The Financial Burden of Tuberculosis for Patients in the Western-Pacific Region
Abstract
:1. Introduction
1.1. What are Catastrophic Costs in the Context of the End TB Strategy?
1.2. How Does “Catastrophic Costs” Differ from “Catastrophic Health Spending”?
2. Costs of TB Care in the Western Pacific Region
2.1. Costs of TB Care in Studies not Using the WHO Methodology to Measure Catastrophic Costs
2.2. Measuring the Costs of TB Care Using Nationally Representative TB Patient Cost Surveys
3. Program and Policy Implications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Implementing the End TB Strategy: The Essentials; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Tanimura, T.; Jaramillo, E.; Weil, D.; Raviglione, M.; Lönnroth, K. Financial burden for tuberculosis patients in low- and middle-income countries: A systematic review. Eur. Respir. J. 2014, 43, 1763–1775. [Google Scholar] [CrossRef] [PubMed]
- Barter, D.; Agboola, S.; Murray, M.; Barnighausen, T. Tuberculosis and poverty: The contribution of patient costs in sub-Saharan Africa—A systematic review. BMC Public Health 2012, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, T.; Boccia, D.; Tovar, M.; Gavino, A.; Zevallos, K.; Montoya, R.; Lonnroth, K.; Evans, C.A. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: A prospective cohort study, Peru. PLoS Med. 2014, 11, e1001675. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Western Pacific Region Office. Regional Framework for Action on Implementation of the End TB Strategy in the Western Pacific: 2016–2020; World Health Organization Western Pacific Region Office: Manila, Philippines, 2016. [Google Scholar]
- World Health Organization. Tuberculosis Patient Cost Surveys: A Handbook; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. Health Financing: Monitoring Sustainable Development Goals—Indicator 3.8.2; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/health_financing/topics/financial-protection/monitoring-sdg/en/ (accessed on 1 May 2019).
- World Health Organization. UHC Service Coverage Index; World Health Organization: Geneva, Switzerland, 2019; Available online: http://apps.who.int/gho/data/node.wrapper.imr?x-id=4834 (accessed on 1 May 2019).
- United Nations. SDG Indicators: Metadata Repository; United Nations: New York, NY, USA, 2019; Available online: https://unstats.un.org/sdgs/metadata/ (accessed on 1 May 2019).
- Hutchison, C.; Khan, M.; Yoong, J.; Lin, X.; Coker, R. Financial barriers and coping strategies: A qualitative study of accessing multidrug-resistant tuberculosis and tuberculosis care in Yunnan, China. BMC Public Health 2017, 17, 221. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Smith, H.; Zhang, T.; Tang, S.; Garner, P. Patient medical costs for tuberculosis treatment and impact on adherence in China: A systematic review. BMC Public Health 2011, 11, 393. [Google Scholar] [CrossRef] [PubMed]
- Nam, V.; Nhung, N.; Hoa, N.; Thuy, H.; Phuong, N.; Anh, N.; Anh, L.T.N.; Trung, V.Q.; Ha, T.V.; Meeyai, A.; et al. Economic burden of multidrug-resistant tuberculosis: A multicenter study across Vietnamese regions. Int. J. Tuberc. Lung Dis. 2018, 22, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, C.; Jiang, H.; Pan, H.-Q.; Zhu, L.-M.; Lu, W. High admission rates and heavy inpatient service costs of urban tuberculosis patients in eastern China. BMC Health Serv. Res. 2019, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Bele, S.; Feng, Y.; Qiu, S.; Lü, J.; Tang, S.; Shen, H.; Wang, J.; Zhu, L. Analysis of the economic burden of diagnosis and treatment of tuberculosis patients in rural China. Int. J. Tuberc. Lung Dis. 2013, 17, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Morishita, F.; Yadav, R.-P.; Eang, M.T.; Saint, S.; Nishikiori, N. Mitigating financial burden of tuberculosis through active case finding targeting household and neighbourhood contacts in Cambodia. PLoS ONE 2016, 11, e0162796. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Li, R.; Wang, X.; Wang, L.; Sun, Q.; Chen, C.; Xu, C.; Su, W.; Zhao, J.; Pang, Y.; et al. The affordability for patients of a new universal MDR-TB coverage model in China. Int. J. Tuberc. Lung Dis. 2016, 20, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, W.; Wang, Q.; Shen, Y.; Gao, J.; Sato, K.D.; Long, Q.; Lucas, H. Non-medical financial burden in tuberculosis care: A cross-sectional survey in rural China. Infect. Dis. Poverty 2016, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Watts, K. Is Australia neglecting the local topography when it comes to catastrophic costs and ending tuberculosis? Trop. Med. Infect. Dis. 2018, 3, 126. [Google Scholar] [CrossRef] [PubMed]
- Lönnroth, K.; Tran, T.-U.; Quy, H.T.; Diwan, V. Can I afford free treatment? Perceived consequences of health care provider choices among people with tuberculosis in Ho Chi Minh City, Vietnam. Soc. Sci. Med. 2001, 52, 935–948. [Google Scholar] [CrossRef]
- Pichenda, K.; Nakamura, K.; Morita, A.; Kizuki, M.; Seino, K.; Takano, T. Non-hospital DOT and early diagnosis of tuberculosis reduce costs while achieving treatment success. Int. J. Tuberc. Lung Dis. 2012, 16, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Rahevar, K.; Nishikiori, N.; Viney, K.; Choi, H.; Biermann, O.; Kin, H.J.; Nou, C.; Kim, S.; Zhu, G.; et al. Action towards universal health coverage and social protection for tuberculosis care and prevention: Workshop on the end TB strategy pillar 2 in the western pacific region 2017. Trop. Med. Int. Health 2019, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.; Hoa, N.; Anh, N.; Ngoc Anh, L.; Siroka, A.; Lönnroth, K.; Garcia, B.I. Measuring catastrophic costs due to tuberculosis in Vietnam. Int. J. Tuberc. Lung Dis. 2018, 22, 983–990. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Systems Universal Health Coverage; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/healthsystems/universal_health_coverage/en/ (accessed on 1 May 2019).
- Lönnroth, K.; Glaziou, P.; Weil, D.; Floyd, K.; Uplekar, M.; Raviglione, M. Beyond UHC: Monitoring health and social protection coverage in the context of tuberculosis care and prevention. PLoS Med. 2014, 11, e1001693. [Google Scholar] [CrossRef] [PubMed]

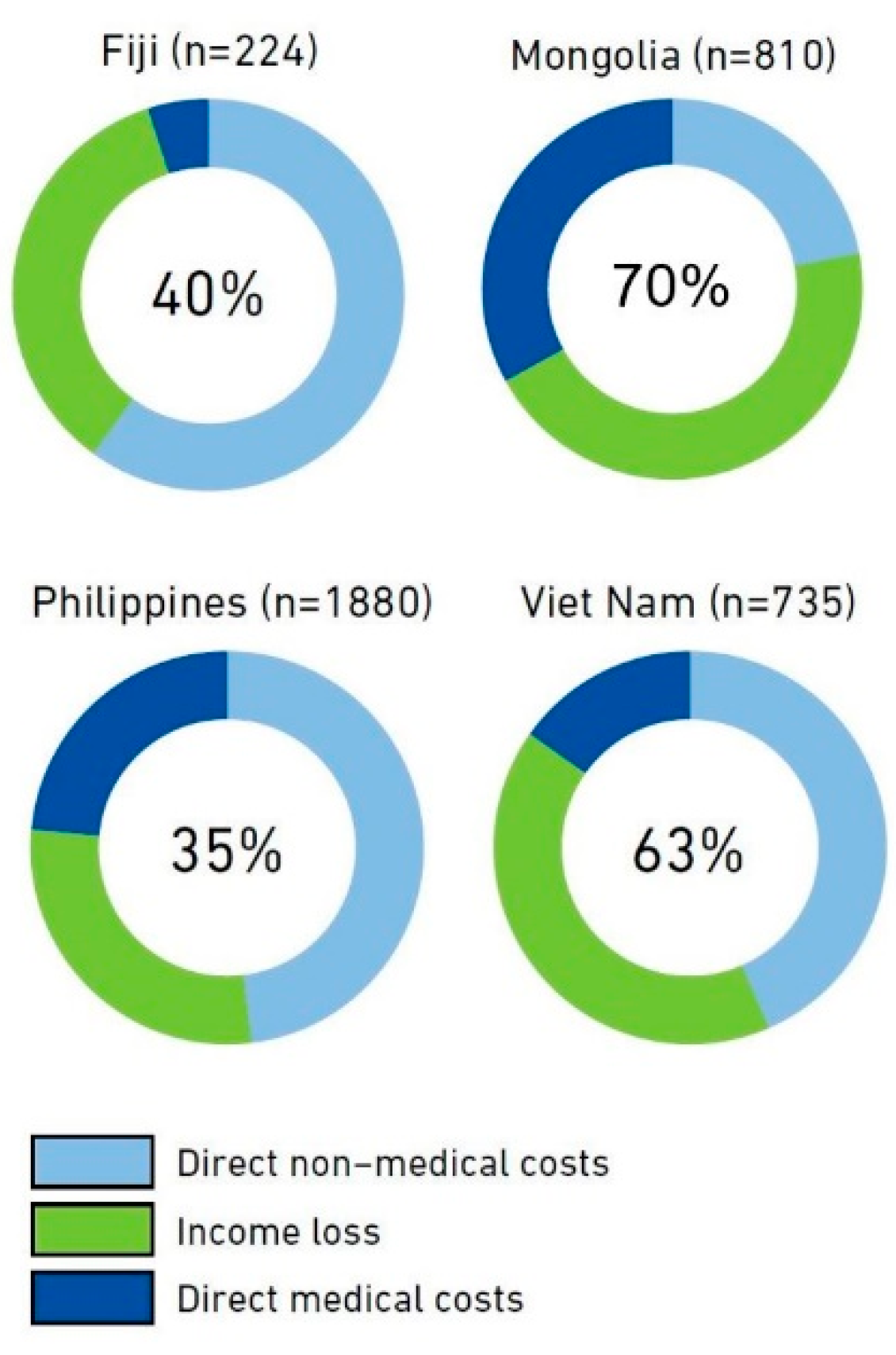
| Country | Catastrophic Out-of-Pocket Health Spending a (SDG 3.8.2) | Catastrophic Costs Related to TB Care | Universal Health Coverage Service Coverage Index (SDG 3.8.1) b |
|---|---|---|---|
| Fiji c | 3.37% | 40% | 66 |
| Mongolia d | 2.39% | 70% | 63 |
| Philippines e | 6.31% | 35% | 58 |
| Vietnam f | 9.81% | 63% | 73 |
| Cost Category | Possible Changes in Service Delivery | TB Patient Social Support and Social Protection Schemes |
|---|---|---|
| Direct medical: before TB diagnosis | Streamline the TB patient pathway:
| Reduce/subsidize/eliminate out-of-pocket payments (OOPs):
|
| Direct medical: after TB diagnosis | Expand free-of-charge or highly subsidized TB service package including TB medicines, ancillary drugs, monitoring of adverse events, preventive treatment: Promote integrated management of comorbidities and risk factors (HIV, diabetes, other lung diseases, tobacco smoking, harmful use of alcohol): Improve the quality of TB care:
| Reduce/subsidize/eliminate OOP:
|
| Direct non-medical | Advocate local health-seeking and for care models bringing services close to patients, including community- and workplace-based care: Improve the quality of nutritional advice and regulate irrational nutritional recommendations by health care providers (e.g., supplements) | Provide assistance via TB program:
Engage NGOs, civil society organizations and patient groups to ensure patient support suitable for the locality |
| Indirect costs (income loss) | Range of interventions to enable earlier diagnosis and patient-centered care delivery that minimize time spent seeking and receiving care (decentralization, shorter waiting times, fewer health care visits, avoid unnecessary hospitalization, etc.): Improve access to social services:
| Facilitate enrolment of eligible patients/households in existing social protection schemes:
Legislate and/or enforce provisions related to social, economic, and labor rights to protect individuals during TB illness and care |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viney, K.; Islam, T.; Hoa, N.B.; Morishita, F.; Lönnroth, K. The Financial Burden of Tuberculosis for Patients in the Western-Pacific Region. Trop. Med. Infect. Dis. 2019, 4, 94. https://doi.org/10.3390/tropicalmed4020094
Viney K, Islam T, Hoa NB, Morishita F, Lönnroth K. The Financial Burden of Tuberculosis for Patients in the Western-Pacific Region. Tropical Medicine and Infectious Disease. 2019; 4(2):94. https://doi.org/10.3390/tropicalmed4020094
Chicago/Turabian StyleViney, Kerri, Tauhidul Islam, Nguyen Binh Hoa, Fukushi Morishita, and Knut Lönnroth. 2019. "The Financial Burden of Tuberculosis for Patients in the Western-Pacific Region" Tropical Medicine and Infectious Disease 4, no. 2: 94. https://doi.org/10.3390/tropicalmed4020094
APA StyleViney, K., Islam, T., Hoa, N. B., Morishita, F., & Lönnroth, K. (2019). The Financial Burden of Tuberculosis for Patients in the Western-Pacific Region. Tropical Medicine and Infectious Disease, 4(2), 94. https://doi.org/10.3390/tropicalmed4020094





