in lungs, spleen and lymphatics; scattered myocarditis | likely southeastern United States, Mexico, and Central America; used as a model to study filarial infection | [15,16,17,18,19] |
| Dirofilaria immitis | companion animals (mainly dogs but also cats, ferrets) and wild animals (wolves, coyotes, foxes, pinnipeds, raccoons, etc.); can also infect humans with D. repens infecting humans to a greater extent than D. immitis | yes | mosquitoes | dirofilariasis/ dirofilariosis, aka. heartworm disease | heart and pulmonary arteries | in dogs: cough, exercise intolerance, fainting, coughing up blood, severe weight loss, congestive heart failure | most countries with temperate, semitropical or tropical climates | [20,21] |
| Dirofilaria repens | subcutaneous dirofilariasis/ dirofilariosis | adults in subcutaneous tissues; mf in peripheral bloodstream | mostly asymptomatic; occasionally pruritus, dermal swelling, subcutaneous nodules containing the parasite, and ocular conjunctivitis | Europe, Asia, Africa | [22] | |||
| Loa loab | humans (experimental models: primates (e.g., baboons), rodents) | no | deerflies, genus Chrysops | loiasis, aka. African eye worm | connective tissue | mostly asymptomatic, eye worm, Calabar swellings, itching, tiredness, muscle and joint pain, hives | West and Central Africa | [23] |
| Ivermectin | Diethylcarbamazine (DEC) | Albendazole |
|---|---|---|
 macrocyclic lactone | 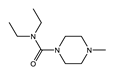 piperazine derivative | 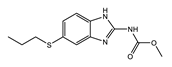 benzimidazole |
| MOA not fully understood; causes paralysis by binding to glutamate-gated chloride channels of parasitic worms affecting their motility, feeding, and reproduction | MOA not fully understood; inhibitor of arachidonic acid metabolism in microfilariae and host; dependent on host iNOS; likely a host innate immunity mediated effect | Blocks parasite microtububle assembly (binds to β-tubulin); most active against intestinal parasites |
| Disease | Areas not Co-endemic with Onchocerciasis | Areas Co-endemic with Onchocerciasis | Areas Co-endemic with Loiasis |
|---|---|---|---|
| Onchocer-ciasis | – | ivermectin (150–200 µg/kg) | not advised; test and not treat strategies investigated |
| Lymphatic filariasis | once a year DEC (6 mg/kg) and albendazole (400 mg); 2018–2019 start triple therapy in select countries | once a year ivermectin (200 µg/kg) with albendazole (400 mg) | twice a year albendazle (400 mg) |
| Loiasis | DEC or albendazole; treatment not always recommended due to risk of SAEs; no programs currently in place to control or eliminate loiasis | ||
| Filarial Nematode | Significance | Wolbachia | |||
|---|---|---|---|---|---|
| Strain | Super-group | Genome Size (Mb) * | Proteins * | ||
| Onchocerca volvulus | clinical | wOv | C | 0.96 | 649 |
| Onchocerca ochengi | advanced screen for drug and vaccine development [3,4] | wOo | C | 0.96 | 651 |
| Onchocerca gutturosa | in vitro screen for drug development [40,41] | wOg | C | – | – |
| Dirofilaria immitis | dog heartworm (veterinary) [42] | wDim | C | 0.92 | 823 |
| Wuchereria bancrofti | clinical | wWb | D | 1.06 (draft) | 961 (draft) |
| Brugia malayi | clinical; rodent efficacy model for drug and vaccine development [4] | wBm | D | 1.08 | 839 |
| Brugia timori | clinical | wBt | D | – | – |
| Brugia pahangi | rodent efficacy model for drug and vaccine development [17] | wBp | D | 1.4 (draft) | 803 (draft) |
| Litomosoides sigmodontis | rodent efficacy model for drug and vaccine development [17,18,43,44,45] | wLs | D | Data available, but not yet published ** | |
| Loa loa | clinical; microfilarial counter-screen for drug development [46] | – | – | – | – |
| Cell Line | Wolbachia | References | |||||
|---|---|---|---|---|---|---|---|
| Cell Line | Species | Markers | Strain | Super-group | Genome Size (Mb) | Proteins | |
| Aa23 | Ae. albopictus | – | wAlb | B | 1.48 | 1205 | [77,78,79,80] |
| C6/36 | Ae. albopictus | – | [81,82,83,84,85,86,87,88,89,90] | ||||
| JW18 | D. melanogaster | Jupiter-GFP | wMel | A | 1.27 | 1100 | [71,72,91] |
| LDW1 | D. melanogaster | Jupiter-GFP, Histone-RFP | [72,75,85,92,93] | ||||
| Quantification Method | Advantages | Disadvantages | Applied to HTS? |
|---|---|---|---|
| Giemsa | simple, inexpensive | non-specific | no |
| Propidium iodide | simple, inexpensive | non-specific | no |
| DAPI | simple, inexpensive | non-specific | 384-well [71] |
| Syto 11 | simple, moderately priced | non-specific | 384-well [89] |
| qPCR | Wolbachia specific | complex, higher expense | 96-well [87,88] |
| qRT-PCR | Wolbachia specific | much more complex, higher expense | no |
| Immuno-fluorescence | Wolbachia specific, simple | higher expense, relies on limited reagent (anti-Wolbachia antibody), more complex than one-reagent protocols (e.g., Syto 11) | 384-well [90] |
| 16S rRNA FISH | Wolbachia specific, simple, inexpensive customizable probes | more complex than one-reagent protocols (e.g., Syto 11) | 1536-well [92,93] |
| AWZ1066S [86] | CBR490 [92] | CBR417 [92] | |
|---|---|---|---|
| Series | azaquinazoline | quinazoline (methylpyridine) | quinazoline (oxadiazole) |
| Structure | 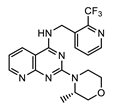 |  | 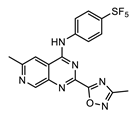 |
| Activity | C6/36 EC50 = 2.5 nM; microfilariae EC50 = 121 nM; | LDW1 EC50 = 33 nM; B. pahangi ovaries ex vivo EC50 < 111 nM | LDW1 EC50 = 24 nM; B. pahangi ovaries ex vivo EC50 = 356 nM |
| Efficacy | >99% wLS depletion in adult female L. sigmodontis: 50 mg/kg bid 7 days >90% wBm depletion in adult female B. malayi: 100 mg/kg bid 7 days | >99% wLS depletion in adult female L. sigmodontis: 200 mg/kg SINGLE DOSE, or 100 mg/kg one dose given per week ×2, or 30 mg/kg qd (quaque die, once a day) for 7 days | >99% wLS depletion in adult female L. sigmodontis: 200 mg/kg SINGLE DOSE, or 100 mg/kg one dose given per week x2, or 60 mg/kg qd for 4 days |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakowski, M.A.; McNamara, C.W. Advances in Antiwolbachial Drug Discovery for Treatment of Parasitic Filarial Worm Infections. Trop. Med. Infect. Dis. 2019, 4, 108. https://doi.org/10.3390/tropicalmed4030108
Bakowski MA, McNamara CW. Advances in Antiwolbachial Drug Discovery for Treatment of Parasitic Filarial Worm Infections. Tropical Medicine and Infectious Disease. 2019; 4(3):108. https://doi.org/10.3390/tropicalmed4030108
Chicago/Turabian StyleBakowski, Malina A., and Case W. McNamara. 2019. "Advances in Antiwolbachial Drug Discovery for Treatment of Parasitic Filarial Worm Infections" Tropical Medicine and Infectious Disease 4, no. 3: 108. https://doi.org/10.3390/tropicalmed4030108
APA StyleBakowski, M. A., & McNamara, C. W. (2019). Advances in Antiwolbachial Drug Discovery for Treatment of Parasitic Filarial Worm Infections. Tropical Medicine and Infectious Disease, 4(3), 108. https://doi.org/10.3390/tropicalmed4030108





