Emergence of Melioidosis in Indonesia and Today’s Challenges
Abstract
1. Introduction
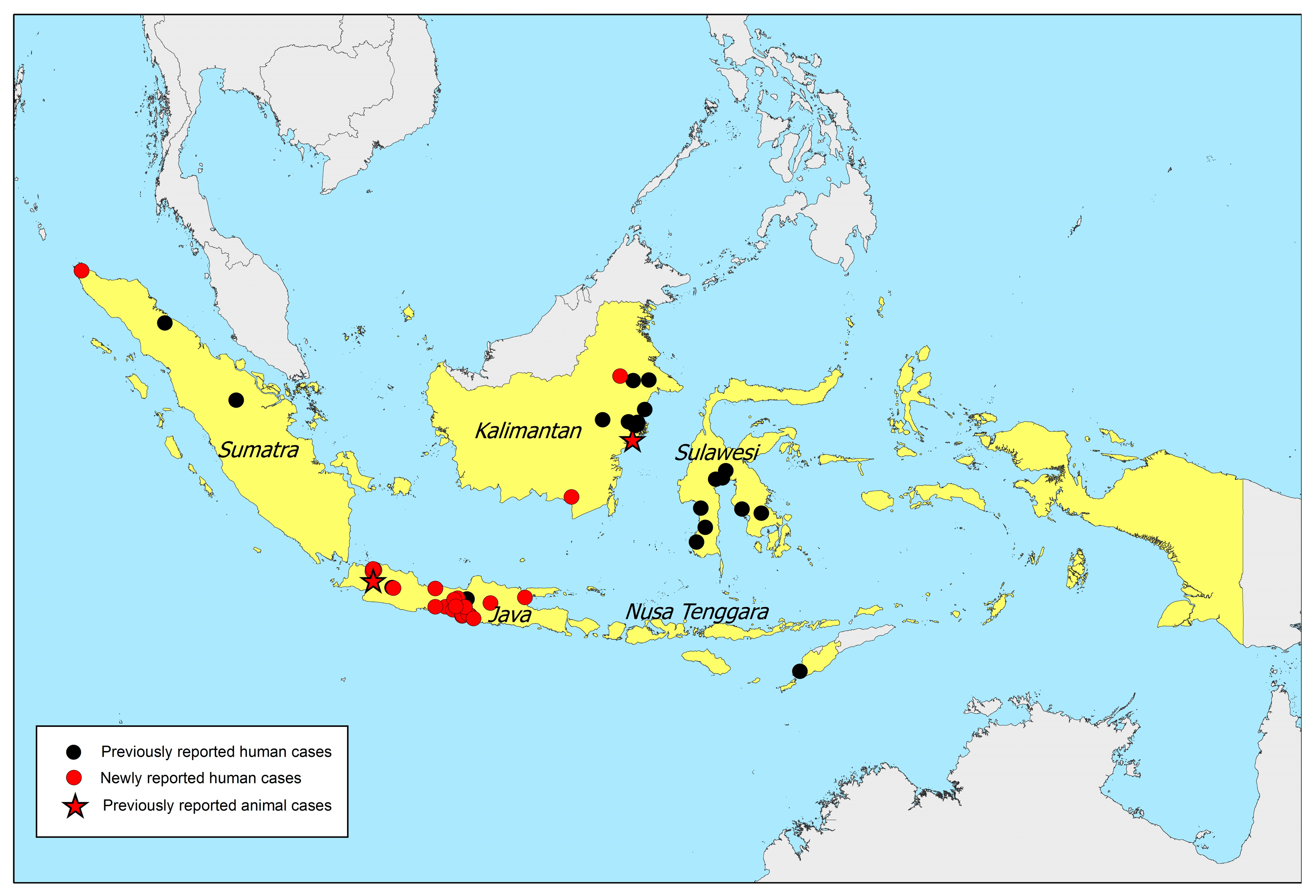
2. Melioidosis Cases and Presence of B. pseudomallei in Indonesia
3. Current Recommendations and Availability of Measures against Melioidosis
4. Surveillance Systems and Reporting of Melioidosis in Indonesia
5. Awareness of Melioidosis in Indonesia
6. Current and Future Challenges
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Soekarnen, M.C.E.; van de Walle, N. Melioidosis op Java. Geneeskd. Tijdschr. Ned. Indie 1932, 72, 1618–1635. [Google Scholar]
- Pet, M.A.; Fossen, A. Melioidosis der inwendige organen (melioidosis of internal organs). Geneeskd. Tijdschr. Ned. Indie 1934, 74, 976–981. [Google Scholar]
- Bezemer, F. Melioidosis op Celebes. Geneeskd. Tijdschr. Ned. Indie 1935, 75, 1577–1579. [Google Scholar]
- Sudibyo, R.M.S. Twee gevallen van huidmelioidosis. Geneeskd. Tijdschr. Ned. Indie 1938, 78, 1424–1444. [Google Scholar]
- Dunlop, S.J. Rapid recovery in a case of melioidosis. Doc. Med. Geogr. Trop. 1952, 4, 296–300. [Google Scholar] [PubMed]
- Athan, E.; Allworth, A.M.; Engler, C.; Bastian, I.; Cheng, A.C. Melioidosis in tsunami survivors. Emerg. Infect. Dis. 2005, 11, 1638–1639. [Google Scholar] [CrossRef] [PubMed]
- Irmawanti-Rahayu, S.; Noorhamdani, A.S.; Santoso, S. Resistance pattern of Burkholderia pseudomallei from clinical isolates at Dr. Saifulanwar General Hospital, Malang-Indonesia. J. Clin. Microbiol. Infect. Dis. 2014, 1, 17–20. [Google Scholar]
- Tauran, P.M.; Sennang, N.; Rusli, B.; Wiersinga, W.J.; Dance, D.; Arif, M.; Limmathurotsakul, D. Emergence of melioidosis in Indonesia. Am. J. Trop. Med. Hyg. 2015, 93, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.J.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Steel, N. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Hoffmaster, A.R.; AuCoin, D.; Baccam, P.; Baggett, H.C.; Baird, R.; Bhengsri, S.; Blaney, D.D.; Brett, P.J.; Brooks, T.J.; Brown, K.A.; et al. Melioidosis diagnostic workshop, 2013. Emerg. Infect. Dis. 2015, 21. [Google Scholar] [CrossRef]
- Doker, T.J.; Quinn, C.L.; Salehi, E.D.; Sherwood, J.J.; Benoit, T.J.; Glass Elrod, M.; Gee, J.E.; Shadomy, S.V.; Bower, W.A.; Hoffmaster, A.R.; et al. Fatal Burkholderia pseudomallei infection initially reported as a Bacillus species, Ohio, 2013. Am. J. Trop. Med. Hyg. 2014, 91, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Teerawattanasook, N.; Tauran, P.M.; Teparrukkul, P.; Wuthiekanun, V.; Dance, D.A.B.; Arif, M.; Limmathurotsakul, D. Capacity and utilization of blood culture in two referral hospitals in Indonesia and Thailand. Am. J. Trop. Med. Hyg. 2017, 97, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Bonne, C.J.; Hennemann, J.P.; Schijveschuurder, W. Een merkwaardig geval van bronchostenose als gevolg van melioidosis. Geneeskd. Tijdschr. Ned. Indie 1939, 79, 877–884. [Google Scholar]
- Brockelmann, C.H. Melioidosis in Java. Z. Tropenmed. Parasitol. 1961, 12, 451–460. [Google Scholar] [PubMed]
- Verbunt, J.A. Decollatio cutis torpida. Melioidosis. Geneeskd. Tijdschr. Ned. Indie 1937, 77, 1318–1319. [Google Scholar]
- Prabandari, S.; Arifin, E.; Rosmanah, L.; Kartika, D.; Narani, A.; Iskandriati, D.; Pamungkas, J. Fatal Burkholderia (Pseudomonas) pseudomallei septicemia in a cynomolgus monkey (Macaca fascicularis) at facility of Primate Research Center, Bogor Agricultural University. In Proceedings of the Kyoto University and Bogor Agricultural University International Symposium, Bogor, Indonesia, 18–21 August 2014. [Google Scholar]
- Pasaribu, A.P.; Pasaribu, S. A case of multiple abdominal abscesses due to melioidosis: First case reported from North Sumatera, Indonesia. In Proceedings of the 15th Asia-Pacific Congress on Clinical Microbiology and Infection, Kuala Lumpur, Malaysia, 26–29 November 2014. [Google Scholar]
- Aman, A.T.; Paramita, D.; Heryono, F.; Mawarti, Y. A Melioidosis case (sub-mandibular abscess) in Yogyakarta. In Proceedings of the Workshop on Melioidosis: Detection, Diagnosis, Treatment and Prevention Using a One Health Approach, Bogor, West Java, Indonesia, 14–16 August 2017. [Google Scholar]
- Tauran, P.M.; Wahyunie, S.; Graciella, M. Indonesian melioidosis cases: Kalimantan, Sulawesi and Nusa Tenggara. In Proceedings of the Workshop on Melioidosis: Detection, Diagnosis, Treatment and Prevention Using A One Health Approach, Bogor, West Java, Indonesia, 14–16 August 2017. [Google Scholar]
- Anggraini, D. Cases of melioidosis in private hospital in Pekanbaru. In Proceedings of the Annual Scientific Meeting (ASM) PAMKI, From Basic Microbiology to Clinical Applied, Approaches to New Technologies in Microbial Diagnostic, Padang, West Sumatra, Indonesia, 12–14 October 2017. [Google Scholar]
- Lestari, D.C.; Ibrahim, F.; Karuniawati, A.; Pratamiutaminingsih, A.; Chrisnawaty, D. Melioidosis Confirmed from Non-human Primate Specimens. In Proceedings of the Annual Scientific Meeting (ASM) PAMKI, From Basic Microbiology to Clinical Applied, Approaches to New Technologies in Microbial Diagnostic, Padang, West Sumatra, Indonesia, 12–14 October 2017. [Google Scholar]
- Thaipadungpanit, J.; Chierakul, W.; Wuthiekanun, V.; Limmathurotsakul, D.; Amornchai, P.; Boonslip, S.; Smythe, L.D.; Limpaiboon, R.; Hoffmaster, A.R.; Day, N.P.; et al. Diagnostic accuracy of real-time PCR assays targeting 16S rRNA and lipL32 genes for human leptospirosis in Thailand: A case-control study. PLoS ONE 2011, 6, e16236. [Google Scholar] [CrossRef]
- Novak, R.T.; Glass, M.B.; Gee, J.E.; Gal, D.; Mayo, M.J.; Currie, B.J.; Wilkins, P.P. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J. Clin. Microbiol. 2006, 44, 85–90. [Google Scholar] [CrossRef] [PubMed]
- MORU. The Map of Melioidosis Cases. Available online: http://www.melioidosis.info/ (accessed on 9 March 2018).
- Lipsitz, R.; Garges, S.; Aurigemma, R.; Baccam, P.; Blaney, D.D.; Cheng, A.C.; Currie, B.J.; Dance, D.; Gee, J.E.; Larsen, J.; et al. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg. Infect. Dis. 2012, 18, e2. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.T.; Hoang, T.S.; Tran, D.A.; Trinh, V.T.; Gohler, A.; Nguyen, T.T.; Hoang, S.N.; Krumkamp, R.; Nguyen, L.T.N.; May, J.; et al. A simple laboratory algorithm for diagnosis of melioidosis in resource-constrained areas: A study from north-central Vietnam. Clin. Microbiol. Infect. 2018, 24, 84. [Google Scholar] [CrossRef] [PubMed]
- Deepaka, R.N.; Crawleyb, B.; Phangc, E. Burkholderia pseudomallei identification: A comparison between the API 20NE and Vitek 2 GN systems. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, S42–S44. [Google Scholar] [CrossRef]
- Zong, Z.; Wang, X.; Deng, Y.; Zhou, T. Misidentification of Burkholderia pseudomallei as Burkholderia cepacia by the VITEK 2 system. J. Med. Microbiol. 2012, 61, 1483–1484. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-B.; Lee, T.; Jeong, J.; Chun, J.-H.; Shin, Y.-W.; Jung, J. Accidental occupational exposure to Burkholderia pseudomallei in South Korea did not result in melioidosis. Infect. Control Hosp. Epidemiol. 2017, 38, 886–888. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koh, T.H.; Yong Ng, L.S.; Foon Ho, J.L.; Sng, L.H.; Wang, G.C.Y.; Valentine Tzer Pin Lin, R. Automated identification systems and Burkholderiapseudomallei. J. Clin. Microbiol. 2003, 41, 1809. [Google Scholar] [CrossRef] [PubMed]
- ISID. The Program for Monitoring Emerging Diseases. Available online: https://www.promedmail.org/ (accessed on 9 March 2018).
- Choy, J.L.; Mayo, M.; Janmaat, A.; Currie, B.J. Animal melioidosis in Australia. Acta Trop. 2000, 74, 153–158. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Thammasart, S.; Warrasuth, N.; Thapanagulsak, P.; Jatapai, A.; Pengreungrojanachai, V.; Anun, S.; Joraka, W.; Thongkamkoon, P.; Saiyen, P.; et al. Melioidosis in animals, Thailand, 2006–2010. Emerg. Infect. Dis. 2012, 18, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Skinner, B.L.; Dietz, S.M.; Blaney, D.; Engel, R.M.; Lathrop, G.W.; Hoffmaster, A.R.; Gee, J.E.; Elrod, M.G.; Powell, N.; et al. Natural infection of Burkholderia pseudomallei in an imported pigtail macaque (Macaca nemestrina) and management of the exposed colony. Comp. Med. 2013, 63, 528–535. [Google Scholar] [PubMed]
- Hicks, C.L.; Kinoshita, R.; Ladds, P.W. Pathology of melioidosis in captive marine mammals. Aust. Vet. J. 2000, 78, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Sprague, L.D.; Neubauer, H. Melioidosis in animals: A review on epizootiology, diagnosis and clinical presentation. J. Vet. Med. B Infect. Dis. Vet. Public Health 2004, 51, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Benoit, T.J.; Blaney, D.D.; Gee, J.E.; Elrod, M.G.; Hoffmaster, A.R.; Doker, T.J.; Bower, W.A.; Walke, H.T. Melioidosis Cases and Selected Reports of Occupational Exposures to Burkholderi apseudomallei—United States, 2008–2013; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2015. [Google Scholar]
- Suntornsut, P.; Wongsuwan, N.; Malasit, M.; Kitphati, R.; Michie, S.; Peacock, S.J.; Limmathurotsakul, D. Barriers and recommended interventions to prevent melioidosis in northeast Thailand: A Focus group study using the behaviour change wheel. PLoS Negl. Trop. Dis. 2016, 10, e0004823. [Google Scholar] [CrossRef] [PubMed]
| Year Presented (References) | Locations | Age(Years)/Gender, Nationality | Clinical Characteristics | Diagnostic Method | Outcome |
|---|---|---|---|---|---|
| 1929 [1] | Cikande, Java | 50/M, Indonesian | Chronic painless nodules in the left thigh | Culture of pus (biochemistry, phenotypic tests and virulence in animal model) | Died |
| 1934 [2] | Jakarta, Java | 38/M, Indonesian | Severe sepsis with pulmonary, splenic and prostatic abscesses | Culture of pus (biochemistry, phenotypic tests and virulence in animal model) | Died |
| 1935 [3] | Surabaya, Java | 25/F, Indonesian | Abscess in the right gluteal region | Culture of pus (biochemistry, phenotypic tests and virulence in animal model) | Fully recovered |
| 1936 [4,16] | Bogor, Java | 60/M, Indonesian | Skin lesion with ulcers on right lower leg after trauma | Culture of pus (biochemistry and phenotypic tests) | Fully recovered |
| 1937 [4] | Jakarta, Java | 55/M, Indonesian | Abscess left foot, originated from minor trauma while farming | Culture of pus (biochemistry and phenotypic tests) | Fully recovered |
| 1938 [14] * | Cimahi, Java | 48/Unknown, European | Pneumonia and splenic abscess | Culture of pus (biochemistry, phenotypic tests and virulence in animal model) | Died |
| 1950 [5] | Surabaya, Java | 28/F, European | Pain in the lower abdomen and high fever | Culture of abscess from the right ovary (biochemistry and phenotypic tests) | Fully recovered |
| 1958 [15] * | Salatiga, Java | Unknown | Diarrhoea | Culture of stool (biochemistry and phenotypic tests) | Died |
| 2005 [6] | Banda Aceh, Sumatra | 4 patients; 15/F, 18 mo/M, 10/F and 13/F | Pneumonia | Culture of sputum (API20NE) | Fully recovered (n = 1) or reported as improving (n = 3) |
| 2011–2013 [7] | Malang, Java | 51 patients (unknown age and sex) | Unknown | Culture of sputum, blood, pus and urine (VITEK2) | Unknown |
| 2012 [17] * | Bogor, Java | 3/Unknown, cynomolgus monkey | General weakness, decreased appetite, dehydration and cough | Culture of pus (VITEK2) | Died |
| 2013–2014 [8] | Luwu Timur (n = 1) and Makassar (n = 2), Sulawesi | 3 patients; 41/M, 45/F and 26/M, Indonesian | Sepsis (n = 1), neck abscess, sepsis and pneumonia (n = 1), and abscess behind the left ear lobe (n = 1) | Culture of blood (n = 1) and pus (n = 2) (VITEK2) | Died (n = 2) or lost to follow-up (n = 1) |
| 2013 [19] * | Yogyakarta, Java | 53/F, Indonesian | Neck abscess, pain and dyspnoea. | Culture of pus (Microbact) | Fully recovered |
| 2014 [18] * | Medan, Sumatra | 13/M, Indonesian | Fever, dry cough, weight loss and abdominal abscesses | Culture of pus (VITEK2) | Fully recovered |
| 2017 [22] * | Samboja, Kalimantan | Unknown age and sex, Borneo orangutan | Loss of appetite, malaise, less active and apparent fever. | Culture of lung, spleen, and livertissue (VITEK2) | Died |
| 2010–2017 [21] * | Pekanbaru, Sumatra | 9 patients (mean age 52 years; range 34–67 years), all males and all Indonesian | Pneumonia, sepsis, abscess, cellulitis, osteomyelitis, pericarditis, seizure and decreased consciousness, and chronic suppurative otitis media with intratemporal complication. | Culture of sputum (n = 4), blood (n = 3) and pus (n = 3) (VITEK2) | Unknown |
| 2014–2017 [20] * | Samarinda, Kalimantan (n = 13), Makassar, Sulawesi (n = 8) and Kupang, Nusa Tenggara (n = 1) | 22 patients (median age 53.5 years; range 4–69 years), 15 males and 7 females, and all Indonesian | Sepsis, pneumonia, alteration of consciousness, and localized abscesses | Culture of blood (n = 11), pus (n = 7), tissue (n = 2) and urine (n = 2) (VITEK2) (PCR assay targeting type III secretion system in 9 cases) | Died (n = 9), Fully recovered (n = 9), and Unknown (n = 4) |
| Year Presented | Locations | Age(years)/Gender, Nationality | Clinical Characteristics | Diagnostic Method | Outcome |
|---|---|---|---|---|---|
| 2010 | Ulin Hospital, Banjarmasin, Kalimantan | Unknown/M | Unknown | Culture of blood (VITEK2) | Died |
| 2010–2017 | Private laboratory, Surabaya, Java | 8 patients (unknown) | Unknown | Culture of sputum (n = 4), blood (n = 2), urine (n = 1) and nasopharyngeal swab (n = 1) (VITEK2) | Unknown |
| 2012–2016 | Hasan Sadikin Hospital, Bandung, Java | 8 patients (unknown) | Unknown | Culture of blood (n = 5), body fluid (n = 3; unknown type of body fluid), pus (n = 1) (VITEK2) | Unknown |
| 2012–2017 | Cipto Mangunkusumo Hospital, Jakarta, Java | 4 patients (unknown) | Unknown | Culture of blood (n = 1), pus (n = 1), sputum (n = 1), cerebrospinal fluid (n = 1) (VITEK2) | Unknown |
| 2012–2017 | Tarakan Hospital, Jakarta, Java | 5 patients, 1 mo/M, 3 mo/M, 10 do/M, 2 mo/M and 59/M | Pneumonia (2), diarrhoea (1), alteration of consciousness (2) | Culture of blood (n = 4) and sputum (n = 1) (Microgen) | Died (n = 2), Fully recovered (n = 3) |
| 2012–2017 | Sardjito Hospital, Yogyakarta, Java | 18 patients (median age 7.5 years; range 1 day–78 years), 13 males and 5 females, and all Indonesian | Sepsis, pneumonia, alteration of conscious, localized abscesses and urinary tract infection. | Culture of blood (n = 11), pus (n = 3), and urine (n = 5) (VITEK2) | Died (n = 7), Fully recovered (n = 11) |
| 2017 | Zainoel Abidin Hospital, Banda Aceh, Sumatra | 33/M | Unknown | Culture of endotracheal secretion (Vitek2) | Unknown |
| Characteristics | Total Patients (n = 42) | Pediatric Patients (n = 15) | Adult Patients (n = 27) |
|---|---|---|---|
| Demographic information | |||
| Median age (IQR and range) | 41.5y (8.8m–56y, 1d–78y | 2m (10d–9.5m, 1d–11y) | 55y (47–59.5y, 21–78y) |
| Male sex | 32 (76%) | 10 (67%) | 22 (82%) |
| Organ involvement * | |||
| Bacteraemia | 25 (60%) | 14 (93%) | 11 (41%) |
| Pneumonia | 11 (25%) | 3 (20%) | 8 (30%) |
| Skin and Soft tissue | 9 (21%) | 1 (7%) | 8 (30%) |
| Genitourinary | 7 (17%) | 0 (0%) | 7 (26%) |
| Osteomyelitis | 1 (3%) | 0 (0%) | 1 (4%) |
| Neurological | 1 (3%) | 0 (0%) | 1 (4%) |
| Known risk factors ** | |||
| Diabetes mellitus | 15 (36%) | 0 (0%) | 15 (56%) |
| Chronic kidney disease | 5 (12%) | 0 (0%) | 5 (19%) |
| Chronic liver disease | 2 (5%) | 0 (0%) | 2 (7%) |
| Malignancy | 2 (5%) | 0 (0%) | 2 (7%) |
| Alcohol abuse | 1 (2%) | 0 (0%) | 1 (4%) |
| Chronic lung disease | 1 (2%) | 0 (0%) | 1 (4%) |
| Malnutrition | 1 (2%) | 1 (7%) | 0 (0%) |
| None known | 21 (50%) | 14 (93%) | 7 (26%) |
| Outcomes | |||
| Full recovery | 23 (55%) | 8 (53%) | 15 (56%) |
| Died | 18 (43%) | 7 (47%) | 11 (41%) |
| Unknown | 1 (2%) | 0 (0%) | 1 (4%) |
| Characteristics | Total (n = 42) |
|---|---|
| Specimens * | |
| Blood | 25 (60%) |
| Pus | 8 (19%) |
| Urine | 7 (17%) |
| Tissue ** | 2 (5%) |
| Sputum | 1 (2%) |
| Diagnostic method | |
| Vitek 2 identification system | 37 (88%) |
| Microgen | 5 (12%) |
| Antibiotic susceptibility test | |
| Not done | 27 (64%) |
| Done *** | |
| Gentamicin (S) | 0/13 (0%) |
| Amoxicillin-clavulanic acid (S) | 2/5 (40%) |
| Ceftazidime (S) | 12/14 (86%) |
| Doxycycline (S) | 7/9 (78%) |
| Meropenem (S) | 14/15 (93%) |
| Imipenem (S) | 2/2 (100%) |
| Trimethoprim-sulfamethoxazole (S) | 6/7 (86%) |
| Bacterial Strain | Year of Isolation/ Location of Isolation | Strain Source/Clinical Manifestations | Outcome |
|---|---|---|---|
| HBPMS00001 | 2015/Konawe, Southeast Sulawesi | Tibial tissue of 55-year old male patient presenting with open wounds with purulent discharge from legs, cough and fatigue | Fully recovered |
| HBPSK00002 | 2016/Samarinda, East Kalimantan | Pus of 55-year-old female patient with unknown clinical characteristics | Unknown |
| HBPMS00003 | 2016/Kolaka, Southeast Sulawesi | Blood of 56-year-old female patient presenting with decreased consciousness, generalized seizure, focal seizure of hand, headache, fever, swollen knee. | Died |
| HBPMS00004 | 2016/Luwu Utara, South Sulawesi | Pus of 39-year-old female patient presenting with lump on neck and weight loss. | Fully recovered |
| HBPMS00005 | 2016/Pinrang, South Sulawesi | Blood of 53-year-old male patient presenting with decreased consciuousness, fever, productive cough, shortness of breath, nausea, vomiting, abdominal pain and bloating. Icteric sclera and skin. Left leg swollen, pain and tenderness. | Died |
| HBPSK00001 | 2016/Kutai Timur, East Kalimantan | Blood of 4-year-old female patient presenting with fever, petechiae, poor appetite, anaemia | Died |
| HBPSK00003 | 2016/Kutai Timur, East Kalimantan | Pus of 37-year-old female patient presenting with skin ulcer on neck, fever | Fully recovered |
| HBPSK00004 | 2017/Kutai Kartanegara, East Kalimantan | Blood of 61-year-old male patient presenting with right hemiplegia, fever, decreased consciousness. | Died |
| HBPSK00005 | 2016/Samarinda, East Kalimantan | Urine of 44-year-old male patient presenting with fever, abscess on knee | Fully recovered |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tauran, P.M.; Wahyunie, S.; Saad, F.; Dahesihdewi, A.; Graciella, M.; Muhammad, M.; Lestari, D.C.; Aryati, A.; Parwati, I.; Loho, T.; et al. Emergence of Melioidosis in Indonesia and Today’s Challenges. Trop. Med. Infect. Dis. 2018, 3, 32. https://doi.org/10.3390/tropicalmed3010032
Tauran PM, Wahyunie S, Saad F, Dahesihdewi A, Graciella M, Muhammad M, Lestari DC, Aryati A, Parwati I, Loho T, et al. Emergence of Melioidosis in Indonesia and Today’s Challenges. Tropical Medicine and Infectious Disease. 2018; 3(1):32. https://doi.org/10.3390/tropicalmed3010032
Chicago/Turabian StyleTauran, Patricia M., Sri Wahyunie, Farahanna Saad, Andaru Dahesihdewi, Mahrany Graciella, Munawir Muhammad, Delly Chipta Lestari, Aryati Aryati, Ida Parwati, Tonny Loho, and et al. 2018. "Emergence of Melioidosis in Indonesia and Today’s Challenges" Tropical Medicine and Infectious Disease 3, no. 1: 32. https://doi.org/10.3390/tropicalmed3010032
APA StyleTauran, P. M., Wahyunie, S., Saad, F., Dahesihdewi, A., Graciella, M., Muhammad, M., Lestari, D. C., Aryati, A., Parwati, I., Loho, T., Pratiwi, D. I. N., Mutiawati, V. K., Loesnihari, R., Anggraini, D., Rahayu, S. I., Wulan, W. N., Antonjaya, U., Dance, D. A. B., Currie, B. J., ... Iskandriati, D. (2018). Emergence of Melioidosis in Indonesia and Today’s Challenges. Tropical Medicine and Infectious Disease, 3(1), 32. https://doi.org/10.3390/tropicalmed3010032







