Latent Tuberculosis Infection among Healthcare Workers in Duhok Province: From Screening to Prophylactic Treatment
Abstract
1. Background
2. Materials and Methods
2.1. Study Design and Data Recruitment
2.2. Sample Collection
2.3. Laboratory Investigations
2.4. Radiological Examination
2.5. Risk Stratifications
- Low risk: administration units, fertility clinic, surgery unit, and the primary health care centers.
- Moderate risk: pharmacy units and radiography units.
- High risk: TB healthcare facilities, medical wards, laboratory units, and emergency units.
2.6. Statistical Analysis
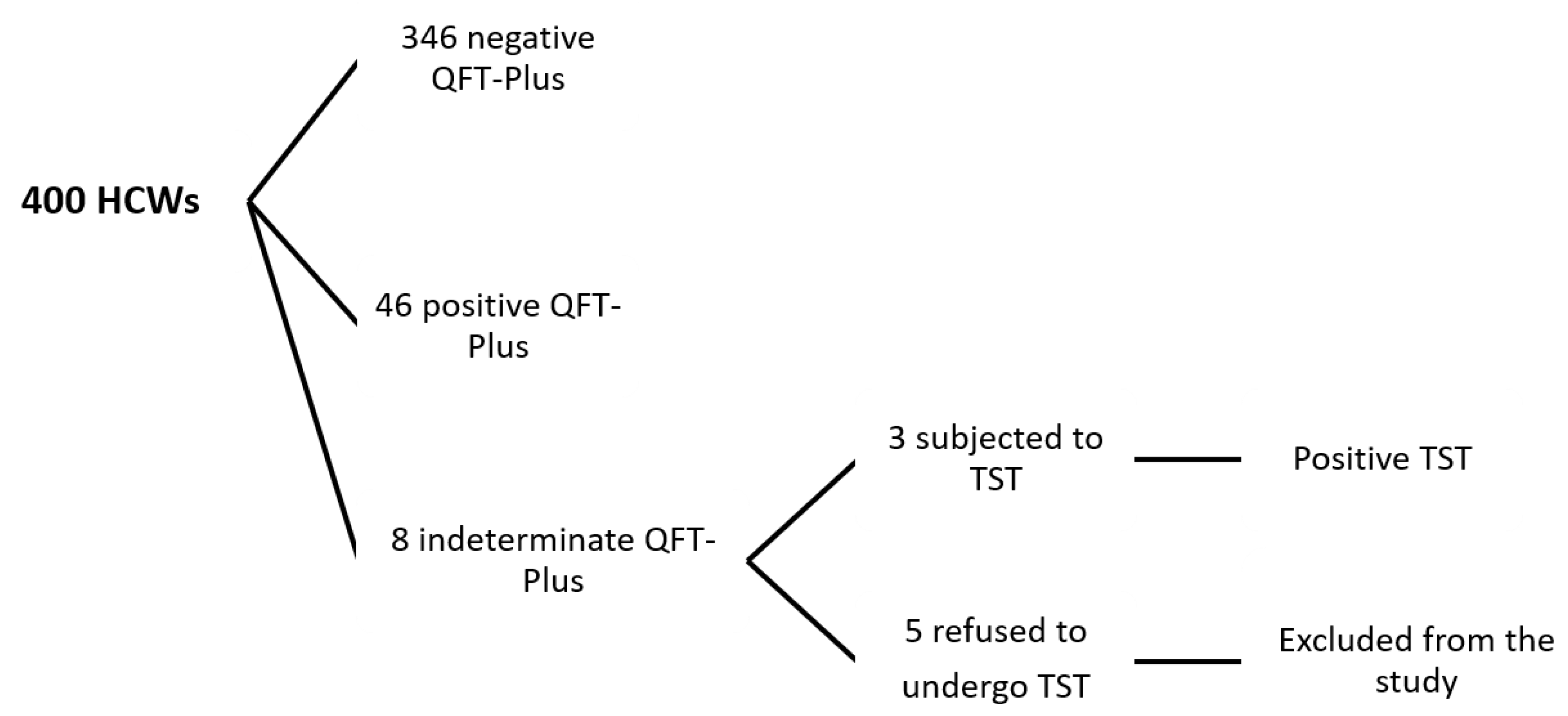
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. What Is DOTS?: A Guide to Understanding the WHO-Recommended TB Control Strategy Known as DOTS; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Houben, R.M.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Kiazyk, S.; Ball, T. Tuberculosis (TB): Latent tuberculosis infection: An overview. Can. Commun. Dis. Rep. 2017, 43, 62. [Google Scholar] [CrossRef]
- Christopoulos, A.I.; Diamantopoulos, A.A.; Dimopoulos, P.A.; Goumenos, D.S.; Barbalias, G.A. Risk factors for tuberculosis in dialysis patients: A prospective multi-center clinical trial. BMC Nephrol. 2009, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, A.; Verma, G.; Humar, A.; Kumar, D. Outcome of latent tuberculosis infection in solid organ transplant recipients over a 10-year period. Transplantation 2014, 98, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Corbett, E.L.; Muzangwa, J.; Chaka, K.; Dauya, E.; Cheung, Y.B.; Munyati, S.S.; Reid, A.; Hakim, J.; Chandiwana, S.; Mason, P.R. Nursing and community rates of Mycobacterium tuberculosis infection among students in Harare, Zimbabwe. Clin. Infect. Dis. 2007, 44, 317–323. [Google Scholar] [CrossRef][Green Version]
- Merza, M.A.; Haji, S.M.; Alsharafani, A.M.H.; Muhammed, S.U. Low prevalence of hepatitis B and C among tuberculosis patients in Duhok Province, Kurdistan: Are HBsAg and anti-HCV prerequisite screening parameters in tuberculosis control program? Int. J. Mycobacteriol. 2016, 5, 313–317. [Google Scholar] [CrossRef]
- Hussein, N.R. Prevalence of HBV, HCV and HIV and Anti-HBs antibodies positivity in healthcare workers in departments of surgery in Duhok City, Kurdistan Region, Iraq. Int. J. Pure Appl. Sci. Technol. 2015, 26, 70. [Google Scholar]
- Al-Orainey, I.O. Diagnosis of latent tuberculosis: Can we do better? Ann. Thorac. Med. 2009, 4, 5. [Google Scholar] [CrossRef]
- World Health Organization. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children; World Health Organization: Geneva, Switzerland, 2014; ISBN 9241548746. [Google Scholar]
- He, G.X.; van den Hof, S.; van der Werf, M.J.; Wang, G.J.; Ma, S.W.; Zhao, D.Y.; Hu, Y.L.; Yu, S.C.; Borgdorff, M.W. Infection control and the burden of tuberculosis infection and disease in health care workers in china: A cross-sectional study. BMC Infect. Dis. 2010, 10, 313. [Google Scholar] [CrossRef]
- Uplekar, M.; Weil, D.; Lonnroth, K.; Jaramillo, E.; Lienhardt, C.; Dias, H.M.; Falzon, D.; Floyd, K.; Gargioni, G.; Getahun, H. WHO’s new end TB strategy. Lancet 2015, 385, 1799–1801. [Google Scholar] [CrossRef]
- World Health Organization. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management; World Health Organization: Geneva, Switzerland, 2018; Available online: http://www.whoint/tb/publications/2018/latent-tuberculosis-infection/en/ (accessed on 6 September 2018).
- World Health Organization. Global Tuberculosis Report 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Castro, K.G.; Goldberg, S.; Jereb, J.A.; LoBue, P.; Mazurek, G.H.; Vernon, A. Updated Guidelines for Using Interferon Gamma Release Assays to Detect Mycobacterium Tuberculosis Infection—United States, 2010; Center for Disease Control and Prevention: Atlanta, GA, USA, 2010. [Google Scholar]
- Konstantinos, A. Testing for tuberculosis. Aust. Prescr. 2010, 33, 12–18. [Google Scholar] [CrossRef]
- Joshi, R.; Reingold, A.L.; Menzies, D.; Pai, M. Tuberculosis among health-care workers in low-and middle-income countries: A systematic review. PLoS Med. 2006, 3, e494. [Google Scholar] [CrossRef] [PubMed]
- Al-Lami, F.; Armean, P.; Al-Ameri, A.H. Prevalence of Latent TB Infection among Health Care Workers in Three Main TB Health Facilities, Baghdad, Iraq, 2013. J. Fac. Med. 2014, 56, 339–342. [Google Scholar]
- Bozkanat, E.; Kaya, H.; Sezer, O.; Caliskan, T.; Kilic, E.; Ciftci, F.; Gümüs, S.; Kartaloglu, Z. Comparison of tuberculin skin test and quantiferon-TB gold in tube test for diagnosis of latent tuberculosis infection in health care workers: A cross sectional study. JPMA 2016, 66, 270–274. [Google Scholar]
- Mostafavi, E.; Nasehi, M.; Shahraki, A.H.; Esmaeili, S.; Ghaderi, E.; Sharafi, S.; Doosti-Irani, A. Comparison of the tuberculin skin test and the QuantiFERON-TB Gold test in detecting latent tuberculosis in health care workers in Iran. Epidemiol. Health 2016, 38, e2016032. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abbas, M.; AlHamdan, N.; Fiala, L.A.; AlEnezy, A.K.; AlQahtani, M.S. Prevalence of latent TB among health care workers in four major tertiary care hospitals in Riyadh, Saudi Arabia. J. Egypt. Public Health Assoc. 2010, 85, 61–71. [Google Scholar] [PubMed]
- Hibah, N.A.A.; Hasan, H.-E.A. Prevalence of latent tuberculosis infection among multinational healthcare workers in Muhayil Saudi Arabia. Egypt. J. Bronchol. 2015, 9, 183. [Google Scholar] [CrossRef]
- Rafiza, S.; Rampal, K.G.; Tahir, A. Prevalence and risk factors of latent tuberculosis infection among health care workers in Malaysia. BMC Infect. Dis. 2011, 11, 19. [Google Scholar] [CrossRef]
- Harada, N.; Nakajima, Y.; Higuchi, K.; Sekiya, Y.; Rothel, J.; Mori, T. Screening for tuberculosis infection using whole-blood interferon-γ and Mantoux testing among Japanese healthcare workers. Infect. Control Hosp. Epidemiol. 2006, 27, 442–448. [Google Scholar] [CrossRef]
- Hung, W.-T.; Lee, S.S.-J.; Sy, C.-L.; Wu, K.-S.; Chen, J.-K.; Tsai, H.-C.; Chen, Y.-S. Prevalence of latent tuberculosis infection in BCG-vaccinated healthcare workers by using an interferon-gamma release assay and the tuberculin skin test in an intermediate tuberculosis burden country. J. Microbiol. Immunol. Infect. 2015, 48, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.-W.; Hong, Y.; Park, J.S.; Bae, I.-G.; Eom, J.S.; Lee, S.-R.; Cho, O.-H.; Choo, E.J.; Heo, J.Y.; Woo, J.H. Prevalence of latent tuberculosis infection among health care workers in South Korea: A multicenter study. Tuberc. Respir. Dis. 2013, 75, 18–24. [Google Scholar] [CrossRef]
- Pai, M.; Gokhale, K.; Joshi, R.; Dogra, S.; Kalantri, S.; Mendiratta, D.K.; Narang, P.; Daley, C.L.; Granich, R.M.; Mazurek, G.H. Mycobacterium tuberculosis infection in health care workers in rural India: Comparison of a whole-blood interferon γ assay with tuberculin skin testing. JAMA 2005, 293, 2746–2755. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, H.; Liu, F.; Pan, L.; Xing, A.; Gu, S.; Du, B.; Sun, Q.; Wei, R.; Zhang, Z. Prevalence and risk factors for latent tuberculosis infection among health care workers in China: A cross-sectional study. PLoS ONE 2013, 8, e66412. [Google Scholar] [CrossRef]
- Schablon, A.; Harling, M.; Diel, R.; Nienhaus, A. Risk of latent TB infection in individuals employed in the healthcare sector in Germany: A multicentre prevalence study. BMC Infect. Dis. 2010, 10, 107. [Google Scholar] [CrossRef]
- Christopher, D.J.; Daley, P.; Armstrong, L.; James, P.; Gupta, R.; Premkumar, B.; Michael, J.S.; Radha, V.; Zwerling, A.; Schiller, I. Tuberculosis infection among young nursing trainees in South India. PLoS ONE 2010, 5, e10408. [Google Scholar] [CrossRef]
- Menzies, D.; Joshi, R.; Pai, M. Risk of tuberculosis infection and disease associated with work in health care settings [state of the art series. Occupational lung disease in high-and low-income countries, edited by M. Chan-Yeung. Number 5 in the series]. Int. J. Tuberc. Lung Dis. 2007, 11, 593–605. [Google Scholar]
- Simou, E.; Britton, J.; Leonardi-Bee, J. Alcohol consumption and risk of tuberculosis: A systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 2018, 22, 1277–1285. [Google Scholar] [CrossRef]
- Szabo, G. Alcohol’s contribution to compromised immunity. Alcohol Health Res. World 1997, 21, 30–41. [Google Scholar]
- Cuhadaroglu, C.; Erelel, M.; Tabak, L.; Kilicaslan, Z. Increased risk of tuberculosis in health care workers: A retrospective survey at a teaching hospital in Istanbul, Turkey. BMC Infect. Dis. 2002, 2, 14. [Google Scholar] [CrossRef]
- Schablon, A.; Beckmann, G.; Harling, M.; Diel, R.; Nienhaus, A. Prevalence of latent tuberculosis infection among health care workers in a hospital for pulmonary diseases. J. Occup. Med. Toxicol. 2009, 4, 1. [Google Scholar] [CrossRef]
- Colson, P.; Hirsch-Moverman, Y.; Bethel, J.; Vempaty, P.; Salcedo, K.; Wall, K.; Miranda, W.; Collins, S.; Horsburgh, C.; Consortium, T.E.S. Acceptance of treatment for latent tuberculosis infection: Prospective cohort study in the United States and Canada. Int. J. Tuberc. Lung Dis. 2013, 17, 473–479. [Google Scholar] [CrossRef]
- Arguello Perez, E.; Seo, S.K.; Schneider, W.J.; Eisenstein, C.; Brown, A.E. Management of latent tuberculosis infection among healthcare workers: 10-year experience at a single center. Clin. Infect. Dis. 2017, 65, 2105–2111. [Google Scholar] [CrossRef]
- Joseph, H.A.; Shrestha-Kuwahara, R.; Lowry, D.; Lambert, L.A.; Panlilio, A.L.; Raucher, B.G.; Holcombe, J.M.; Poujade, J.; Rasmussen, D.M.; Wilce, M. Factors influencing health care workers’ adherence to work site tuberculosis screening and treatment policies. Am. J. Infect. Control 2004, 32, 456–461. [Google Scholar] [CrossRef]
- Steele, M.A.; Burk, R.F.; DesPrez, R.M. Toxic hepatitis with isoniazid and rifampin: A meta-analysis. Chest 1991, 99, 465–471. [Google Scholar] [CrossRef]
| Parameter | No. (%) * |
|---|---|
| Age | |
| Mean ± SD | 33.4 ± 9.25 |
| Gender | |
| Male | 193 (48.9) |
| Female | 202 (51.1) |
| Cigarette smokers | |
| Yes | 75 (19.0) |
| No | 320 (81.0) |
| Alcohol consumption | |
| Yes | 18 (4.6) |
| No | 377 (95.4) |
| Diabetics | |
| Yes | 15 (3.8) |
| No | 380 (96.2) |
| BCG vaccination | |
| Yes | 384 (97.2) |
| No | 11 (2.8) |
| History of TB contact | |
| Yes | 38 (9.6) |
| No | 357 (90.4) |
| Years of employment | |
| Mean ± SD | 9.09 ± 8.39 |
| Nationality | |
| Iraqi | 387 (98.0) |
| Syrian | 6 (1.5) |
| Turkish | 2 (0.5) |
| Occupation | |
| Medical doctors | 38 (9.6) |
| Pharmacists | 11 (2.8) |
| Dentists | 4 (1.0) |
| Nurses | 132 (33.4) |
| Paramedics | 92 (23.3) |
| Laboratory Technicians | 60 (15.2) |
| Administrative staff | 39 (9.9) |
| Cleaners | 19 (4.8) |
| Variable | IGRA Positive (No. = 49) No. (%) | IGRA Negative (No. = 346) No. (%) | Total (No. = 39) No. (%) | Odd Ratio (95% CI) | p Value |
|---|---|---|---|---|---|
| Age group (year) | |||||
| ≤29 * | 10 (20.4) | 163 (47.1) | 173 (43.8) | - | - |
| 30–39 | 21 (42.9) | 99 (28.6) | 120 (30.4) | 3.46 (1.56–7.64) | 0.0013 |
| 40–49 | 13 (26.5) | 63 (18.2) | 76 (19.2) | 3.36 (1.40–8.06) | 0.0045 |
| ≥50 | 5 (10.2) | 21 (6.1) | 26 (6.6) | 3.88 (1.21–12.45) | 0.0154 |
| Gender | |||||
| Male | 30 (61.2) | 163 (47.1) | 193 (48.9) | 1.77 (0.96–3.27) | 0.06 |
| Female | 19 (38.8) | 183 (52.9) | 202 (51.1) | ||
| Cigarette smokers | |||||
| Yes | 9 (18.4) | 66 (19.1) | 75 (19) | 0.96 (0.44–2.06) | 0.91 |
| No | 40 (81.6) | 280 (80.9) | 320 (81) | ||
| Alcohol consumption | |||||
| Yes | 5 (10.2) | 13 (3.8) | 18 (4.6) | 2.91 (0.99–8.56) | 0.05 |
| No | 44 (89.8) | 333 (96.2) | 377 (95.4) | ||
| Diabetics | |||||
| Yes | 2 (4.1) | 13 (3.8) | 15 (3.8) | 1.09 (0.24–4.98) | 0.91 |
| No | 47 (95.9) | 333 (96.2) | 380 (96.2) | ||
| BCG status | |||||
| Yes | 48 (98) | 336 (97.1) | 384 (97.2) | 1.43 (0.18–11.41) | 0.74 |
| no | 1(2) | 10 (2.9) | 11 (2.8) | ||
| History of close TB contact | |||||
| Yes | 6 (12.2) | 32 (9.2) | 38 (9.6) | 1.37 (0.54–3.47) | 0.51 |
| No | 43 (87.8) | 314 (90.8) | 357 (90.4) | ||
| Nationality | |||||
| Iraqi | 48 (98) | 339 (98) | 387 (98) | NA | 0.25 ** |
| Syrian | 0 (0) | 6(1.7) | 6 (1.5) | ||
| Turkey | 1 (2) | 1 (0.3) | 2 (0.5) | ||
| Years of employment | |||||
| ≤1 * | 2 (4.1) | 45 (13) | 47 (11.9) | – | – |
| 2–5 | 13 (26.5) | 133 (38.4) | 146 (37) | 2.20 (0.48–10.12) | 0.301 |
| 6–10 | 11 (22.5) | 67 (19.4) | 78 (19.7) | 3.69 (0.78–17.46) | 0.099 |
| ≥11 | 23 (46.9) | 101 (29.2) | 124 (31.4) | 5.12 (1.16–22.67) | 0.018 |
| Risk stratification of workplaces | |||||
| Low * | 8 (16.3) | 95 (27.4) | 103 (26.1) | - | - |
| Moderate | 3 (6.1) | 53 (15.3) | 56 (14.2) | 0.67 (0.17–2.642) | 0.57 |
| High | 38 (77.6) | 198 (57.2) | 236 (59.7) | 2.28 (1.02–5.07) | 0.044 |
| Occupation | |||||
| Medical doctors | 9 (18.4) | 29 (8.4) | 38 (9.6) | 2.46 (1.09–5.57) | 0.03 |
| Pharmacists | 2 (4.1) | 9 (2.6) | 11 (2.8) | 1.59 (0.33–7.60) | 0.55 |
| Dentists | 0 (0) | 4 (1.2) | 4 (1) | 0.77 (0.05–14.50) | 0.86 ** |
| Nurses | 16 (32.6) | 116 (33.5) | 132 (33.4) | 0.96 (0.51–1.82) | 0.9 |
| Paramedics | 8 (16.3) | 84 (24.3) | 92 (23.3) | 0.61 (0.27–1.35) | 0.22 |
| Laboratory technicians | 6 (12.3) | 54 (15.6) | 60 (15.2) | 0.76 (0.31–1.86) | 0.54 |
| Administrative staff | 3 (6.1) | 36 (10.4) | 39 (9.9) | 2.70 (0.93–7.84) | 0.59 |
| Cleaners | 5 (10.2) | 14 (4) | 19 (4.8) | 0.56 (0.17–1.90) | 0.35 |
| Variables | Odd Ratio (95% CI) | p Value |
|---|---|---|
| Age group | ||
| ≤29 | - | 0.121 |
| 30–39 | 0.288 (0.105–0.794) | 0.016 |
| 40–49 | 0.330 (0.100–1.090) | 0.069 |
| ≥50 | 0.332 (0.074–1.499) | 0.152 |
| Alcohol consumption | ||
| Yes | 0.325 (0.098–1.077) | 0.066 |
| No | ||
| Employment years | ||
| ≤1 | - | 0.920 |
| 2–5 | 0.590 (0.120–2.889) | 0.515 |
| 6–10 | 0.545 (0.094–3.164) | 0.499 |
| ≥11 | 0.546 (0.091–3.256) | 0.506 |
| Risk stratification of workplaces | ||
| Low | - | 0.150 |
| Moderate | 0.563 (0.177–1.792) | 0.331 |
| High | 0.436 (0.188–1.007) | 0.052 |
| Job as a doctor | 0.468 (0.203–1.082) | 0.076 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almufty, H.B.; Abdulrahman, I.S.; Merza, M.A. Latent Tuberculosis Infection among Healthcare Workers in Duhok Province: From Screening to Prophylactic Treatment. Trop. Med. Infect. Dis. 2019, 4, 85. https://doi.org/10.3390/tropicalmed4020085
Almufty HB, Abdulrahman IS, Merza MA. Latent Tuberculosis Infection among Healthcare Workers in Duhok Province: From Screening to Prophylactic Treatment. Tropical Medicine and Infectious Disease. 2019; 4(2):85. https://doi.org/10.3390/tropicalmed4020085
Chicago/Turabian StyleAlmufty, Hind Bahzad, Ibtesam Salih Abdulrahman, and Muayad Aghali Merza. 2019. "Latent Tuberculosis Infection among Healthcare Workers in Duhok Province: From Screening to Prophylactic Treatment" Tropical Medicine and Infectious Disease 4, no. 2: 85. https://doi.org/10.3390/tropicalmed4020085
APA StyleAlmufty, H. B., Abdulrahman, I. S., & Merza, M. A. (2019). Latent Tuberculosis Infection among Healthcare Workers in Duhok Province: From Screening to Prophylactic Treatment. Tropical Medicine and Infectious Disease, 4(2), 85. https://doi.org/10.3390/tropicalmed4020085





