Tick-, Flea-, and Louse-Borne Diseases of Public Health and Veterinary Significance in Nigeria
Abstract
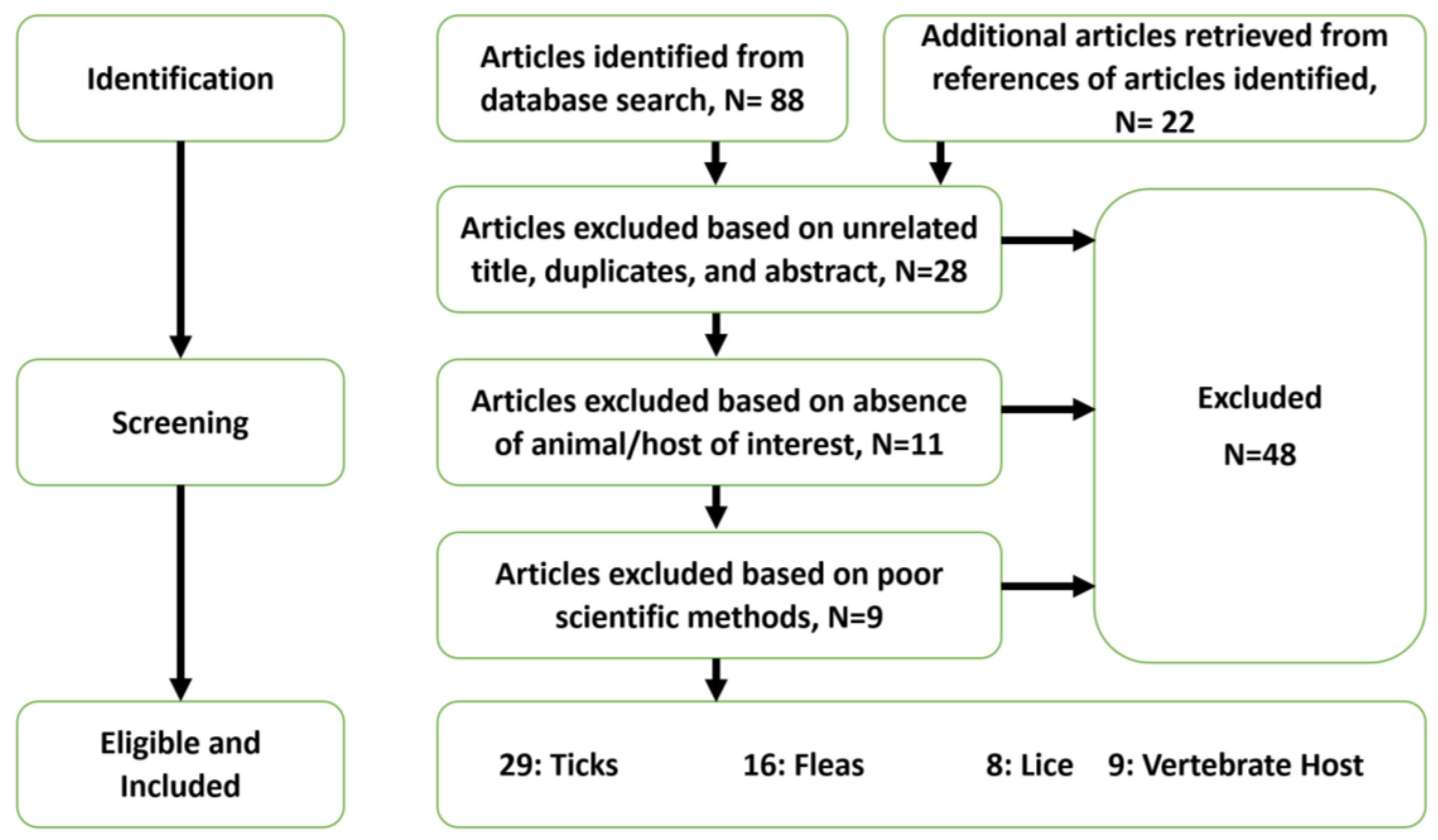
1. Introduction
Nigeria: Demographics, Geography, Climate, Ecology, and Practices
2. Ticks in Nigeria
2.1. Summary of Tick Species Known to Be Present in Nigeria
2.2. Impacts of Ticks and Tick-Borne Diseases on Dogs and Cats
2.3. Ticks and Tick-Borne Diseases in Large Livestock Animals
2.4. Ticks and Tick-Borne Diseases in Other Livestock Animals
2.5. Ticks and Tick-Borne Diseases in Humans
3. Fleas in Nigeria
3.1. Fleas and Flea-Borne Pathogens in Rats
3.2. Fleas and Flea-Borne Pathogens in Dogs and Cats
3.3. Fleas and Flea-Borne Pathogens in Livestock
3.4. Fleas and Flea-Borne Pathogens in Humans
4. Lice and Louse-Borne Diseases in Nigeria
5. Conclusions and Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ogden, N.H.; Lindsay, L.R. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Lendrum, D.; Manga, L.; Bagayoko, M.; Sommerfeld, J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos. Trans. R. Soc. B 2015, 370, 20130552. [Google Scholar] [CrossRef] [PubMed]
- Parham, P.E.; Waldock, J.; Christophides, G.K.; Hemming, D.; Agusto, F.; Evans, K.J.; Fefferman, N.; Gaff, H.; Gumel, A.; LaDeau, S. Climate, environmental and socio-economic change: Weighing up the balance in vector-borne disease transmission. Philos. Trans. R. Soc. B 2015, 370, 20130551. [Google Scholar] [CrossRef] [PubMed]
- Atehmengo, N.L.; Nnagbo, C.S. Emerging animal parasitic diseases: A global overview and appropriate strategies for their monitoring and surveillance in Nigeria. Open Microbiol. J. 2014, 8, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Socolovschi, C.; Edouard, S.; Fenollar, F.; Mouffok, N.; Bassene, H.; Diatta, G.; Tall, A.; Niangaly, H.; Doumbo, O.; et al. Common epidemiology of Rickettsia felis infection and malaria, Africa. Emerg. Infect. Dis. 2013, 19, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Socolovschi, C.; Labruna, M.B.; Mediannikov, O.; Kernif, T.; Abdad, M.Y.; Stenos, J.; Bitam, I.; Fournier, P.-E.; et al. Update on tick-borne rickettsioses around the world: A geographic approach. Clin. Microbiol. Rev. 2013, 26, 657–702. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Mediannikov, O.; Parola, P.; Raoult, D. Rickettsia felis: The complex journey of an emergent human pathogen. Trends Parasitol. 2016, 32, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Aung, A.K.; Spelman, D.W.; Murray, R.J.; Graves, S. Rickettsial infections in southeast Asia: Implications for local populace and febrile returned travelers. Am. J. Trop. Med. Hyg. 2014, 91, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, P. Tick-borne diseases of domestic animals in northern Nigeria I. Historical review, 1923–1966. Trop. Anim. Health Prod. 1977, 9, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, P.; Ilemobade, A.A. Tick-borne diseases of domestic animals in northern Nigeria. II. Research summary, 1966 to 1976. Trop. Anim. Health Prod. 1977, 9, 211–218. [Google Scholar] [CrossRef] [PubMed]
- National Geographic Atlas of the World-Nigeria Facts. Available online: http://travel.nationalgeographic.com/travel/countries/nigeria-facts/ (accessed on 11 July 2017).
- Kirk-Greene, A.H.; Udo, R.K. Nigeria. Available online: https://www.britannica.com/place/Nigeria (accessed on 20 July 2017).
- Average Monthly Rainfall, Sunshine, Temperatures, Humidity, Wind Speed. Available online: https://weather-and-climate.com/average-monthly-Rainfall-Temperature-Sunshine-in-Nigeria (accessed on 13 July 2017).
- Falola, T.O.; Ajayi, J.F.A.; Kirk-Greene, A.H.M.; Udo, R.K. Nigeria. Available online: https://www.britannica.com/place/Nigeria/Climate (accessed on 28 June 2017).
- Blanchard, L.P. Nigeria’s Boko Haram: Frequently asked questions. Curr. Politics Econ. Afr. 2014, 7, 109–140. [Google Scholar] [CrossRef]
- Akinboade, O.A.; Akinboade, C.Y. The effect of Babesia bigemina infections caused by cattle ticks on Nigerian economy. Rev. Elev. Med. Vet. Pays Trop. 1985, 38, 250–252. [Google Scholar] [CrossRef]
- Akinboade, O.; Dipeolu, O. Comparison of blood smear and indirect fluorescent antibody techniques in detection of haemoparasite infections in trade cattle in Nigeria. Vet. Parasitol. 1984, 14, 95–104. [Google Scholar] [CrossRef]
- Dipeolu, O.; Amoo, A. The presence of kinetes of a Babesia species in the haemolymph smears of engorged Hyalomma ticks in Nigeria. Vet. Parasitol. 1984, 17, 41–46. [Google Scholar] [CrossRef]
- Jegede, O.; Obeta, S.; Faisal, B. Infection of dogs with Babesia canis in Gwagwalada metropolis of Federal Capital Territory, Abuja, Nigeria. Sokoto J. Vet. Sci. 2014, 12, 37–41. [Google Scholar] [CrossRef]
- Konto, M.; Biu, A.; Ahmed, M.; Charles, S. Prevalence and seasonal abundance of ticks on dogs and the role of Rhipicephalus sanguineus in transmitting Babesia species in Maidugiri, Northeastern Nigeria. Vet. World 2014, 7, 119–124. [Google Scholar] [CrossRef]
- Paul, B.; Bello, A.; Ngari, O.; Mana, H.; Gadzama, M.; Abba, A.; Malgwi, K.; Balami, S.; Dauda, J.; Abdullahi, A. Risk factors of haemoparasites and some haematological parameters of slaughtered trade cattle in Maiduguri, Nigeria. J. Vet. Med. Anim. Health 2016, 8, 83–88. [Google Scholar] [CrossRef]
- Opara, M.; Santali, A.; Mohammed, B.; Jegede, O. Prevalence of haemoparasites of small ruminants in Lafia Nassarawa state: A guinea savannah zone of Nigeria. J. Vet. Adv. 2016, 6, 1251–1257. [Google Scholar] [CrossRef]
- Ogo, N.I.; de Mera, I.G.F.; Galindo, R.C.; Okubanjo, O.O.; Inuwa, H.M.; Agbede, R.I.; Torina, A.; Alongi, A.; Vicente, J.; Gortázar, C. Molecular identification of tick-borne pathogens in Nigerian ticks. Vet. Parasitol. 2012, 187, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Baneth, G.; Mumcuoglu, K.Y.; Waziri, N.E.; Eyal, O.; Guthmann, Y.; Harrus, S. Molecular detection and characterization of tick-borne pathogens in dogs and ticks from Nigeria. PLoS Negl. Trop. Dis. 2013, 7, e2108. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Morick, D.; Mumcuoglu, K.Y.; Harrus, S. Prevalence and diversity of Bartonella species in commensal rodents and ectoparasites from Nigeria, West Africa. PLoS Negl. Trop. Dis. 2013, 7, e2246. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Picozzi, K.; de Bronsvoort, B.M.; Majekodunmi, A.; Dongkum, C.; Balak, G.; Igweh, A.; Welburn, S.C. Ixodid ticks of traditionally managed cattle in central Nigeria: Where Rhipicephalus (Boophilus) microplus does not dare (yet?). Parasites Vectors 2013, 6, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Adamu, M.; Troskie, M.; Oshadu, D.O.; Malatji, D.P.; Penzhorn, B.L.; Matjila, P.T. Occurrence of tick-transmitted pathogens in dogs in Jos, Plateau state, Nigeria. Parasites Vectors 2014, 7, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kamani, J.; Baneth, G.; Apanaskevich, D.; Mumcuoglu, K.; Harrus, S. Molecular detection of Rickettsia aeschlimannii in Hyalomma spp. ticks from camels (Camelus dromedarius) in Nigeria, West Africa. Med. Vet. Entomol. 2015, 29, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003. [Google Scholar]
- Dipeolu, O. Studies on ticks of veterinary importance in Nigeria VI. Comparisons of oviposition and the hatching of eggs of Hyalomma species. Vet. Parasitol. 1983, 13, 251–265. [Google Scholar] [CrossRef]
- Dipeolu, O. Studies on ticks of veterinary importance in Nigeria. XVI. The oviposition pattern of engorged Boophilus and Hyalomma species when subjected in the laboratory to artificially created factors. Acarologia 1984, 25, 232–240. [Google Scholar] [PubMed]
- Dipeolu, O. Development of ixodid ticks under natural conditions in Nigeria. Trop. Anim. Health Prod. 1984, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Dipeolu, O. Studies on ticks of veterinary importance in Nigeria XII. Oviposition and eclosion in five species of ixodid ticks in contrasting habitats. Exp. Appl. Acarol. 1985, 1, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Dipeolu, O.; Adeyafa, C. Studies on ticks of veterinary importance in Nigeria. VIII. Differences observed in the biology of ticks which fed on different domestic animal hosts. Folia Parasitol. 1984, 31, 53–61. [Google Scholar] [PubMed]
- Dipeolu, O.; Amoo, A.; Akinboade, O. Studies on ticks of veterinary importance in Nigeria: Intrinsic factors influencing oviposition and egg-hatch of Amblyomma variegatum under natural conditions. Folia Parasitol. 1991, 38, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Adejinmi, J.O. Effect of water flooding on the oviposition capacity of engorged adult females and hatchability of eggs of dog ticks: Rhipicephalus sanguineus and Haemaphysalis leachi leachi. J. Parasitol. Res. 2011, 2011, 824162. [Google Scholar] [CrossRef] [PubMed]
- Adejinmi, J.O.; Akinboade, O.A. Sizes and developmental viability of sequentially oviposited eggs of dog ticks: Rhipicephalus sanguineus and Haemaphysalis leachi leachi. Afr. J. Med. Med. Sci. 2012, 41, 55–60. [Google Scholar] [PubMed]
- Bayer, W.; Maina, J.A. Seasonal pattern of tick load in Bunaji cattle in the subhumid zone of Nigeria. Vet. Parasitol. 1984, 15, 301–307. [Google Scholar] [CrossRef]
- Dipeolu, O.O.; Akinboade, O.A.; Ogunji, F.O. Observations on the epidemiology of house infesting Rhipicephalus sanguineus in a household in Lagos, Nigeria. Bull. Anim. Health Prod. Afr. 1982, 30, 29–30. [Google Scholar] [PubMed]
- Lorusso, V.; Gruszka, K.A.; Majekodunmi, A.; Igweh, A.; Welburn, S.C.; Picozzi, K. Rickettsia africae in Amblyomma variegatum ticks, Uganda and Nigeria. Emerg. Infect. Dis. 2013, 19, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Musa, H.I.; Jajere, S.M.; Adamu, N.B.; Atsanda, N.N.; Lawal, J.R.; Adamu, S.G.; Lawal, E.K. Prevalence of tick infestation in different breeds of cattle in Maiduguri, Northeastern Nigeria. Bangladesh J. Vet. Med. 2014, 12, 161–166. [Google Scholar] [CrossRef]
- Ugbomoiko, U.S.; Obiamiwe, B.A. Distribution and incidence of ectoparasites on small mammals in a rainforest belt of Southern Nigeria. Angew. Parasitol. 1991, 32, 143–148. [Google Scholar] [PubMed]
- Omudu, E.; Amuta, E. Parasitology and urban livestock farming in Nigeria: Prevalence of ova in faecal and soil samples and animal ectoparasites in Makurdi. J. S. Afr. Vet. Assoc. 2007, 78, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Abah, O.O.I.; Audu, P.A. Prevalence of brown dog tick (Rhipicephalus sanguineus) infestation of dogs in Lokoja metropolis, Kogi State, North-Central Nigeria. Niger. J. Parasitol. 2013, 34, 91–98. [Google Scholar]
- Obadiah, H.; Shekaro, A. Survey of tick infestation in cattle in Zaria abattoir, Nigeria. J. Vet. Adv. 2012, 2, 81–87. [Google Scholar]
- Agbolade, O.; Soetan, E.; Awesu, A.; Ojo, J.; Somoye, O.; Raufu, S. Ectoparasites of domestic dogs in some Ijebu communities, Southwest Nigeria. World Appl. Sci. J. 2008, 3, 916–920. [Google Scholar]
- James-Rugu, N.N.; Jidayi, S. A survey on the ectoparasites of some livestock from some areas of Borno and Yobe States. Niger. Vet. J. 2004, 25, 48–55. [Google Scholar] [CrossRef]
- Opara, M.N.O.; Ezeh, N.O. Ixodid ticks of cattle in Borno and Yobe states of Northeastern Nigeria: Breed and coat colour preference. Anim. Res. Int. 2016, 8, 1359–1365. [Google Scholar]
- Tongjura, J.; Amuga, G.; Ombugadu, R.; Azamu, Y.; Mafuiya, H. Ectoparasites infesting livestock in three local government areas (LGAs) of Nasarawa State, Nigeria. Sci. World J. 2012, 7, 15–17. [Google Scholar]
- Ameen, S.; Odetokun, I.; Ghali-Muhammed, L.; Azeez, O.; Raji, L.; Kolapo, T.; Adedokun, R. Status of ticks infestation in ruminant animals in Ogbomoso area of Oyo State, Nigeria. J. Environ. Issues Agric. Dev. Ctries. 2014, 6, 48–53. [Google Scholar]
- Ugochukwu, E.I.; Nnadozie, C.C. Ectoparasitic infestation of dogs in Bendel state, Nigeria. Int. J. Zoonoses 1985, 12, 308–312. [Google Scholar] [PubMed]
- Ugbomoiko, U.S.; Ariza, L.; Heukelbach, J. Parasites of importance for human health in Nigerian dogs: High prevalence and limited knowledge of pet owners. BMC Vet. Res. 2008, 4, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Amuta, E.U.; Houmsou, R.S.; Ogabiela, M. Tick infestation of dogs in Makurdi metropolis, Benue state, Nigeria. Int. J. Vet. Med. 2010, 7, 3. [Google Scholar]
- Isaac, C.; Igbinosa, I.; Nmorsi, O. Parasites and pathogens of ticks (Rhipicephalus species Acari: Ixodidae) among dogs in Edo state, Nigeria. Niger. J. Parasitol. 2016, 37, 129–134. [Google Scholar] [CrossRef]
- Ikpeze, O.; Eneanya, C.; Chinweoke, O.; Aribodor, D.; Anyasodor, A. Species diversity, distribution and predilection sites of ticks (Acarina: Ixodidae) on trade cattle at Enugu and Anambra States, South-Eastern Nigeria. Zoologist 2011, 9, 1–8. [Google Scholar]
- Okoli, I.C.; Okoli, C.G.; Opara, M. Environmental and multi-host infestation of the brown dog tick, Rhipicephalus sanguineus in Owerri, South-East Nigeria—A case report. Vet. Arch. 2006, 76, 93–100. [Google Scholar]
- Arong, G.A.; Adetunji, B.A.; Mowang, D.A.; Odu, A.E. Comparative distribution of ticks on dogs in the Calabar Metropolis, South-South Nigeria. Eur. J. Zool. Res. 2013, 2, 14–18. [Google Scholar]
- Ugochukwu, E.; Apeh, A. Prevalence of ectoparasites of small ruminants in Nsukka, Nigeria. Int. J. Zoonoses 1985, 12, 313–317. [Google Scholar] [PubMed]
- Unsworth, K. The ixodid parasites of cattle in Nigeria, with particular reference to the northern territories. Ann. Trop. Med. Parasitol. 1952, 46, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Obeta, S.; Idris, H.; Azare, B.; Simon, M.; Jegede, C. Prevalence of haemoparasites of dogs in Federal Capital Territory, Abuja, Nigeria. Niger. Vet. J. 2009, 30, 73–76. [Google Scholar]
- Kamani, J.; Lee, C.-C.; Haruna, A.M.; Chung, P.-J.; Weka, P.R.; Chung, Y.-T. First detection and molecular characterization of Ehrlichia canis from dogs in Nigeria. Res. Vet. Sci. 2013, 94, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Wijnveld, M.; Majekodunmi, A.O.; Dongkum, C.; Fajinmi, A.; Dogo, A.G.; Thrusfield, M.; Mugenyi, A.; Vaumourin, E.; Igweh, A.C. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasites Vectors 2016, 9, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, S.; Dipeolu, O. Prevalence of Anaplasma marginale, Babesia bigemina and B. bovis in Nigerian cattle using serological methods. Vet. Parasitol. 1986, 22, 147–149. [Google Scholar] [CrossRef]
- Iwuala, M.; Ejezie, G. Control of arthropod vectors of disease in Nigeria—A review. Bull. Anim. Health Prod. Afr. 1980, 28, 197–213. [Google Scholar] [PubMed]
- Adekunle, O.; Oladele, O.; Olukaiyeja, T. Indigenous control methods for pests and diseases of cattle in Northern Nigeria. Livest. Res. Rural Dev. 2002, 14, 66–75. [Google Scholar]
- Isere, E.E.; Fatiregun, A.A.; Ajayi, I.O. An overview of disease surveillance and notification system in Nigeria and the roles of clinicians in disease outbreak prevention and control. Niger. Med. J. 2015, 56, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.; Idris, S.; Sabitu, K.; Shehu, A.; Sambo, M. Emergency preparedness and the capability to identify outbreaks: A case study of Sabon gari local government area, Kaduna state. Ann. Niger. Med. 2010, 4, 21–27. [Google Scholar] [CrossRef]
- Abubakar, A.A.; Sambo, M.N.; Idris, S.H.; Sabitu, K.; Nguku, P. Assessment of integrated disease surveillance and response strategy implementation in selected local government areas of Kaduna state. Ann. Niger. Med. 2013, 7, 14–19. [Google Scholar] [CrossRef]
- Nguku, P.; Oyemakinde, A.; Sabitu, K.; Olayinka, A.; Ajayi, I.; Fawole, O.; Babirye, R.; Gitta, S.; Mukanga, D.; Waziri, N.; et al. Training and service in public health, Nigeria. Field epidemiology and laboratory training, 2008–2014. Pan Afr. Med. J. 2014, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Murray, S. Dog’s Dinners Prove Popular in Nigeria. Available online: http://news.bbc.co.uk/1/hi/world/africa/6419041.stm (accessed on 20 October 2017).
- Oboegbulem, S.I.; Nwakonobi, I.E. Population density and ecology of dogs in Nigeria: A pilot study. Rev. Sci. Tech. 1989, 8, 733–745. [Google Scholar] [CrossRef]
- Rangel, M.; Cardenas Lara, J.; De Aluja, A. Canine population of Mexico City: An estimative study. Anim. Regul. Stud. 1981, 3, 281–290. [Google Scholar]
- Bögel, K.; Meslin, F. Economics of human and canine rabies elimination: Guidelines for programme orientation. Bull. World Health Organ. 1990, 68, 281–291. [Google Scholar] [PubMed]
- Demma, L.J.; Traeger, M.S.; Nicholson, W.L.; Paddock, C.D.; Blau, D.M.; Eremeeva, M.E.; Dasch, G.A.; Levin, M.L.; Singleton, J., Jr.; Zaki, S.R. Rocky mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med. 2005, 353, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Sykes, T. Establishment of the tropical dog tick, Rhipicephalus sanguineus, in a house in London. Vet. Rec. 1985, 116, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Omobowale, O.; Tozuka, M.; Ohta, K.; Matsuu, A.; Nottidge, H.O.; Hirata, H.; Ikadai, H.; Oyamada, T. Molecular survey of Babesia canis in dogs in Nigeria. J. Vet. Med. Sci. 2007, 69, 1191–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, J.-F.; Liu, W.; Zheng, Y.-C.; Huo, Q.-B.; Tang, K.; Zuo, S.-Y.; Liu, K.; Jiang, B.-G.; Yang, H. Human infection with Candidatus Neoehrlichia mikurensis, China. Emerg. Infect. Dis. 2012, 18, 1636–1639. [Google Scholar] [CrossRef] [PubMed]
- Naitou, H.; Kawaguchi, D.; Nishimura, Y.; Inayoshi, M.; Kawamori, F.; Masuzawa, T.; Hiroi, M.; Kurashige, H.; Kawabata, H.; Fujita, H. Molecular identification of Ehrlichia species and ‘Candidatus Neoehrlichia mikurensis’ from ticks and wild rodents in Shizuoka and Nagano prefectures, Japan. Microbiol. Immunol. 2006, 50, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Derdáková, M.; Václav, R.; Pangrácova-Blaňárová, L.; Selyemová, D.; Koči, J.; Walder, G.; Špitalská, E. Candidatus Neoehrlichia mikurensis and its co-circulation with Anaplasma phagocytophilum in Ixodes ricinus ticks across ecologically different habitats of central Europe. Parasites Vectors 2014, 7, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Reye, A.L.; Arinola, O.G.; Hübschen, J.M.; Muller, C.P. Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl. Environ. Microbiol. 2012, 78, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- Jensenius, M.; Fournier, P.-E.; Raoult, D. Tick-borne rickettsioses in international travellers. Int. J. Infect. Dis. 2004, 8, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Mediannikov, O.; Trape, J.-F.; Diatta, G.; Parola, P.; Fournier, P.-E.; Raoult, D. Rickettsia africae, Western Africa. Emerg. Infect. Dis. 2010, 16, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, M. An investigation into the health status and diseases of wild dogs (Lycaon pictus) in the Kruger National Park. J. S. Afr. Vet. Assoc. 1995, 66, 18–27. [Google Scholar] [PubMed]
- Kelly, P.; Marabini, L.; Dutlow, K.; Zhang, J.; Loftis, A.; Wang, C. Molecular detection of tick-borne pathogens in captive wild felids, Zimbabwe. Parasites Vectors 2014, 7, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Berentsen, A.; Shock, B.C.; Teixiera, M.; Dunbar, M.R.; Becker, M.S.; Yabsley, M.J. Prevalence and diversity of Babesia, Hepatozoon, Ehrlichia, and Bartonella in wild and domestic carnivores from Zambia, Africa. Parasitol. Res. 2014, 113, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Noden, B.H.; Soni, M. Vector-borne diseases of small companion animals in Namibia: Literature review, knowledge gaps and opportunity for a One Health approach. J. S. Afr. Vet. Assoc. 2015, 86, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rabana, J.L.; Kumshe, H.A.; Kamani, J.; Hafsat, G.; Turaki, U.A.; Dilli, H.K. Effects of Parasitic Infections on Erythrocyte Indices of Camels in Nigeria. Vet. Res. Forum 2011, 2, 59–63. [Google Scholar]
- Bourn, D.; Wint, W.; Blench, R.; Woolley, E. Nigerian livestock resources survey. World Anim. Rev. 1994, 78, 49–58. [Google Scholar]
- Onusi, A. Retreat on Livestock and Dairy Development in Nigeria—Keynote Address Delivered by the Hon. Minister of Agriculture and Rural Development, Chief Audu Ogbeh. Available online: http://fmard.gov.ng/retreat-on-livestock-and-dairy-development-in-nigeria-keynote-address-delivered-by-the-hon-minister-of-agriculture-and-rural-development-chief-audu-ogbeh/ (accessed on 7 June 2017).
- Adesina, A. Nigeria’s Agriculture Minister on the Country’s Historical Failures, and a Reform Agenda to Push Nigeria into the Big League of Agriculture Producers. Available online: http://www.thisisafricaonline.com/Analysis/Interview-Akinwumi-Adesina-Minister-of-Agriculture-Nigeria (accessed on 28 September 2017).
- Mohammed, I.; Hoffmann, I. Management of draught camels (Camelus dromedarius) in crop-livestock production systems in Northwest Nigeria. Livest. Res. Rural Dev. 2006, 18. Available online: http://www.lrrd.cipav.org.co/lrrd18/1/moha18016.htm (accessed on 18 December 2017).
- Egbe-Nwiyi, T. Haematological and pathological studies of camel babesiosis in Nigeria. Bull. Anim. Health Prod. Afr. 1994, 42, 287–290. [Google Scholar]
- Anderson, B.E.; Tzianabos, T. Comparative sequence analysis of a genus-common rickettsial antigen gene. J. Bacteriol. 1989, 171, 5199–5201. [Google Scholar] [CrossRef] [PubMed]
- Isoun, T.; Akpokodje, J.; Ikede, B.; Fayemi, O. Heartwater in imported brown Swiss breed of cattle in western Nigeria. Bull. Epizoot. Dis. Afr. 1974, 22, 331–334. [Google Scholar] [PubMed]
- Ilemobade, A. Heartwater in Nigeria. I. The susceptibility of different local breeds and species of domestic ruminants to heartwater. Trop. Anim. Health Prod. 1977, 9, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Ilemobade, A.; Blotkamp, C. Heartwater in Nigeria. II. The isolation of Cowdria ruminantium from live and dead animals and the importance of routes of inoculation. Trop. Anim. Health Prod. 1978, 10, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Okoh, A.; Oyetunde, I.; Ibu, J. Heartwater infection (cowdriosis) in a sitatunga (Tragelaphus spekei) in Nigeria. J. Wildl. Dis. 1987, 23, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Mahan, S.; Peter, T.; Semu, S.; Simbi, B.; Norval, R.; Barbet, A. Laboratory reared Amblyomma hebraeum and Amblyomma variegatum ticks differ in their susceptibility to infection with Cowdria ruminantium. Epidemiol. Infect. 1995, 115, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ilemobade, A.; Leeflang, P. Epidemiology of heartwater in Nigeria. Revue D’élevage et de Médecine Vétérinaire des Pays Tropicaux 1977, 30, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Morrison, L.J.; Zhou, L.; Magona, J.W.; Jongejan, F.; Sugimoto, C. Development of multiple-locus variable-number tandem-repeat analysis for rapid genotyping of Ehrlichia ruminantium and its application to infected Amblyomma variegatum collected in heartwater endemic areas in Uganda. Parasitology 2012, 139, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Adakal, H.; Stachurski, F.; Konkobo, M.; Zoungrana, S.; Meyer, D.F.; Pinarello, V.; Aprelon, R.; Marcelino, I.; Alves, P.M.; Martinez, D. Efficiency of inactivated vaccines against heartwater in Burkina Faso: Impact of Ehrlichia ruminantium genetic diversity. Vaccine 2010, 28, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, J.L. Increased pathogenicity of an Ehrlichia-like agent after passage through Amblyomma hebraeum: A preliminary report. Onderstepoort J. Vet. Res. 1990, 57, 233–237. [Google Scholar] [PubMed]
- Aubry, P.; Geale, D. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Folkers, C.; Kuil, H. Blood-parasites in cattle, sheep and goats in Northern Nigeria. Bull. Epizoot. Dis. Afr. 1967, 15, 121–123. [Google Scholar] [PubMed]
- Dipeolu, O.O. Survey of blood parasites in domestic animals in Nigeria. Bull. Anim. Health Prod. Afr. 1975, 23, 155–164. [Google Scholar]
- Vesco, U.; Knap, N.; Labruna, M.B.; Avšič-Županc, T.; Estrada-Peña, A.; Guglielmone, A.A.; Bechara, G.H.; Gueye, A.; Lakos, A.; Grindatto, A. An integrated database on ticks and tick-borne zoonoses in the tropics and subtropics with special reference to developing and emerging countries. Exp. Appl. Acarol. 2011, 54, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Nduaka, O.; Ikeme, M.M. Human skin lesions in east central state, Nigeria due to the larvae of Amblyomma variegatum (Fabricius, 1794). Niger. Med. J. 1973, 3, 140–143. [Google Scholar] [PubMed]
- Fagbemi, B.O.; Ogunji, F.; Dipeolu, O.O. Anthropophilic deflection of Ctenocephalides canis (dog flea). Int. J. Zoonoses 1981, 8, 97–99. [Google Scholar] [PubMed]
- Ejezie, G.C. The parasitic diseases of school children in Lagos state, Nigeria. Acta Trop. 1981, 38, 79–84. [Google Scholar] [PubMed]
- Arene, F.O.I. The prevalence of sand flea (Tunga penetrans) among primary and post-primary school pupils in Choba area of the Niger Delta. Public Health 1984, 98, 282–283. [Google Scholar] [CrossRef]
- Ugbomoiko, U.S.; Ariza, L.; Ofoezie, I.E.; Heukelbach, J. Risk factors for tungiasis in Nigeria: Identification of targets for effective intervention. PLoS Negl. Trop. Dis. 2007, 1, e87. [Google Scholar] [CrossRef] [PubMed]
- Ugbomoiko, U.S.; Ofoezie, I.E.; Heukelbach, J. Tungiasis: High prevalence, parasite load, and morbidity in a rural community in Lagos State, Nigeria. Int. J. Dermatol. 2007, 46, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Chukwu, C. Prevalence of fleas on dogs in Anambra state of Nigeria. Int. J. Zoonoses 1985, 12, 192–195. [Google Scholar] [PubMed]
- Koehler, P.G.; Pereira, R.; Kaufman, P. Sticktight Flea, Echidnophaga Gallinacea; University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS: Gainsville, FL, USA, 1991. [Google Scholar]
- Mafiana, C.; Osho, M.; Sam-Wobo, S. Gastrointestinal helminth parasites of the black rat (Rattus rattus) in Abeokuta, southwest Nigeria. J. Helminthol. 1997, 71, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Ogunniyi, T.; Balogun, H.; Shasanya, B. Ectoparasites and endoparasites of peridomestic house-rats in Ile-Ife, Nigeria and implication on human health. Iran J. Parasitol. 2014, 9, 134–140. [Google Scholar] [PubMed]
- Pearse, A. Ecology of the ectoparasites of Nigerian rodents and insectivores. J. Mammal. 1929, 10, 229–239. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B. Bartonellosis, One Health and all creatures great and small. Vet. Dermatol. 2017, 28, 96-e21. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H.T.; Brouqui, P.; Faugere, B.; Raoult, D. Prevalence of antibodies to Coxiella burnetii, Rickettsia conorii, and Rickettsia typhi in seven African countries. Clin. Infect. Dis. 1995, 21, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Rakotonanahary, R.J.L.; Harrison, A.; Maina, A.N.; Jiang, J.; Richards, A.L.; Rajerison, M.; Telfer, S. Molecular and serological evidence of flea-associated typhus group and spotted fever group rickettsial infections in Madagascar. Parasites Vectors 2017, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Noden, B.H.; Davidson, S.; Smith, J.L.; Williams, F. First detection of Rickettsia typhi and Rickettsia felis in fleas collected from client-owned companion animals in the southern Great Plains. J. Med. Entomol. 2017, 54, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Maina, A.N.; Fogarty, C.; Krueger, L.; Macaluso, K.R.; Odhiambo, A.; Nguyen, K.; Farris, C.M.; Luce-Fedrow, A.; Bennett, S.; Jiang, J.; et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS ONE 2016, 11, e0160604. [Google Scholar] [CrossRef] [PubMed]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010, 14, e667–e676. [Google Scholar] [CrossRef] [PubMed]
- Dobler, G.; Pfeffer, M. Fleas as parasites of the family Canidae. Parasites Vectors 2011, 4, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Opasina, B. Ctenocephalides canis infestation of goats. Trop. Anim. Health Prod. 1983, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Ugbomoiko, U.S.; Ariza, L.; Babamale, A.O.; Heukelbach, J. Prevalence and clinical aspects of tungiasis in South-West Nigerian schoolchildren. Trop. Dr. 2017, 47, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Shaibu, S.; Oyetunde, I.; Jwander, L.; Tanko, J.; Ikpa, L.; Adamu, J. Flea bite dermatitis in a herd of dairy calves in Vom Nigeria. Niger. Vet. J. 2011, 32. [Google Scholar] [CrossRef]
- Krasnov, B.R. Functional and Evolutionary Ecology of Fleas. A Model for Ecological Parazitology; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Dipeolu, O.O.; Ayoade, G.O. The epizootiology of infestation of sheep with Ctenocephalides canis in a livestock farm in Nigeria. Bull. Anim. Health Prod. Afr. 1982, 30, 31–34. [Google Scholar] [PubMed]
- Raoult, D.; La Scola, B.; Enea, M.; Fournier, P.E.; Roux, V.; Fenollar, F.; Galvao, M.A.; de Lamballerie, X. A flea-associated Rickettsia pathogenic for humans. Emerg. Infect. Dis. 2001, 7, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.-M.; Bourry, O.; Davoust, B.; Raoult, D. Bartonella quintana and Rickettsia felis in Gabon. Emerg. Infect. Dis. 2005, 11, 1742–1744. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Osorio, C.E.; Zavala-Velázquez, J.E.; León, J.J.A.; Zavala-Castro, J.E. Rickettsia felis as emergent global threat for humans. Emerg. Infect. Dis. 2008, 14, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Berrelha, J.; Briolant, S.; Muller, F.; Rolain, J.M.; Marie, J.L.; Pagés, F.; Raoult, D.; Parola, P. Rickettsia felis and Rickettsia massiliae in Ivory Coast, Africa. Clin. Microbiol. Infect. 2009, 15, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Reif, K.E.; Macaluso, K.R. Ecology of Rickettsia felis: A review. J. Med. Entomol. 2009, 46, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Socolovschi, C.; Mediannikov, O.; Sokhna, C.; Tall, A.; Diatta, G.; Bassene, H.; Trape, J.-F.; Raoult, D. Rickettsia felis-associated uneruptive fever, Senegal. Emerg. Infect. Dis. 2010, 16, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.L.; Jiang, J.; Omulo, S.; Dare, R.; Abdirahman, K.; Ali, A.; Sharif, S.K.; Feikin, D.R.; Breiman, R.F.; Njenga, M.K. Human infection with Rickettsia felis, Kenya. Emerg. Infect. Dis. 2010, 16, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Udoudo, M.G.M.; Umoh, G.S.; Akpaeti, A.J. Malaria and agricultural production in Nigeria. Asian Dev. Policy Rev. 2016, 4, 91–99. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2015; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Yemi, K. Annual Abstract of Statistics, 2012; National Bureau of Statistics: Beijing, China, 2012; pp. 176–177.
- Tungiasis. Available online: https://www.cdc.gov/dpdx/tungiasis/index.html (accessed on 13 July 2017).
- Sackal, C.; Laudisoit, A.; Kosoy, M.; Massung, R.; Eremeeva, M.E.; Karpathy, S.E.; Van Wyk, K.; Gabitzsch, E.; Zeidner, N.S. Bartonella spp. and Rickettsia felis in fleas, Democratic Republic of Congo. Emerg. Infect. Dis. 2008, 14, 1972–1974. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinade, A.F.; Oyejide, C.O. Pediculosis capitis among rural and urban schoolchildren in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 590–592. [Google Scholar] [CrossRef]
- Jinadu, M.K. Pediculosis humanus capitis among primary school children in Ile-ife, Nigeria. J. R. Soc. Health 1985, 105, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Ebomoyi, E. Pediculosis capitis among primary schoolchildren in urban and rural areas of Kwara state, Nigeria. J. Sch. Health 1988, 58, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Ebomoyi, E.W. Pediculosis capitis among urban school children in Ilorin, Nigeria. J. Natl. Med. Assoc. 1994, 86, 861–864. [Google Scholar] [PubMed]
- Arene, F.O.; Ukaulor, A.L. Prevalence of head louse (Pediculus capitis) infestation among inhabitants of the Niger Delta. Trop. Med. Parasitol. 1985, 36, 140–142. [Google Scholar] [PubMed]
- Gboeloh, L.B.; Elele, K. Incidence of head lice (Pediculus humanus capitis) among primary school children in five rural schools in Khana local government area, Rivers state, Nigeria. Res. Zool. 2013, 3, 75–79. [Google Scholar] [CrossRef]
- Okwa, O.O.; Omoniyi, O.A.O. The prevalence of head lice (Pediculus humanus capitus) and bed bugs (Cimex hemipterus) in selected human settlement areas in southwest, Lagos state, Nigeria. J. Parasitol. Vector Biol. 2010, 2, 8–13. [Google Scholar]
- Montgomery, T.H.L.; Budden, F.H. Typhus in Northern Nigeria. I. Epidemiological studies. Trans. R. Soc. Trop. Med. Hyg. 1947, 41, 327–337. [Google Scholar] [CrossRef]
- Findlay, G.M.; Elmes, B.G.T. Typhus in Northern Nigeria. II. Laboratory investigations. Trans. R. Soc. Trop. Med. Hyg. 1947, 41, 339–352. [Google Scholar] [CrossRef]
- Montgomery, T.H.L.; Budden, F.H. Typhus in Northern Nigeria. III. Clinical studies. Trans. R. Soc. Trop. Med. Hyg. 1947, 41, 353–362. [Google Scholar] [CrossRef]
- Emejuaiwe, S.O.; Njoku-Obi, A.N. Serological evidence for the presence of rickettsial infections in parts of Nigeria. Niger. Med. J. 1978, 8, 514–517. [Google Scholar] [PubMed]
- Thiga, J.W.; Mutai, B.K.; Eyako, W.K.; Ng’ang’a, Z.; Jiang, J.; Richards, A.L.; Waitumbi, J.N. High seroprevalence of antibodies against spotted fever and scrub typhus bacteria in patients with febrile illness, Kenya. Emerg. Infect. Dis. 2015, 21, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Galan, M.; Razzauti, M.; Bard, E.; Bernard, M.; Brouat, C.; Charbonnel, N.; Dehne-Garcia, A.; Loiseau, A.; Tatard, C.; Tamisier, L.; et al. 16S rRNA amplicon sequencing for epidemiological surveys of bacteria in wildlife. mSystems 2016, 1, e00032-16. [Google Scholar] [CrossRef] [PubMed]
- Sharp, N.A.D. Epidemic disease in West Africa: The menace of the future. Trans. R. Soc. Trop. Med. Hyg. 1925, 19, 256–264. [Google Scholar] [CrossRef]
- Sunantaraporn, S.; Sanprasert, V.; Pengsakul, T.; Phumee, A.; Boonserm, R.; Tawatsin, A.; Thavara, U.; Siriyasatien, P. Molecular survey of the head louse Pediculus humanus capitis in Thailand and its potential role for transmitting Acinetobacter spp. Parasites Vectors 2015, 8, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Abdissa, A.; Diatta, G.; Trape, J.-F.; Angelakis, E.; Mediannikov, O.; La Scola, B.; Raoult, D. Detection of Acinetobacter baumannii in human head and body lice from Ethiopia and identification of new genotypes. Int. J. Infect. Dis. 2012, 16, e680–e683. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Rolain, J.-M.; Diatta, G.; Azza, S.; Samb, B.; Mediannikov, O.; Gassama Sow, A.; Diene, S.M.; Fenollar, F.; Raoult, D. Carbapenem resistance and Acinetobacter baumannii in Senegal: The paradigm of a common phenomenon in natural reservoirs. PLoS Negl. Trop. Dis. 2012, 7, e39495. [Google Scholar] [CrossRef] [PubMed]
- Olaitan, A.O.; Berrazeg, M.; Fagade, O.E.; Adelowo, O.O.; Alli, J.A.; Rolain, J.M. Emergence of multidrug-resistant Acinetobacter baumannii producing OXA-23 carbapenemase, Nigeria. Int. J. Infect. Dis. 2013, 17, e469–e470. [Google Scholar] [CrossRef] [PubMed]
- Nwadike, V.U.; Ojide, C.K.; Kalu, E.I. Multidrug resistant acinetobacter infection and their antimicrobial susceptibility pattern in a nigerian tertiary hospital ICU. Afr. J. Infect. Dis. 2014, 8, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Odewale, G.; Adefioye, O.J.; Ojo, J.; Adewumi, F.A.; Olowe, O.A. Multidrug resistance of Acinetobacter baumannii in Ladoke Akintola University Teaching Hospital, Osogbo, Nigeria. Eur. J. Microbiol. Immunol. 2016, 6, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Mugnier, P.D.; Poirel, L.; Naas, T.; Nordmann, P. Worldwide dissemination of the bla(OXA-23) carbapenemase gene of Acinetobacter baumannii. Emerg. Infect. Dis. 2010, 16, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Naas, T.; Nordmann, P. Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob. Agents Chemother. 2010, 54, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Nordmann, P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin. Microbiol. Infect. 2006, 12, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Sangaré, A.K.; Boutellis, A.; Drali, R.; Socolovschi, C.; Barker, S.C.; Diatta, G.; Rogier, C.; Olive, M.-M.; Doumbo, O.K.; Raoult, D. Detection of Bartonella quintana in African body and head lice. Am. J. Trop. Med. Hyg. 2014, 91, 294–301. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Nigeria Crisis. Available online: http://www.who.int/emergencies/nigeria/en/ (accessed on 14 September 2017).
- Raoult, D.; Ndihokubwayo, J.B.; Tissot-Dupont, H.; Roux, V.; Faugere, B.; Abegbinni, R.; Birtles, R.J. Outbreak of epidemic typhus associated with trench fever in Burundi. Lancet 1998, 352, 353–358. [Google Scholar] [CrossRef]
- Umulisa, I.; Omolo, J.; Muldoon, K.A.; Condo, J.; Habiyaremye, F.; Uwimana, J.M.; Muhimpundu, M.A.; Galgalo, T.; Rwunganira, S.; Dahourou, A.G.; et al. A mixed outbreak of epidemic typhus fever and trench fever in a youth rehabilitation center: Risk factors for illness from a case-control study, Rwanda, 2012. Am. J. Trop. Med. Hyg. 2016, 95, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Dittrich, S.; Lopez, J.; Phuklia, W.; Martinez-Valdebenito, C.; Velasquez, K.; Blacksell, S.D.; Paris, D.H.; Abarca, K. Endemic scrub typhus in South America. N. Engl. J. Med. 2016, 375, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Izzard, L.; Fuller, A.; Blacksell, S.D.; Paris, D.H.; Richards, A.L.; Aukkanit, N.; Nguyen, C.; Jiang, J.; Fenwick, S.; Day, N.P.; et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J. Clin. Microbiol. 2010, 48, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Horton, K.C.; Jiang, J.; Maina, A.; Dueger, E.; Zayed, A.; Ahmed, A.A.; Pimentel, G.; Richards, A.L. Evidence of Rickettsia and Orientia infections among abattoir workers in Djibouti. Am. J. Trop. Med. Hyg. 2016, 95, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Cosson, J.F.; Galan, M.; Bard, E.; Razzauti, M.; Bernard, M.; Morand, S.; Brouat, C.; Dalecky, A.; Ba, K.; Charbonnel, N.; et al. Detection of Orientia sp. DNA in rodents from Asia, West Africa and Europe. Parasites Vectors 2015, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Kolo, A.O.; Sibeko-Matjila, K.P.; Maina, A.N.; Richards, A.L.; Knobel, D.L.; Matjila, P.T. Molecular detection of zoonotic Rickettsiae and Anaplasma spp. in domestic dogs and their ectoparasites in Bushbuckridge, South Africa. Vector-Borne Zoonotic Dis. 2016, 16, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Esemu, S.N.; Ndip, L.M.; Ndip, R.N. Ehrlichia species, probable emerging human pathogens in sub-Saharan Africa: Environmental exacerbation. Rev. Environ. Health 2011, 26, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Premaratna, R.; Halambarachchige, L.P.; Nanayakkara, D.M.; Chandrasena, T.G.; Rajapakse, R.P.; Bandara, N.K.; de Silva, H.J. Evidence of acute rickettsioses among patients presumed to have chikungunya fever during the chikungunya outbreak in Sri Lanka. Int. J. Infect. Dis. 2011, 15, e871–e873. [Google Scholar] [CrossRef] [PubMed]
- Hoogstraal, H. Review Article 1: The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef] [PubMed]
- Bukbuk, D.N.; Dowall, S.D.; Lewandowski, K.; Bosworth, A.; Baba, S.S.; Varghese, A.; Watson, R.J.; Bell, A.; Atkinson, B.; Hewson, R. Serological and virological evidence of Crimean-Congo haemorrhagic fever virus circulation in the human population of Borno State, northeastern Nigeria. PLoS Negl. Trop. Dis. 2016, 10, e0005126. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.L.; Causey, O.R.; Carey, D.E.; Reddy, S.; Cooke, A.R.; Akinkugbe, F.M.; David-West, T.S.; Kemp, G.E. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann. Trop. Med. Parasitol. 1975, 69, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lutomiah, J.; Musila, L.; Makio, A.; Ochieng, C.; Koka, H.; Chepkorir, E.; Mutisya, J.; Mulwa, F.; Khamadi, S.; Miller, B.R. Ticks and tick-borne viruses from livestock hosts in arid and semiarid regions of the eastern and northeastern parts of Kenya. J. Med. Entomol. 2014, 51, 269–277. [Google Scholar] [CrossRef] [PubMed]

| Tick Species Collected | Vertebrate Host (Number) | Location | Infestation a (%) | Pathogen (%) b | Detection Method | Study [Reference] | |
|---|---|---|---|---|---|---|---|
| Host Blood | Tick | ||||||
| Hyalomma impeltatum | Camels (170) | Kano | 59.9 | Rickettsia | Rickettsia | PCR | Kamani et al. 2015 [29] |
| - | - | - | - | aeschlimannii (18.8) | aeschlimannii (4.2–50.0) c | - | - |
| Hyalomma rufipes | - | - | 20.3 | - | Rickettsia | PCR | - |
| - | - | - | - | - | aeschlimannii (56.3–94.1) c | - | - |
| Hyalomma dromedarii | - | - | 18.8 | - | Rickettsia | PCR | - |
| - | - | - | - | - | aeschlimannii (13.3) c | - | - |
| Hyalomma impressum | - | - | 1 | - | ND | PCR | - |
| Rhipicephalus sanguineus | Dogs (181) | Plateau | NR | Hepatozoon canis (41.4) | Ehrlichia canis (23.7) | PCR | Kamani et al. 2013 [25] |
| Haemaphysalis leachi | - | Rivers | NR | Ehrlichia canis (12.7) | Hepatozoon canis (21.1) | PCR | - |
| Rhipicephalus turanicus | - | Kaduna | NR | Rickettsia spp. (8.8) | Rickettsia spp. (10.5) | PCR | - |
| - | - | Kwara | - | Babesia rossi (6.6) | Candidatus Neoehrlichia | - | - |
| - | - | - | - | - | mikurensis (5.3) | - | - |
| - | - | - | - | Anaplasma platys (6.6) | Anaplasma platys (1.9) | - | - |
| - | - | - | - | Rickettsia conorii | Rickettsia conorii | - | - |
| - | - | - | - | israelensis (NR) | israelensis (NR) d | - | - |
| Rhipicephalus sanguineus | Dogs (100) | Plateau | 73 | Babesia rossi (53) e | NT | PCR & RLB | Adamu et al. 2014 [28] |
| Haemaphysalis leachi | - | - | 18 | Theileria spp. (12.5) e | NT | - | - |
| Rhipicephalus turanicus | - | - | 2 | Ehrlichia canis (6.9) e | NT | - | - |
| - | - | - | - | Anaplasma spp. (6.9) e | - | - | - |
| - | - | - | - | Theileria equi (4.2) e | - | - | - |
| Amblyomma variegatum | Dogs (NR) | Plateau | 70.2 | NT | Babesia spp.(3.9) f | PCR | Ogo et al. 2012 [24] |
| Rhipicephalus | Cattle (NR) | Nassarawa | 20.6 | NT | Babesia bigemina (1.3) f | PCR | - |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus sanguineus | - | - | 9.2 | - | Babesia divergens (0.6) f | PCR | - |
| - | - | - | - | - | Anaplasma marginale (20.0) g | - | - |
| - | - | - | - | - | Rickettsia africae (4.4–7.8) h | - | - |
| Rhipicephalus sanguineus | Rattus rattus (48) | Plateau | 46.5 | Bartonella spp. (18.8) | Bartonella spp. (10.0) i | PCR & Blood culture j | Kamani et al. 2013 [26] |
| Haemaphysalis leachi | R. norvegicus (121) | - | 3.5 | Bartonella spp. (29.8) | ND | PCR & Blood culture j | - |
| - | Mus musculus (6) | - | - | - | ND | PCR & Blood culture j | - |
| - | Cricetomys gambianus (2) | - | - | - | Bartonella spp. (50.0) | PCR & Blood culture j | - |
| Hyalomma rufipes | Cattle (NR) | Nigeria | 34 k | NT | Babesia kinetes (88) | Haemolymph | Dipeolu et al. 1984 [19] |
| - | - | - | - | - | - | smear stain | - |
| Hyalomma truncatum | - | - | 32 k | NT | Babesia kinetes (56) | Haemolymph | - |
| - | - | - | - | - | - | smear stain | - |
| Hyalomma impressum | - | - | 19 k | NT | Babesia kinetes (36) | Haemolymph | - |
| - | - | - | - | - | - | smear stain | - |
| Hyalomma marginatum | - | - | 18 k | NT | Babesia kinetes (72) | Haemolymph | - |
| - | - | - | - | - | - | smear stain | - |
| Hyalomma impeltatum | - | - | 7 k | NT | Babesia kinetes (43) | Haemolymph | - |
| - | - | - | - | - | - | smear stain | - |
| Amblyomma variegatum | Cattle (NR) | Plateau | 141 k | NT | Rickettsia africae (62) | PCR | Lorusso et al. 2013 [41] |
| Rhipicephalus | Cattle (120) | Nigeria | NR | Babesia bigemina (80) | NT | Blood smear stain | Akinboade et al. 1985 [17] |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Rhipicephalus | Dogs (400) | Borno | 88 | Babesia canis (12.0) | NT | Blood smear stain | Konto et al. 2014 [21] |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Rhipicephalus sanguineus | - | - | 10.8 | - | NT | - | - |
| Hyalomma spp. | - | - | 0.9 | - | NT | - | - |
| Amblyomma variegatum | - | - | 0.3 | - | NT | - | - |
| Rhipicephalus | Cattle (205) | Borno | 63.4 l | NT | NT | N/A | Musa et al. 2014 [42] |
| (Boophilus) microplus | - | - | - | - | - | - | - |
| Amblyomma variegatum | - | - | - | - | NT | - | - |
| Hyalomma spp. | - | - | - | - | NT | - | - |
| Rhipicephalus sanguineus | - | - | - | - | NT | - | - |
| Ornithodorus spp. | - | - | - | - | NT | - | - |
| Amblyomma variegatum | Arvincanthis | Delta | 7.4 | NT | NT | N/A | Ugbomoiko et al. 1991 [43] |
| - | niloticus (NR) | - | - | - | - | - | - |
| Ixodes sp. | Lophuromys | Edo | 5.1 | NT | NT | - | - |
| - | sikapusi (NR) | - | - | - | - | - | - |
| - | Mastomys | - | - | NT | - | - | - |
| - | natalensis (NR) m | - | - | - | - | - | - |
| - | Mus minutoides (NR) | - | - | NT | - | - | - |
| - | Leminiscomys | - | - | NT | - | - | - |
| - | striatus (NR) m | - | - | - | - | - | - |
| Rhipicephalus | Cattle (228) | Plateau | 41.4 | NT | NT | N/A | Lorusso et al. 2013 [27] |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus | - | - | 15.4 | - | NT | - | - |
| (Boophilus) annulatus | - | - | - | - | - | - | - |
| Rhipicephalus guilhoni | - | - | 12 | - | NT | - | |
| Rhipicephalus | - | - | 7.6 | - | NT | - | - |
| (Boophilus) geigyi | - | - | - | - | - | - | - |
| Hyalomma truncatum | - | - | 7.4 | - | NT | - | - |
| Amblyomma variegatum | - | 6.3 | - | NT | - | - | |
| Rhipicephalus | - | - | 4.1 | - | NT | - | |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Rhipicephalus | - | - | 4 | - | NT | - | - |
| simus group | - | - | - | - | - | - | - |
| Rhipicephalus turanicus | - | - | 1.2 | NT | - | - | |
| Rhipicephalus sanguineus | - | - | 0.3 | - | NT | - | - |
| Hyalomma rufipes | - | - | 0.2 | - | NT | - | - |
| Rhipicephalus lunulatus | - | - | <0.1 | - | NT | - | - |
| Rhipicephalus spp. | Dogs (44) | Benue | 34.1 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Rhipicephalus | - | - | 29.5 | NT | NT | - | - |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Amblyomma spp. | - | - | 22.7 | NT | NT | - | - |
| Amblyomma spp. | Goats (45) | Benue | 20.1 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Rhipicephalus spp. | - | - | 13.3 | NT | NT | - | - |
| Rhipicephalus | - | - | 11.1 | NT | NT | - | - |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Hyalomma spp. | - | - | 8.9 | NT | NT | - | - |
| Amblyomma spp. | Cattle (43) | Benue | 25.6 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Hyalomma spp. | - | - | 18.6 | NT | NT | - | - |
| Rhipicephalus | - | - | 16.3 | NT | NT | - | - |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Rhipicephalus spp. | - | - | 9.3 | NT | NT | - | - |
| Amblyomma spp. | Pigs (44) | Benue | 20.5 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Hyalomma spp. | - | - | 20.5 | NT | NT | - | - |
| Rhipicephalus | - | - | 15.9 | NT | NT | - | - |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Amblyomma spp. | Sheep (45) | Benue | 13.3 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Rhipicephalus | - | - | 4.4 | NT | NT | - | - |
| (Boophilus) spp. | - | - | - | - | - | - | - |
| Hyalomma spp. | - | - | 8.9 | NT | NT | - | - |
| Rhipicephalus sanguineus | Dogs (200) | Kogi | 80 | NT | NT | N/A | Abah et al. 2013 [45] |
| Amblyomma variegatum | Cattle (120) | Kaduna | 22.5 | NT | NT | N/A | Obadiah et al. 2012 [46] |
| Rhipicephalus | - | - | 17.5 | - | NT | - | - |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Hyalomma sp. | - | - | 6.7 | - | NT | - | - |
| Rhipicephalus sanguineus | - | - | 3.3 | - | NT | - | - |
| Rhipicephalus sanguineus | Dogs (202) | Ogun | 89.6 | NT | NT | N/A | Agbolade et al. 2008 [47] |
| Haemaphysalis leachi | - | 78.7 | - | NT | - | - | |
| Hyalomma rufipes | Camels (1600) | Borno | 34.9 | NT | NT | N/A | James-Rugu et al. 2004 [48] |
| Hyalomma dromedarii | - | Yobe | 30.4 | - | NT | - | - |
| Hyalomma truncatum | - | - | 10.6 | - | NT | - | - |
| Rhipicephalus | - | - | 24.2 | - | NT | - | - |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Amblyomma variegatum | Dogs (230) | Borno | 23.5 | NT | NT | N/A | James-Rugu et al. 2004 [48] |
| Rhipicephalus spp. | - | Yobe | 21.2 | - | NT | - | - |
| Rhipicephalus (Boophilus) | - | - | 40.8 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Amblyomma lepidum | - | - | 7.1 | - | NT | - | - |
| Haemaphysalis leachi | - | - | 6.9 | - | NT | - | - |
| Amblyomma variegatum | Cattle (2200) | Borno | 11.4 | NT | NT | N/A | James-Rugu et al. 2004 [48] |
| Amblyomma lepidum | - | Yobe | 1.97 | - | NT | - | - |
| Hyalomma truncatum | - | - | 17.9 | - | NT | - | - |
| Hyalomma rufipes | - | - | 11.5 | - | NT | - | - |
| Haemaphysalis leachi | - | - | 8.8 | - | NT | - | - |
| Rhipicephalus evertsi | - | - | 17.8 | - | NT | - | - |
| Rhipicephalus (Boophilus) | - | - | 21.8 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus sanguineus | - | - | 8.9 | - | NT | - | - |
| Amblyomma variegatum | Sheep (500) | Borno | 50.0 | NT | NT | N/A | James-Rugu et al. 2004 [48] |
| Rhipicephalus (Boophilus) | - | Yobe | 50.0 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Amblyomma variegatum | Cattle (3150) | Borno | 43.8 | NT | NT | N/A | Opara et al. 2016 [49] |
| Hyalomma sp. | - | Yobe | 24.7 | - | NT | - | - |
| Rhipicephalus (Boophilus) | - | - | 21.9 | - | NT | - | - |
| microplus | - | - | - | - | - | - | - |
| Dermacentor variabilis | - | - | 9.6 | - | NT | - | - |
| Amblyomma variegatum | Cattle (1200) | Nasarawa | 20.8 | NT | NT | N/A | Tongjura et al. 2012 [50] |
| Amblyomma lepidum | - | - | 14.3 | - | NT | - | - |
| Rhipicephalus (Boophilus) | - | - | 14.3 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 12.8 | - | NT | - | - |
| annulatus | - | - | - | - | - | - | - |
| Hyalomma truncatum | - | - | 6.8 | - | NT | - | - |
| Amblyomma variegatum | Sheep (1200) | Nasarawa | 18.2 | NT | NT | N/A | Tongjura et al. 2012 [50] |
| Amblyomma lepidum | - | - | 8.6 | - | NT | - | - |
| Hyalomma truncatum | - | - | 5.6 | - | NT | - | - |
| Rhipicephalus (Boophilus) | - | - | 10.0 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 7.6 | - | NT | - | - |
| annulatus | - | - | - | - | - | - | - |
| Amblyomma variegatum | Goats (1200) | Nasarawa | 15.5 | NT | NT | N/A | Tongjura et al. 2012 [50] |
| Rhipicephalus (Boophilus) | - | - | 7.1 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Amblyomma lepidum | - | - | 6.6 | - | NT | - | - |
| Rhipicephalus (Boophilus) | - | - | 6.4 | - | NT | - | - |
| annulatus | - | - | - | - | - | - | - |
| Hyalomma truncatum | - | - | 3.7 | - | NT | - | - |
| Rhipicephalus evertsi | Cattle (317) | Oyo | 37.6 | NT | NT | N/A | Ameen et al. 2014 [51] |
| evertsi | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 22.1 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 17.8 | - | NT | - | - |
| annulatus | - | - | - | - | - | - | - |
| Amblyomma variegatum | - | - | 15.8 | - | NT | - | - |
| Rhipicephalus | - | - | 10.7 | - | NT | - | - |
| appendiculatus | - | - | - | - | - | - | - |
| Haemaphysalis leachi | - | - | 5.7 | - | NT | - | - |
| Rhipicephalus evertsi | Goats (210) | Oyo | 33.3 | NT | NT | N/A | Ameen et al. 2014 [51] |
| evertsi | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 24.8 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 18.1 | - | NT | - | - |
| annulatus | - | - | - | - | - | - | - |
| Amblyomma variegatum | - | - | 11.9 | - | NT | - | - |
| Rhipicephalus | - | - | 6.2 | - | NT | - | - |
| appendiculatus | - | - | - | - | - | - | - |
| Haemaphysalis leachi | - | - | 2.9 | - | NT | - | - |
| Rhipicephalus evertsi | Sheep (104) | Oyo | 28.8 | NT | NT | N/A | Ameen et al. 2014 [51] |
| evertsi | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 18.3 | - | NT | - | - |
| decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus (Boophilus) | - | - | 11.5 | - | NT | - | - |
| annulatus | - | - | - | - | - | - | - |
| Amblyomma variegatum | - | - | 9.6 | - | NT | - | - |
| Rhipicephalus | - | 8.7 | - | NT | - | - | |
| appendiculatus | - | - | - | - | - | - | - |
| Haemaphysalis leachi | - | - | 4.8 | - | NT | - | - |
| Rhipicephalus sanguineus | Dogs (820) | Edo | 19.5 | NT | NT | N/A | Ugochukwu et al. 1985 [52] |
| - | - | Delta | - | - | - | - | - |
| Rhipicephalus sanguineus | Dogs (396) | Kwara | 19.2 | NT | NT | N/A | Ugbomoiko et al. 2008 [53] |
| Ixodes sp. | - | - | 4.5 | - | NT | - | - |
| Rhipicephalus sanguineus | Dogs (130) | Benue | 80.5 | NT | NT | N/A | Amuta et al. 2010 [54] |
| Rhipicephalus (Boophilus) | - | - | 14.6 | - | NT | - | - |
| annulatus | - | - | - | - | - | - | - |
| Hyalomma truncatum | - | - | 4.9 | - | NT | - | - |
| Rhipicephalus sanguineus | Dogs (157) | Edo | 53.6 | NT | NT | N/A | Isaac et al. 2016 [55] |
| Rhipicephalus pulchellus | - | - | 42.3 | - | NT | - | - |
| Rhipicephalus | - | - | 7.4 | - | NT | - | - |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Hyalomma truncatum | Cattle (450) | Anambra | 34.8 | NT | NT | N/A | Ikpeze et al. 2011 [56] |
| Rhipicephalus | - | Enugu | 23.1 | - | NT | - | - |
| appendiculatus | - | - | - | - | - | - | - |
| Rhipicephalus | - | - | 22.1 | - | NT | - | - |
| (Boophilus) annulatus | - | - | - | - | - | - | - |
| Amblyomma variegatum | - | - | 20.1 | - | NT | - | - |
| Rhipicephalus sanguineus | Humans (11) | Imo | NR | NT | NT | N/A | Okoli et al. 2006 [57] |
| - | - | Dogs (2) | - | - | NT | - | - |
| - | - | Sheep (3) | - | - | NT | - | - |
| Rhipicephalus sanguineus | Dogs (150) | Cross River | NR | NT | NT | N/A | Arong et al. 2013 [58] |
| Haemaphysalis leachi | - | - | - | - | NT | - | - |
| Rhipicephalus | - | - | - | - | NT | - | - |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Rhipicephalus | Goats (NR) | Enugu | NR | NT | NT | N/A | Ugochukwu et al. 1985 [59] |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Amblyomma variegatum | - | - | NR | - | NT | - | - |
| Amblyomma variegatum | Cattle (NR) | Kano | NR | NT | NT | N/A | Unsworth K. 1952 [60] |
| Amblyomma splendidum | - | Katsina | NR | - | NT | - | - |
| Rhipicephalus | - | Plateau | NR | - | NT | - | - |
| (Boophilus) decoloratus | - | - | - | - | - | - | - |
| Hyalomma spp. | - | Niger | NR | - | NT | - | - |
| Rhipicephalus spp. | - | Sokoto | NR | - | NT | - | - |
| - | - | Adamawa | - | - | - | - | - |
| - | - | Bauchi | - | - | - | - | - |
| - | - | Borno | - | - | - | - | - |
| - | - | Kwara | - | - | - | - | - |
| NR | Cattle (120) | Borno | N/A | Anaplasma spp. (5.8) | N/A | Blood smear stain | Paul et al. 2016 [22] |
| - | - | - | - | Babesia spp. (4.2) | - | - | - |
| NR | Goats (72) | Nassarawa | N/A | Anaplasma spp. (15.3) | N/A | Blood smear stain | Opara et al. 2016 [23] |
| - | - | - | - | Babesia spp. (5.6) | - | - | - |
| NR | Sheep (32) | Nassarawa | N/A | Anaplasma spp. (9.4) | N/A | Blood smear stain | Opara et al. 2016 [23] |
| - | - | - | - | Babesia spp. (3.1) | - | - | - |
| NR | Dogs (101) | Abuja | N/A | Babesia canis (8.9) | N/A | Blood smear stain | Jegede et al. 2014 [20] |
| NR | Dogs (120) | Abuja | N/A | Babesia spp. | N/A | Blood smear stain | Obeta et al. 2009 [61] |
| NR | Dogs (100) | Plateau | N/A | Ehrlichia canis (11) | N/A | PCR | Kamani et al. 2013 [62] |
| NR | Cattle (704) | Plateau | N/A | Theileria mutans (61.8) | N/A | PCR, RLB | Lorusso et al. 2016 [63] |
| - | - | - | - | Theileria velifera (49.4) | - | - | - |
| - | - | - | - | Anaplasma marginale (38.1) | - | - | - |
| - | - | - | - | Theileria taurotragi (36.9) | - | - | - |
| - | - | - | - | Anaplasma sp. (Omatjenne) (33.9) | - | - | - |
| - | - | - | - | Anaplasma centrale (8.1) | - | - | - |
| - | - | - | - | Babesia bigemina (8.1) | - | - | - |
| - | - | - | - | Rickettsia spp. (2.7) | - | - | - |
| - | - | - | - | Babesia bovis (2.3) | - | - | - |
| - | - | - | - | Erhlichia ruminantium (1.1) | - | - | - |
| - | - | - | - | Anaplasma platys (3.8) | - | - | - |
| NR | Cattle (100) | Oyo | N/A | Babesia bigemina (9, 93.0) n | N/A | Blood smear stain | Akinboade et al. 1984 [18] |
| - | - | - | - | - | - | IFA | - |
| - | - | - | - | Anaplasma marginale (8.9, 68.0) n | - | Blood smear stain | - |
| - | - | - | - | - | - | IFA | - |
| - | - | - | - | Babesia bovis (3.3, 54.5) n | - | Blood smear stain | - |
| - | - | - | - | - | - | IFA | - |
| - | - | - | - | Anaplasma centrale (0.75) | - | Blood smear stain | - |
| - | - | - | - | Eperythrozoon (0.75) | - | Blood smear stain | - |
| - | - | - | - | Theleria spp. (0.75) | - | Blood smear stain | - |
| NR | Cattle (500) | Nigeria | N/A | Anaplasma marginale | N/A | IFA, CT, CA | Ajayi et al. 1986 [64] |
| - | - | - | - | (79.4, 40.0, 25.0) | - | - | - |
| - | - | - | - | Babesia bigemina (29.4) | - | IFA | - |
| - | - | - | - | Babesia bovis (14.1) | - | IFA | - |
| Species Collected | Vertebrate Host (Number) | Location | Infestation (%) | Pathogen (%) | Detection Method | Study [Reference] | |
|---|---|---|---|---|---|---|---|
| Host Blood | Fleas | ||||||
| Xenopsylla cheopsis | Rattus rattus (48) | Plateau | NR | Bartonella spp. (18.8) | Bartonella spp. (50) a | PCR, Blood culture b | Kamani et al. 2013 [26] |
| Ctenophthalmus sp. | R. norvegicus (121) | - | NR | Bartonella spp. (29.8) | Bartonella spp. (66.7) c | PCR, Blood culture b | - |
| - | Mus musculus (6) | - | - | ND | - | PCR, Blood culture b | - |
| - | Cricetomys gambianus (2) | - | - | Bartonella spp. (50.0) | - | PCR, Blood culture b | - |
| Ctenocephalides canis | Goats (2) | Oyo | NR | Babesia motasi | NT | NR | Opasina. 1983 [126] |
| Ctenocephalides canis | Dogs (396) | Kwara | 32.1 | NT | NT | N/A | Ugbomoiko et al. 2008 [53] |
| Pulex irritans | - | - | 6.6 | NT | - | - | - |
| Tunga penetrans | - | - | 0.5 | NT | - | - | - |
| Ctenocephalides sp. | Dogs (44) | Benue | 38.6 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Pulex sp. | - | - | 6.8 | NT | NT | - | - |
| Tunga sp. | Pigs (44) | Benue | 77.3 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Tunga sp. | Goats (45) | Benue | 22.2 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Tunga sp. | Sheep (45) | Benue | 26.7 | NT | NT | N/A | Omudu et al. 2007 [44] |
| Tunga penetrans | Humans (5595) | Lagos | 49.5 | NT | NT | N/A | Ejezie G. 1981 [110] |
| Tunga penetrans | Humans (480) | Rivers | 30.4 | NT | NT | N/A | Arene, F. O. I. 1984 [111] |
| Xenopsylla cheopsis | Arvincanthis | Delta | 22.4 | NT | NT | N/A | Ugbomoiko et al. 1991 [43] |
| - | niloticus (NR) | - | - | - | - | - | - |
| Xenopsylla braziliensis | Lophuromys | Edo | 5.1 | NT | NT | - | - |
| - | sikapusi (NR) d | - | - | - | - | - | |
| - | Mastomys | - | - | NT | - | - | - |
| - | natalensis (NR) | - | - | - | - | - | - |
| - | Mus minutoides (NR) d | - | - | NT | - | - | - |
| - | Leminiscomys | - | - | NT | - | - | - |
| - | striatus (NR) d | - | - | - | - | - | - |
| - | Rattus rattus (NR) | - | - | NT | - | - | - |
| Tunga penetrans | Humans (547) | Lagos | 45.2 | NT | NT | N/A | Ugbomoiko et al. 2007 [113] |
| Tunga penetrans | Humans (545) | Lagos | 22.4 | NT | NT | N/A | Ugbomoiko et al. 2017 [127] |
| Ctenocephalides canis | Dogs (202) | Ogun | 13.4 | NT | NT | N/A | Agbolade et al. 2008 [47] |
| Xenopsylla cheopsis | Rattus rattus (50) | Osun | 36 | NT | NT | N/A | Ogunniyi et al. 2014 [117] |
| Ctenocephalides canis | Dogs (338) | Anambra | 26.3 | NT | NT | N/A | Chukwu et al. 1985 [114] |
| Echidnophaga gallinacean | - | - | 2.1 | - | NT | - | - |
| Ctenocephalides canis | Dogs (820) | Edo | NR | NT | NT | N/A | Ugochukwu et al. 1985 [52] |
| - | - | Delta | - | - | - | - | - |
| Ctenocephalides canis | Humans (NR) | Oyo | NR | NT | NT | N/A | Fagbemi et al. 1981 [109] |
| Ctenocephalides felis | Cattle (NR) | NR | NR | NT | NT | N/A | Shaibu et al. 2011 [128] |
| Species Collected | Vertebrate Host (Number) | Location | Infestation (%) | Pathogen (%) | Detection Method | Study [Reference] | |
|---|---|---|---|---|---|---|---|
| Host Blood | Lice | ||||||
| Pediculus humanus corporis | Humans (126) | Plateau | NR | Rickettsia prowazekii (100) a | NT | Weil-Felix | Montgomery et al. 1947 [152] |
| Pediculus humanus capitis | Humans (2333) | Oyo | 4.8 b | NT | NT | N/A | Ogunrinade et al. 1984 [143] |
| Pediculus humanus capitis | Humans (2704) | Osun | 12.7 b | NT | NT | N/A | Jinadu, M. K. 1985 [144] |
| Pediculus humanus capitis | Humans (7360) | Abia, Imo | 5.7 b | NT | NT | N/A | Arene et al. 1985 [147] |
| - | - | Akwa Ibom | - | - | - | - | - |
| - | - | Bayelsa, Delta | - | - | - | - | - |
| - | - | Cross River | - | - | - | - | - |
| - | - | Edo, Ondo | - | - | - | - | - |
| - | - | Rivers | - | - | - | - | - |
| Pediculus humanus capitis | Humans (6882) | Kwara | 3.7 b | NT | NT | N/A | Ebomoyi, E. 1994 [146] |
| Pediculus humanus capitis | Humans (2898) | Kwara | 2.0 b | NT | NT | N/A | Ebomoyi, E. 1988 [145] |
| Pediculus humanus capitis | Humans (1000) | Kwara | 8.8 b | NT | NT | N/A | Okwa et al. 2010 [149] |
| Pediculus humanus capitis | Humans (726) | Rivers | 45.6 b | NT | NT | N/A | Gboeloh et al. 2013 [148] |
| NR | Humans (NR) | Enugu | N/A | Rickettsia prowazekii | N/A | Weil-Felix | Emejuaiwe et al. 1978 [153] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oguntomole, O.; Nwaeze, U.; Eremeeva, M.E. Tick-, Flea-, and Louse-Borne Diseases of Public Health and Veterinary Significance in Nigeria. Trop. Med. Infect. Dis. 2018, 3, 3. https://doi.org/10.3390/tropicalmed3010003
Oguntomole O, Nwaeze U, Eremeeva ME. Tick-, Flea-, and Louse-Borne Diseases of Public Health and Veterinary Significance in Nigeria. Tropical Medicine and Infectious Disease. 2018; 3(1):3. https://doi.org/10.3390/tropicalmed3010003
Chicago/Turabian StyleOguntomole, Oluwaseun, Ugochukwu Nwaeze, and Marina E. Eremeeva. 2018. "Tick-, Flea-, and Louse-Borne Diseases of Public Health and Veterinary Significance in Nigeria" Tropical Medicine and Infectious Disease 3, no. 1: 3. https://doi.org/10.3390/tropicalmed3010003
APA StyleOguntomole, O., Nwaeze, U., & Eremeeva, M. E. (2018). Tick-, Flea-, and Louse-Borne Diseases of Public Health and Veterinary Significance in Nigeria. Tropical Medicine and Infectious Disease, 3(1), 3. https://doi.org/10.3390/tropicalmed3010003




