The Epidemiology of Malaria in Kutubu, Southern Highlands Province, Papua New Guinea, before and during a Private Sector Initiative for Malaria Control
Abstract
:1. Introduction
2. Methods
2.1. Intervention: Marasin Stoa Kipa (MSK): 2007–2013
- (1)
- implement the MSK program in malaria endemic communities with no functional health facilities or access to care;
- (2)
- implement the MSK program in areas where health facility function was challenged;
- (3)
- motivate the MSK with financial incentives according to quantity and quality of work;
- (4)
- embed local management of the MSK program with a community-based organization, such as the church;
- (5)
- develop the MSK as an integral component of local health services (public private partnerships);
- (6)
- develop a monitoring and evaluation (M&E) system to evaluate progress and adjust elements of the program according to specific community needs where applicable.
2.2. Data on Reported Cases
3. Results
3.1. Access and Use of HFs and MSK Providers
3.2. Number of Cases Reported
3.3. Test Positivity Rate
3.4. Average Cases per Month by Facility Type
3.5. Species Distribution
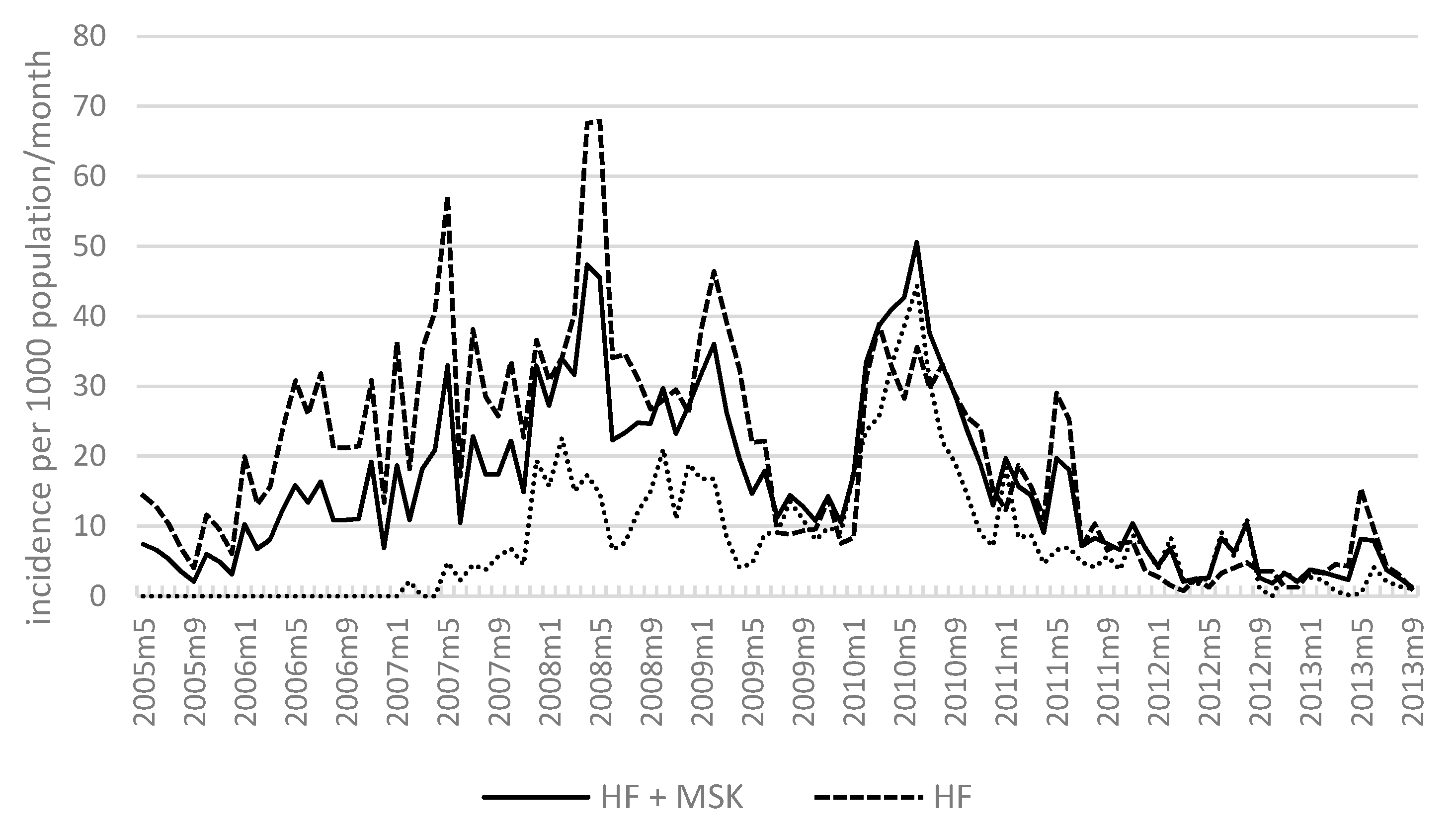
3.6. Overall Malaria Incidence in Kutubu (May 2005–September 2013)
3.7. Malaria Incidence: Health Facilities and MSKs
4. Discussion
- (1)
- the sequential increase in the number of MSK sites and thus increased case detection
- (2)
- climate variation
- (3)
- population mobility and economic development
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Ethical Considerations
References
- National Department of Health. 2015 Sector Performance Annual Review: Assessment of Sector Performance 2011–2015; National Report; National Department of Health: Port Moresby, Papua New Guinea, 2016.
- WHO. World Malaria Report 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- National Department of Health. Papua New Guinea National Health Plan 2011–2020; National Department of Health: Port Moresby, Papua New Guinea, 2010.
- Hetzel, M.; Choudhury, A.; Pulford, J.; Ura, Y.; Whittaker, M.; Siba, P.; Mueller, I. Progress in mosquito net coverage in Papua New Guinea. Malar. J. 2014, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, M.; Gideon, G.; Lote, N.; Makita, L.; Siba, P.; Mueller, I. Ownership and usage of mosquito nets after four years of large-scale free distribution in Papua New Guinea. Malar. J. 2012, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, M.W.; Morris, H.; Tarongka, N.; Barnadas, C.; Pulford, J.; Makita, L.; Siba, P.M.; Mueller, I. Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Trop. Med. Int. Health 2015, 20, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Pulford, J.; Kurumop, S.F.; Ura, Y.; Siba, P.M.; Mueller, I.; Hetzel, M.W. Malaria case management in Papua New Guinea following the introduction of a revised treatment protocol. Malar. J. 2013, 12, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betuela, I.; Maraga, S.; Hetzel, M.W.; Tandrapah, T.; Sie, A.; Yala, S.; Kundi, J.; Siba, P.; Reeder, J.C.; Mueller, I. Epidemiology of malaria in the Papua New Guinean highlands. Trop. Med. Int. Health 2012, 17, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Mueller, I.; Bockarie, M.; Alpers, M.; Smith, T. The epidemiology of malaria in Papua New Guinea. Trends Parasitol. 2003, 19, 253–259. [Google Scholar] [CrossRef]
- Maraga, S.; Pluss, B.; Schopflin, S.; Sie, A.; Iga, J.; Ousari, M.; Yala, S.; Meier, G.; Reeder, J.C.; Mueller, I. The epidemiology of malaria in the Papua New Guinea highlands: 7. Southern Highlands Province. PNG Med. J. 2011, 54, 35–47. [Google Scholar]
- Hii, J.; Dyke, T.; Dagoro, H.; Sanders, R.C. Health impact assessments of malaria and Ross River virus infection in the Southern Highlands Province of Papua New Guinea. PNG Med. J. 1997, 1, 14–25. [Google Scholar]
- Spencer, T.; Spencer, M.; Jemesen, M.; Tommerup, J. Special patrol report: Malaria in the Mt Hagen area. PNG Med. J. 1956, 1, 110–113. [Google Scholar] [CrossRef]
- Parkinson, A. Malaria in Papua New Guinea. PNG Med. J. 1974, 17, 8–16. [Google Scholar]
- Peters, W.; Christian, S.H. Studies on the epidemiology of malaria in New Guinea. VI. Unstable highlands malaria. The clinical picture. Trans. R. Soc. Trop. Med. Hyg. 1960, 54, 542–548. [Google Scholar] [CrossRef]
- Pulford, J.; Mueller, I.; Siba, P.; Hetzel, M. Malaria case management in Papua New Guinea prior to the introduction of a revised treatment protocol. Malar. J. 2012, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, M.; Page-Sharp, M.; Bala, N.; Pulford, J.; Betuela, I.; Davis, T.M.; Lavu, E. Quality of antimalarial drugs and antibiotics in Papua New Guinea: A survey of the health facility supply chain. PLoS ONE 2014, 9, e96810. [Google Scholar] [CrossRef] [PubMed]
- Pulford, J.; Smith, I.; Mueller, I.; Siba, P.M.; Hetzel, M.W. Health worker compliance with a “test and treat” malaria case management protocol in Papua New Guinea. PLoS ONE 2016, 11, e0158780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Statistics Office. PNG 2011 Census Final Figures—Summary Data for Persons and Households at National, Provincial, District and Statistical LLG Area Level; National Statistics Office: Waigani, Papua New Guinea, 2011.
- The National Research Institute: Papua New Guinea District and Provincial Profiles; National Library Service of Papua New Guinea: Boroko, Papua New Guinea, 2010.
- Bellamy, J.; McAlpine, J. Papua New Guinea Inventory of Natural Resources, Population Distribution and Land Use Handbook; Papua New Guinea Resource Information System (PNGRIS): Port Moresby, Papua New Guinea, 1996; p. 2. [Google Scholar]
- The National Research Institute. Topographic Base Map; The National Research Institute: Port Moresby, Papua New Guinea, 2015.
- United States Defense Mapping Agency. Vector Map Level; online database; United States Defense Mapping Agency: Springfield, VA, USA, 2000.
- Mueller, I.; Namuigi, P.; Kundi, J.; Ivivi, R.; Tandrapah, T.; Bjorge, S.; Reeder, J.C. Epidemic malaria in the highlands of Papua New Guinea. Am. J. Trop. Med. Hyg. 2005, 72, 554–560. [Google Scholar] [PubMed]
- Mueller, I.; Ousari, M.; Yala, S.; Ivivi, R.; Sie, A.; Reeder, J.C. The epidemiology of malaria in the Papua New Guinea highlands: 4. Enga Province. PNG Med. J. 2006, 49, 115–125. [Google Scholar]
- Mueller, I.; Yala, S.; Ousari, M.; Kundi, J.; Ivivi, R.; Saleu, G.; Sie, A.; Reeder, J.C. The epidemiology of malaria in the Papua New Guinea highlands: 6. Simbai and Bundi, Madang Province. PNG Med. J. 2007, 50, 123–133. [Google Scholar]
- Mueller, I.; Sie, A.; Ousari, M.; Iga, J.; Yala, S.; Ivivi, R.; Reeder, J.C. The epidemiology of malaria in the Papua New Guinea highlands: 5. Aseki, Menyamya and Wau-Bulolo, Morobe Province. PNG Med. J. 2007, 50, 111–122. [Google Scholar]
- Mueller, I.; Kundi, J.; Bjorge, S.; Namuigi, P.; Saleu, G.; Riley, I.D.; Reeder, J.C. The epidemiology of malaria in the Papua New Guinea highlands: 3. Simbu Province. PNG Med. J. 2004, 47, 159–173. [Google Scholar]
- Mueller, I.; Bjorge, S.; Poigeno, G.; Kundi, J.; Tandrapah, T.; Riley, I.D.; Reeder, J.C. The epidemiology of malaria in the Papua New Guinea highlands: 2. Eastern Highlands Province. PNG Med. J. 2003, 46, 166–179. [Google Scholar]
- Mueller, I.; Taime, J.; Ivivi, R.; Yala, S.; Bjorge, S.; Riley, I.D.; Reeder, J.C. The epidemiology of malaria in the Papua New Guinea highlands: 1. Western Highlands Province. PNG Med. J. 2003, 46, 16–31. [Google Scholar]
- Ahorlu, C.K.; Koram, K.; Ahorlu, C.; de Savigny, D.; Weiss, M.G. Socio-cultural determinants of treatment delay for childhood malaria in southern Ghana. Trop. Med. Int. Health 2006, 11, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.A.; Jones, C. A critical review of behavioural issues related to malaria control in sub-Saharan Africa: What contributions have social scientists made? Soc. Sci. Med. 2004, 59, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Kazembe, L.N.; Appleton, C.C.; Kleinschmidt, I. Choice of treatment for fever at household level in Malawi: Examining spatial patterns. Malar. J. 2007, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Dzator, J.; Asafu-Adjaye, J. A study of malaria care provider choice in Ghana. Health Policy 2004, 69, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiseman, V.; Scott, A.; Conteh, L.; McElroy, B.; Stevens, W. Determinants of provider choice for malaria treatment: Experiences from The Gambia. Soc. Sci. Med. 2008, 67, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, A. Anthropological and socio-medical health care research in developing countries. Soc. Sci. Med. 1983, 17, 147–161. [Google Scholar] [CrossRef]
- McCombie, S.C. Treatment seeking for malaria: A review of recent research. Soc. Sci. Med. 1996, 43, 933–945. [Google Scholar] [CrossRef]
- Tanner, M.; Vlassoff, C. Treatment-seeking behaviour for malaria: A typology based on endemicity and gender. Soc. Sci. Med. 1998, 46, 523–532. [Google Scholar] [CrossRef]
- Hetzel, M.W.; Alba, S.; Fankhauser, M.; Mayumana, I.; Lengeler, C.; Obrist, B.; Nathan, R.; Makemba, A.M.; Mshana, C.; Schulze, A.; et al. Malaria risk and access to prevention and treatment in the paddies of Kilombero Valley, Tanzania. Malar. J. 2008, 7, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofoed, P.-E.; Rodrigues, A.; Co, F.; Hedegaard, K.; Rombo, L.; Aaby, P. Which children come to the health centre for treatment of malaria? Acta Trop. 2004, 90, 17–22. [Google Scholar]
- Phommanivong, V.; Thongkham, K.; Deyer, G.; Rene, J.P.; Barennes, H. An assessment of early diagnosis and treatment of malaria by village health volunteers in the Lao PDR. Malar. J. 2010, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Kloos, H.; Chama, T.; Abemo, D.; Tsadik, K.G.; Belay, S. Utilization of pharmacies and pharmaceutical drugs in Addis Ababa, Ethiopia. Soc. Sci. Med. 1986, 22, 653–672. [Google Scholar] [CrossRef]
- Davy, C.P.; Sicuri, E.; Ome, M.; Lawrence-Wood, E.; Siba, P.; Warvi, G.; Mueller, I.; Conteh, L. Seeking treatment for symptomatic malaria in Papua New Guinea. Malar. J. 2010, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mueller, I.; Smith, T.; Mellor, S.; Rare, L.; Genton, B. The effect of distance from home on attendance at a small rural health centre in Papua New Guinea. Int. J. Epidemiol. 1998, 27, 878–884. [Google Scholar] [CrossRef]
- Moir, J.S.; Tulloch, J.L.; Vrbova, H.; Jolley, D.J.; Heywood, P.F.; Alpers, M.P. The role of voluntary village aides in the control of malaria by presumptive treatment of fever. PNG Med. J. 1985, 28, 257–266. [Google Scholar]
- Tsukahara, T.; Ogura, S.; Sugahara, T.; Sekihara, M.; Furusawa, T.; Kondo, N.; Mita, T.; Endo, H.; Hombhanje, F. The choice of healthcare providers for febrile children after introducing non-professional health workers in a malaria endemic area in Papua New Guinea. Front. Public Health 2015, 3, 275. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Whittaker, M. Beyond mobile populations: A critical review of the literature on malaria and population mobility and suggestions for future directions. Malar. J. 2014, 13, 307. [Google Scholar] [CrossRef] [PubMed]
- Mueller, I.; Kaiok, J.; Reeder, J.C.; Cortes, A. The population structure of Plasmodium falciparum and Plasmodium vivax during an epidemic of malaria in the Eastern Highlands of Papua New Guinea. Am. J. Trop. Med. Hyg. 2002, 67, 459–464. [Google Scholar] [PubMed]
- Radford, A.J.; van Leeuwen, H.; Christian, S.H. Social aspects in the changing epidemiology of malaria in the highlands of New Guinea. Ann. Trop. Med. Parasitol. 1976, 70, 11–23. [Google Scholar] [CrossRef] [PubMed]
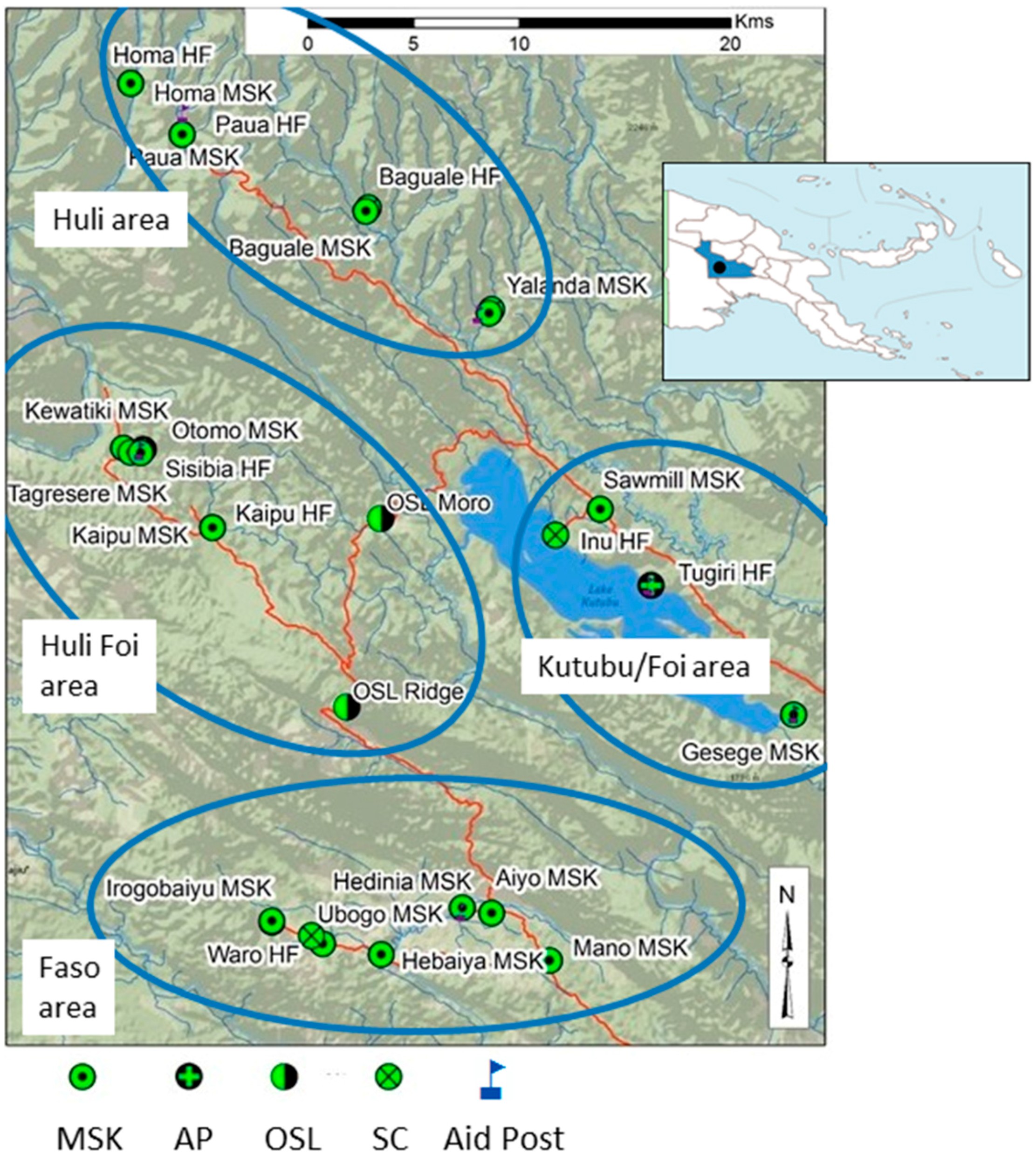
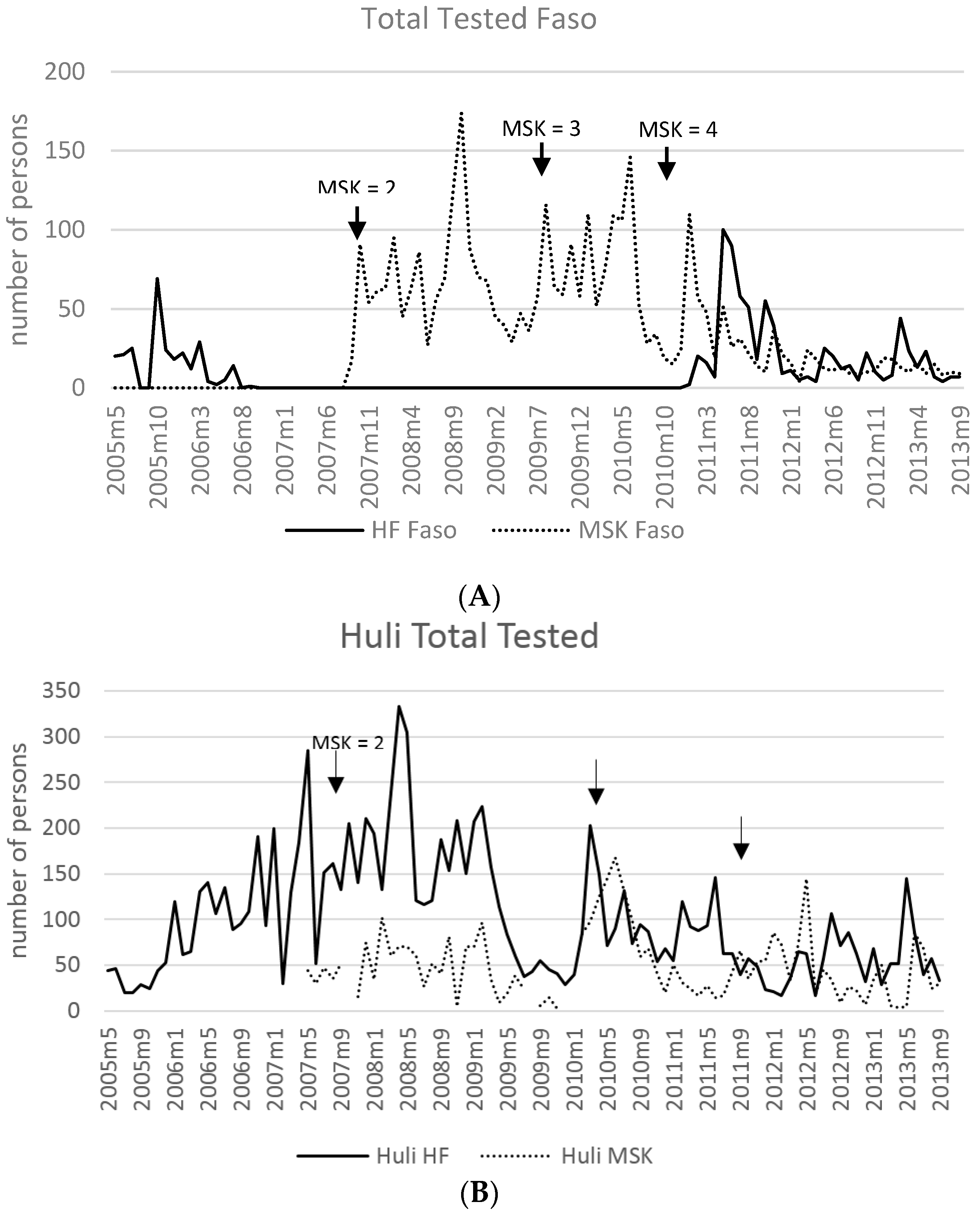
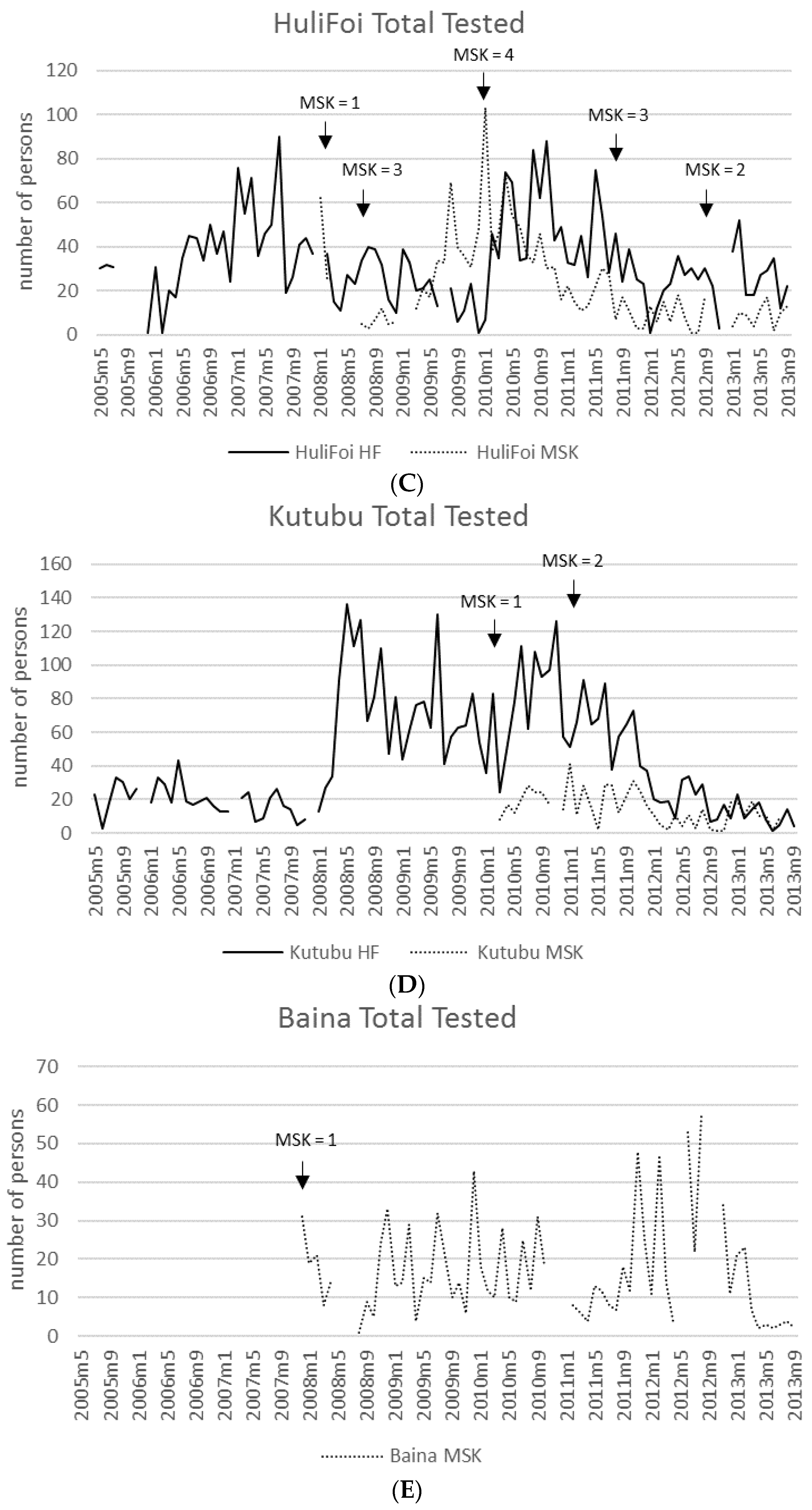
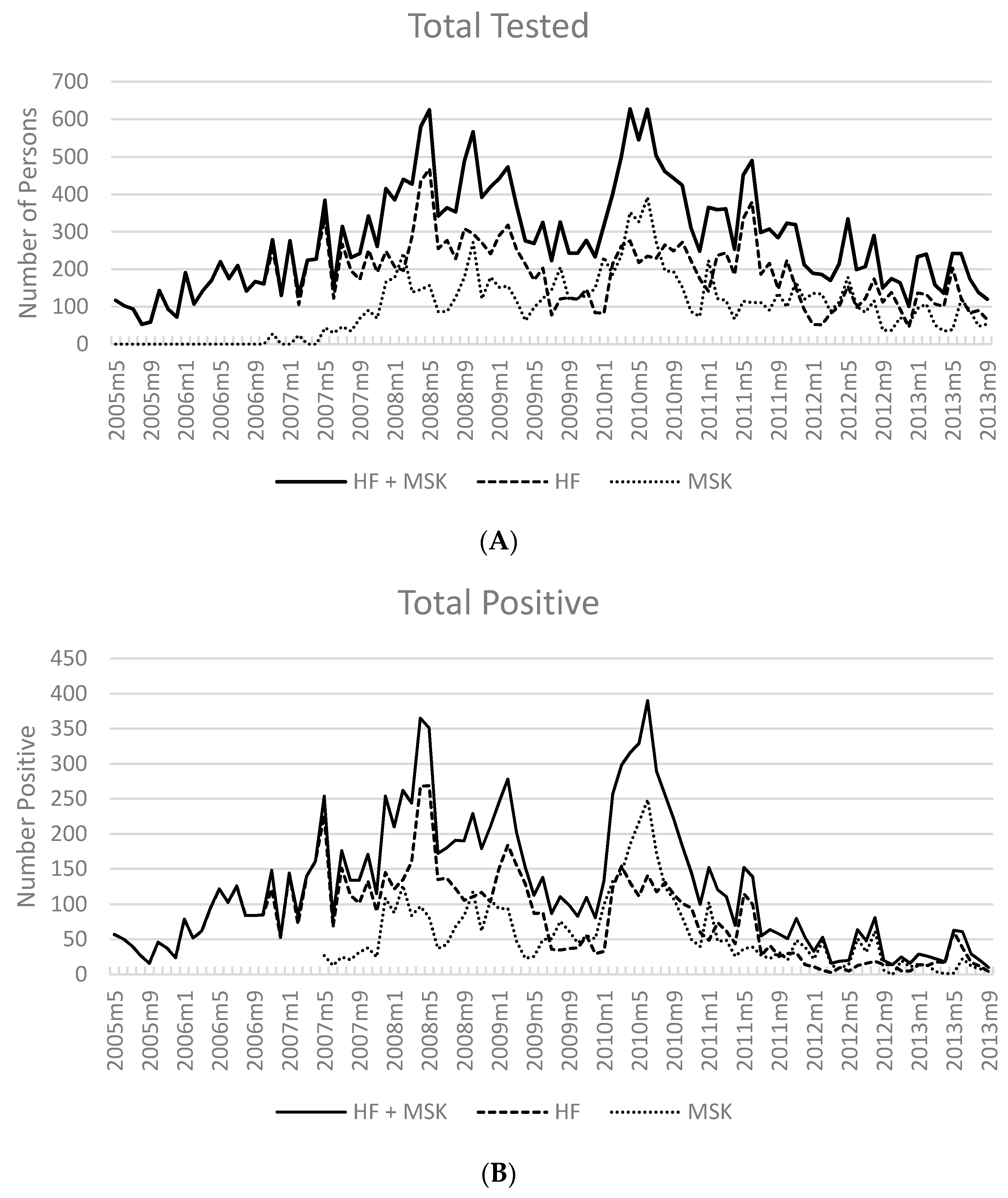
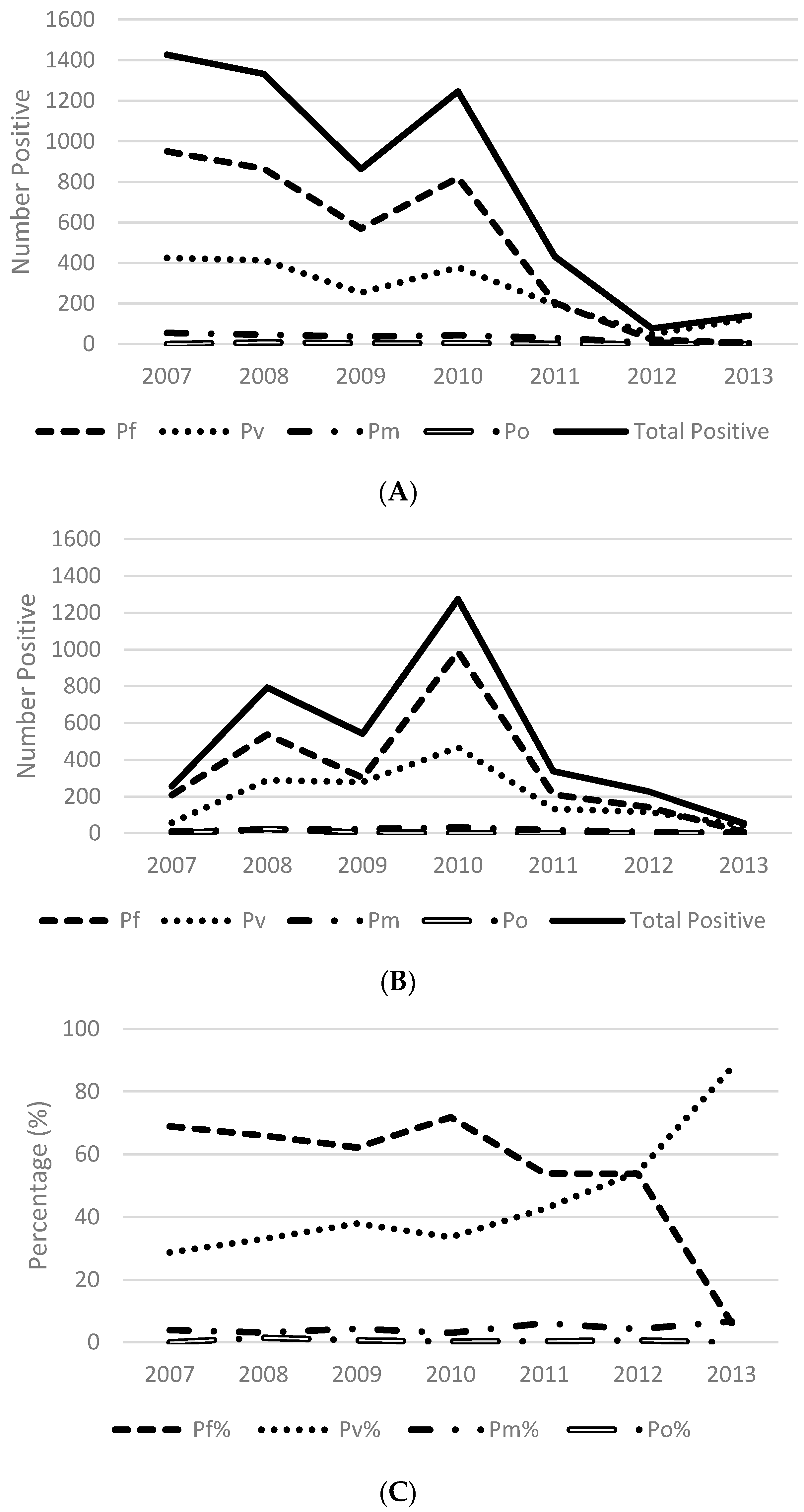
| Year | # HF | Reporting Months * | Total Tested | Total Pos | (%) | Pf or Pf Mix | (%) | P Other | (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2007 | 5 | 48/60 | 2623 | 1546 | 58.9 | 1142 | 73.9 | 404 | 26.1 |
| 2008 | 6 | 69/72 | 3470 | 1787 | 51.5 | 1266 | 70.8 | 521 | 29.2 |
| 2009 | 6 | 64/72 | 2122 | 1025 | 48.3 | 721 | 70.3 | 304 | 29.7 |
| 2010 | 7 | 67/84 | 2700 | 1313 | 48.6 | 897 | 68.3 | 416 | 31.7 |
| 2011 | 9 | 90/108 | 2541 | 614 | 24.2 | 314 | 51.1 | 300 | 48.9 |
| 2012 | 8 | 79/96 | 1227 | 121 | 9.9 | 48 | 39.7 | 73 | 60.3 |
| 2013 ** | 8 | 70/96 | 1043 | 194 | 18.6 | 38 | 19.6 | 156 | 80.4 |
| Total | 15726 | 6600 | 42.0 | 4426 | 67.1 | 2174 | 32.9 |
| Year | # MSK | Reporting Months * | Total Tested | Total Pos | (%) | Pf or Pf Mix | (%) | P Other | (%) |
|---|---|---|---|---|---|---|---|---|---|
| 2007 | 6 | 20/72 | 576 | 302 | 52.4 | 244 | 80.8 | 58 | 19.2 |
| 2008 | 7 | 68/84 | 1914 | 996 | 52.0 | 673 | 67.6 | 323 | 32.4 |
| 2009 | 11 | 80/132 | 1493 | 644 | 43.1 | 339 | 52.6 | 305 | 47.4 |
| 2010 | 13 | 123/156 | 2549 | 1488 | 58.4 | 1014 | 68.1 | 474 | 31.9 |
| 2011 | 14 | 129/168 | 1429 | 485 | 33.9 | 314 | 64.7 | 171 | 35.3 |
| 2012 | 13 | 116/156 | 1103 | 284 | 25.7 | 182 | 64.1 | 102 | 35.9 |
| 2013 ** | 12 | 83/144 | 623 | 84 | 13.5 | 30 | 35.7 | 54 | 64.3 |
| Total | 9687 | 4283 | 44.2 | 2796 | 65.3 | 1487 | 34.7 |
| Indicator | Mean | sd | N * |
|---|---|---|---|
| RDT num. tested | 28.59 | 31.27 | 559 |
| RDT num. pos Pf or Pfmix | 8.66 | 14.13 | 559 |
| RDT num. pos P other only | 2.58 | 4.15 | 559 |
| RDT num. pos any | 11.24 | 17.24 | 559 |
| MIC num. tested | 30.67 | 32.47 | 559 |
| MIC num. pos Pf or Pfmix | 7.85 | 12.94 | 559 |
| MIC num. pos P other only | 3.79 | 5.32 | 559 |
| MIC num. pos any | 11.65 | 17.04 | 559 |
| RDT or MIC num. tested | 31.35 | 32.00 | 559 |
| RDT or MIC num. pos Pf or Pfmix | 9.67 | 14.97 | 559 |
| RDT or MIC num. pos P other only | 4.58 | 6.28 | 559 |
| RDT or MIC num. pos any | 14.23 | 19.91 | 559 |
| Indicator | Mean | sd | N * |
|---|---|---|---|
| RDT num. tested | 14.89 | 15.93 | 620 |
| RDT num. pos.Pf or Pfmix | 4.35 | 7.43 | 620 |
| RDT num. pos P other only | 1.51 | 2.97 | 620 |
| RDT num. pos any | 5.86 | 9.19 | 620 |
| MIC num. tested | 15.88 | 16.19 | 620 |
| MIC num. pos Pf or Pfmix | 3.89 | 6.75 | 620 |
| MIC num. pos P other only | 2.01 | 3.15 | 620 |
| MIC num. pos any | 5.90 | 9.04 | 620 |
| RDT or MIC num. tested | 15.38 | 14.20 | 620 |
| RDT or MIC num. pos Pf or Pfmix | 4.73 | 7.58 | 620 |
| RDT or MIC num. pos P other only | 2.47 | 3.78 | 620 |
| RDT or MIC num. pos any | 7.20 | 10.37 | 620 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feterl, M.; Graves, P.; Seehofer, L.; Warner, J.; Wood, P.; Miles, K.; Hutton, R. The Epidemiology of Malaria in Kutubu, Southern Highlands Province, Papua New Guinea, before and during a Private Sector Initiative for Malaria Control. Trop. Med. Infect. Dis. 2017, 2, 2. https://doi.org/10.3390/tropicalmed2010002
Feterl M, Graves P, Seehofer L, Warner J, Wood P, Miles K, Hutton R. The Epidemiology of Malaria in Kutubu, Southern Highlands Province, Papua New Guinea, before and during a Private Sector Initiative for Malaria Control. Tropical Medicine and Infectious Disease. 2017; 2(1):2. https://doi.org/10.3390/tropicalmed2010002
Chicago/Turabian StyleFeterl, Marshall, Patricia Graves, Liesel Seehofer, Jeffery Warner, Peter Wood, Kevin Miles, and Ross Hutton. 2017. "The Epidemiology of Malaria in Kutubu, Southern Highlands Province, Papua New Guinea, before and during a Private Sector Initiative for Malaria Control" Tropical Medicine and Infectious Disease 2, no. 1: 2. https://doi.org/10.3390/tropicalmed2010002
APA StyleFeterl, M., Graves, P., Seehofer, L., Warner, J., Wood, P., Miles, K., & Hutton, R. (2017). The Epidemiology of Malaria in Kutubu, Southern Highlands Province, Papua New Guinea, before and during a Private Sector Initiative for Malaria Control. Tropical Medicine and Infectious Disease, 2(1), 2. https://doi.org/10.3390/tropicalmed2010002






