A Transdisciplinary Approach to Managing Emerging and Resurging Mosquito-Borne Diseases in the Western Pacific Region
Abstract
:1. Introduction
1.1. Mosquito-Borne Diseases in the Western Pacific Region
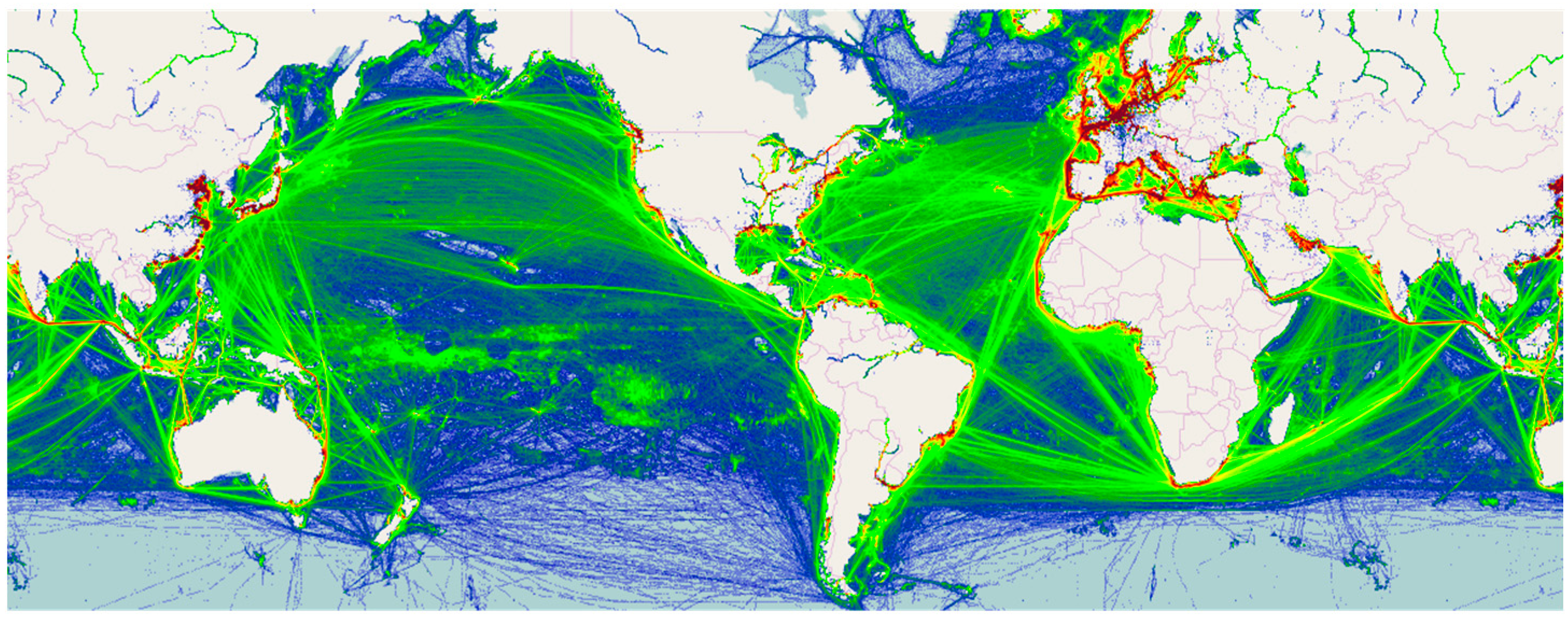
1.2. Biosecurity
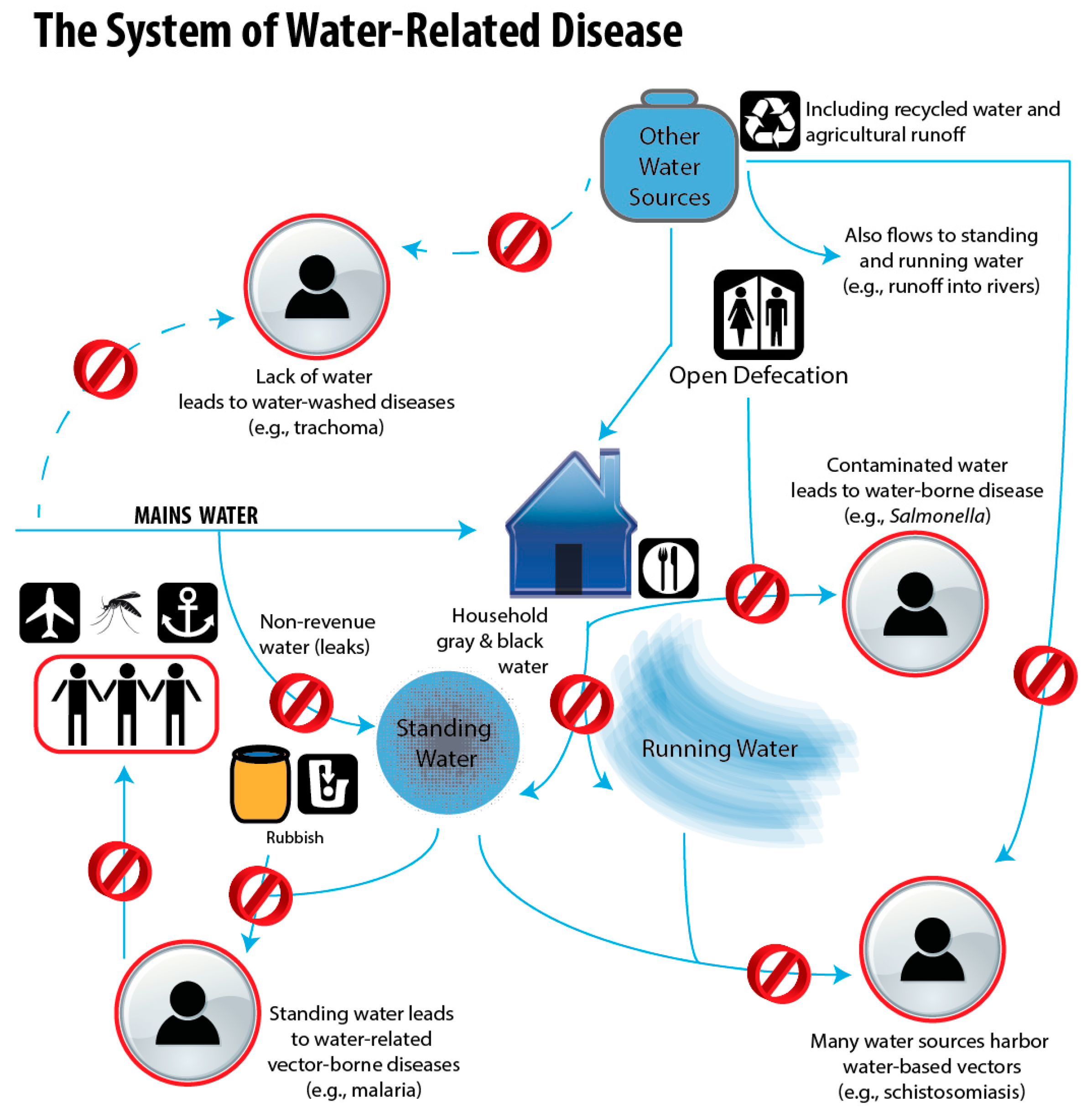
1.3. Water-Related Diseases
1.4. Insecticides: the Silver Bullet?
1.5. A New Mosquito-Borne Pathogen Management Approach for the Western Pacific Region
2. Exploring Mosquito and Pathogen Populations through Citizen Science
3. A Systems-Based Approach to Reducing Mosquito-Borne Disease
3.1. The “One Health” Approach
3.2. Integrating the One Health Approach into Citizen Science Programs
3.3. Integrating the One Health Approach into Biosecurity Measures
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- WHO Regional Office for the Western Pacific (WPRO). Available online: http://www.who.int/about/regions/wpro/en/ (accessed on 21 June 2016).
- Roth, A.; Mercier, A.; Lepers, C.; Hoy, D.; Duituturaga, S.; Benyon, E.; Guillaumot, L.; Souares, Y. Concurrent outbreaks of dengue, chikungunya and Zika virus infections-an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Eurosurveillance 2014, 19, 20929. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Gubler, D.J. Geographic expansion of dengue: The impact of international travel. Med. Clin. N. Am. 2008, 92, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for the Western Pacific (WPRO). Regional Malaria Situation; WPRO|WHO Western Pacific Region: Manila, Philippines, 2016. [Google Scholar]
- LaPointe, D.A. Current and potential impacts of mosquitoes and the pathogens they vector in the Pacific Region. Proc. Hawaii. Entomol. Soc. 2007, 39, 75–81. [Google Scholar]
- WHO Regional Office for the Western Pacific (WPRO). World Malaria Report 2015; World Health Organization Western Pacific Regional Office, 2015. [Google Scholar]
- William, T.; Jelip, J.; Menon, J.; Anderios, F.; Mohammad, R.; Awang Mohammad, T.A.; Grigg, M.J.; Yeo, T.W.; Anstey, N.M.; Barber, B.E.; et al. Changing epidemiology of malaria in Sabah, Malaysia: Increasing incidence of Plasmodium knowlesi. Malar. J. 2014, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Yusof, R.; Lau, Y.; Mahmud, R.; Fong, M.; Jelip, J.; Ngian, H.; Mustakim, S.; Mat Hussin, H.; Marzuki, N.; Mohd Ali, M.; et al. High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar. J. 2014, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D. The changing epidemiology of yellow fever and dengue, 1900 to 2003: Full circle? Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Ortiz, K.; Ansari, A.; Gershwin, M.E. The Zika outbreak of the 21st century. J. Autoimmun. 2016, 68, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. Countries with Current or Recent Local Transmission of Zika Virus. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-zika-countries.htm (accessed on 21 June 2016).
- Saiz, J.-C.; Vázquez-Calvo, Á.; Blázquez, A.B.; Merino-Ramos, T.; Escribano-Romero, E.; Martín-Acebes, M.A. Zika virus: The latest newcomer. Front. Microbiol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Cao-Lormeau, V.M.; Gubler, D.J. Zika virus: Following the path of dengue and chikungunya? Lancet 2015, 386, 243–244. [Google Scholar] [CrossRef]
- Sonic HealthPlus Pty Ltd. Chikungunya Island-Hopping across Pacific. Available online: http://www.travelvax.com.au/latest-news/30-risks-and-illnesses/156-chikungunya-something-nasty-coming-this-way (accessed on 1 December 2016).
- Parham, P.E.; Waldock, J.; Christophides, G.K.; Michael, E. Climate change and vector-borne diseases of humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Schaffner, F.; Versteirt, V.; Hendrickx, G.; Zeller, H.; Bortel, W. Van A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Randolph, S.E. Climate change and vector-borne diseases. Adv. Parasitol. 2006, 62, 345–381. [Google Scholar] [PubMed]
- National Research Council. Globalization, Biosecurity, and the Future of the Life Sciences; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- White, G.F.; Bradley, D.J.; White, A.U.; Ahmed, T. Drawers of Water: Domestic Water Use in East Africa; University of Chicago Press: Chicago, IL, USA, 1972. [Google Scholar]
- Han, M.; Hashemi, S.; Joo, S.H.; Kim, T. Novel integrated systems for controlling and prevention of mosquito-borne diseases caused by poor sanitation and improper water management. J. Environ. Chem. Eng. 2016, 4, 3718–3723. [Google Scholar] [CrossRef]
- Von Hedemann, N.; Robbins, P.; Butterworth, M.K.; Landau, K.; Morin, C.W. Managing mosquito spaces: Citizen self-governance of disease vectors in a desert landscape. Health Place 2017, 43, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Barik, T.K. Ecologically Sound Mosquito Vector Control in River Basins; Springer: Berlin, Germany, 2015; pp. 749–761. [Google Scholar]
- Dada, N.; Vannavong, N.; Seidu, R.; Lenhart, A.; Stenstrom, T.A.; Chareonviriyaphap, T.; Overgaard, H.J. Relationship between Aedes aegypti production and occurrence of Escherichia coli in domestic water storage containers in rural and sub-urban villages in Thailand and Laos. Acta Trop. 2013, 126, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ballera, J.E.; Zapanta, M.J.; de los Reyes, V.C.; Sucaldito, M.N.; Tayag, E. Investigation of chikungunya fever outbreak in Laguna, Philippines, 2012. Western Pac. Surveill. Response 2015, 6, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.; Barbara, J. Pacific Urbanisation: Changing Times; The Australian National University: Canberra, Australia, 2015. [Google Scholar]
- Storey, D. Urbanisation in the Pacific. In State Society and Governance in Melanesia; AusAID Research Paper; Australian National University: Canberra, Australia, 2006. [Google Scholar]
- Gubler, D.J. Dengue, urbanization and globalization: The unholy trinity of the 21st century. Trop. Med. Health 2011, 39, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Schrecongost, A.; Wong, K. Unsettled: Water and Sanitation in Urban Settlement Communities of the Pacific; Water and Sanitation Program; The World Bank: Washington, DC, USA, 2015. [Google Scholar]
- Kyle, J.L.; Harris, E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 2008, 62, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Takken, W. Do insecticide-treated bednets have an effect on malaria vectors? Trop. Med. Int. Health 2002, 7, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Winch, P.; Kendall, C.; Gubler, D. Effectiveness of community participation in vector-borne disease control. Health Policy Plan. 1992, 7, 342–351. [Google Scholar] [CrossRef]
- Essé, C.; Utzinger, J.; Tschannen, A.B.; Raso, G.; Pfeiffer, C.; Granado, S.; Koudou, B.G.; N’Goran, E.K.; Cissé, G.; Girardin, O.; et al. Social and cultural aspects of “malaria” and its control in central Côte d’Ivoire. Malar. J. 2008, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Barrington, D.J.; Sridharan, S.; Saunders, S.G.; Souter, R.T.; Shields, K.F.; Bartram, J.; Kearton, A.; Hughes, R.K. Improving community health through holistic marketing exchanges: Insights from a participatory action research study on water, sanitation, and hygiene in three Melanesian countries. Soc. Sci. Med. 2016, 171, 84–93. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Hardy, M.C. Spider-venom peptides: Structure, pharmacology, and potential for control of insect pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Gentz, M.C.; Murdoch, G.; King, G.F. Tandem use of selective insecticides and natural enemies for effective, reduced-risk pest management. Biol. Control 2010, 52, 208–215. [Google Scholar] [CrossRef]
- Hardy, M.C. Resistance is not futile: It shapes insecticide discovery. Insects 2014, 5, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Field, L.M. Gene amplification and insecticide resistance. Pest Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Heckel, D.G. Insecticide resistance after Silent Spring. Science 2012, 337, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Diabate, A.; Baldet, T.; Chandre, F.; Akoobeto, M.; Guiguemde, T.R.; Darriet, F.; Brengues, C.; Guillet, P.; Hemingway, J.; Small, G.J.; et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am. J. Trop. Med. Hyg. 2002, 67, 617–622. [Google Scholar] [PubMed]
- Windley, M.J.; Herzig, V.; Dziemborowicz, S.A.; Hardy, M.C.; King, G.F.; Nicholson, G.M. Spider-venom peptides as bioinsecticides. Toxins 2012, 4, 191–227. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 1998, 4, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [PubMed]
- McIver, L.; Kim, R.; Woodward, A.; Hales, S.; Spickett, J.; Katscherian, D.; Hashizume, M.; Honda, Y.; Kim, H.; Iddings, S.; et al. Health impacts of climate change in Pacific Island Countries: A regional assessment of vulnerabilities and adaptation priorities. Environ. Health Perspect. 2016, 124, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Boulding, K.E. General systems theory—The skeleton of science. Manag. Sci. 1956, 2, 197–208. [Google Scholar] [CrossRef]
- Barrington, D.J. Inadequate water, sanitation and hygiene in the South Pacific: How might it be impacting children? Rev. Environ. Health 2016, 31, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.Y.; Bi, P.; Cazelles, B.; Zhou, S.; Huang, S.Q.; Yang, J.; Pei, Y.; Wu, X.X.; Fu, S.H.; Tong, S.L.; et al. How environmental conditions impact mosquito ecology and Japanese encephalitis: An eco-epidemiological approach. Environ. Int. 2015, 79, 17–24. [Google Scholar] [CrossRef] [PubMed]
- James, P.J. Issues and advances in the integrated control of sheep lice. Anim. Prod. Sci. 2010, 50, 435–439. [Google Scholar] [CrossRef]
- Bonney, R.; Cooper, C.B.; Dickinson, J.; Kelling, S.; Phillips, T.; Rosenberg, K.V.; Shirk, J. Citizen science: A developing tool for expanding science knowledge and scientific literacy. BioScience 2009, 59, 977–984. [Google Scholar] [CrossRef]
- Tulloch, A.I.T.; Possingham, H.P.; Joseph, L.N.; Szabo, J.; Martin, T.G. Realising the full potential of citizen science monitoring programs. Biol. Conserv. 2013, 165, 128–138. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen science as an ecological research tool: Challenges and benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Gallo, T.; Waitt, D. Creating a successful citizen science model to detect and report invasive species. BioScience 2011, 61, 459–465. [Google Scholar] [CrossRef]
- Lucky, A.; Savage, A.M.; Nichols, L.M.; Castracani, C.; Shell, L.; Grasso, D.A.; Mori, A.; Dunn, R.R. Ecologists, educators, and writers collaborate with the public to assess backyard diversity in The School of Ants Project. Ecosphere 2014, 5, 1–23. [Google Scholar] [CrossRef]
- Delaney, D.G.; Sperling, C.D.; Adams, C.S.; Leung, B. Marine invasive species: Validation of citizen science and implications for national monitoring networks. Biol. Invasions 2008, 10, 117–128. [Google Scholar] [CrossRef]
- Walker, K. The BowerBird Bugle; Museum Victoria: Melbourne, Australia, 2015. [Google Scholar]
- Rosewell, A.; Ropa, B.; Randall, H.; Dagina, R.; Hurim, S.; Bieb, S.; Datta, S.; Ramamurthy, S.; Mola, G.; Zwi, A.B.; et al. Mobile phone-based syndromic surveillance system, Papua New Guinea. Emerg. Infect. Dis. 2013, 19, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Crall, A.W.; Jordan, R.; Holfelder, K.; Newman, G.J.; Graham, J.; Waller, D.M. The impacts of an invasive species citizen science training program on participant attitudes, behavior, and science literacy. Public Underst. Sci. 2013, 22, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.W.; De Rosa, C.; Howze, E.H.; Baldwin, G.T. Understanding wicked problems: A key to advancing environmental health promotion. Health Educ. Behav. 2004, 31, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Whittaker, M.; Tanner, M. One Health: The Theory and Practice of Integrated Health Approaches; CABI: Wallingford, CT, USA, 2015. [Google Scholar]
- Hadwen, W.L.; Powell, B.; MacDonald, M.C.; Elliott, M.; Chan, T.; Gernjak, W.; Aalbersberg, W.G. Putting WASH in the water cycle: Climate change, water resources and the future of water, sanitation and hygiene challenges in Pacific Island Countries. J. Water Sanit. Hyg. Dev. 2015, 5, 183–191. [Google Scholar] [CrossRef]
- Saunders, S.G.; Barrington, D.J.; Sridharan, S.; Meo, S.; Hadwen, W.; Shields, K.F.; Souter, R.T.; Bartram, J. Addressing water, sanitation and hygiene challenges in Pacific Island Countries: A participatory systems mapping approach to empower informal settlement community action. Habitat Int. 2016, 55, 159–166. [Google Scholar] [CrossRef]
- Patz, J.A.; Campbell-Lendrum, D.; Holloway, T.; Foley, J.A. Impact of regional climate change on human health. Nature 2005, 438, 310–317. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Plan for Insecticide Resistance Management in Malaria Vectors; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Reason, P.; Bradbury, H. (Eds.) The SAGE Handbook of Action Research: Participative Inquiry and Practice, 2nd ed.; SAGE Publishing: Thousand Oaks, CA, USA, 2008.
- Okurut, K.; Kulabako, R.N.; Chenoweth, J.; Charles, K. Assessing demand for improved sustainable sanitation in low-income informal settlements of urban areas: A critical review. Int. J. Environ. Health Res. 2015, 25, 81–95. [Google Scholar] [CrossRef] [PubMed]
- The Locally-Managed Marine Area Network About the LMMA. Available online: http://lmmanetwork.org/who-we-are/vision/ (accessed on 1 December 2016).
- Veitayaki, J.; Aalbersberg, W.G.L.; Tawake, A.; Rupeni, E.; Tabunakawai, K. Mainstreaming Resource Conservation: The Fiji Locally Managed Marine Area Network and Its Influence on National Policy Development; Resource Management in Asia-Pacific Working Paper: Canberra, Australia, 2003. [Google Scholar]
- MacLaren, D.; Kekeubata, E. Reorienting health services through community health promotion in Kwaio, Solomon Islands. Promot. Educ. 2007, 14, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Gentz, M.C. A review of chemical control options for invasive social insects in island ecosystems. J. Appl. Entomol. 2009, 133, 229–235. [Google Scholar] [CrossRef]
- Allsopp, R. Options for vector control against trypanosomiasis in Africa. Trends Parasitol. 2001, 17, 15–19. [Google Scholar] [CrossRef]
- Knight, A.L.; Flexner, L. Disruption of mating in codling moth (Lepidoptera: Tortricidae) by chlorantranilipole, an anthranilic diamide insecticide. Pest Manag. Sci. 2007, 63, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Mau, R.; Jang, E.; Vargas, R. The Hawaii area-wide fruit fly pest management programme: Influence of partnerships and a good education programme. In Area-Wide Control of Insect Pests; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 671–683. [Google Scholar]
- Wyss, J.H. Screwworm eradication in the Americas. Ann. N. Y. Acad. Sci. 2006, 916, 186–193. [Google Scholar] [CrossRef]
- Lax, A.R.; Osbrink, W.L.A. United States Department of Agriculture Agriculture Research Service research on targeted management of the Formosan subterranean termite Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). Pest Manag. Sci. 2003, 59, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Pound, J.M.; Miller, J.A.; George, J.E.; Fish, D. The United States Department of Agriculture northeast area-wide tick control project: history and protocol. Vector Borne Zoonotic Dis. 2009, 9, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Vreysen, M.J.B.; Seck, M.T.; Sall, B.; Bouyer, J. Tsetse flies: Their biology and control using area-wide integrated pest management approaches. J. Invertebr. Pathol. 2013, 112, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Gentz, M.C.; Rubinoff, D.; Grace, J.K. Phylogenetic analysis of subterranean termites (Coptotermes spp., Isoptera: Rhinotermitidae) reveals the origins of both Hawaiian and North American invasions. Proc. Hawaii. Entomol. Soc. 2008, 40, 1–9. [Google Scholar]
- MarineTraffic. Available online: http://www.marinetraffic.com (accessed on 1 March 2016).
| Genus | Bites During | Vectors |
|---|---|---|
| Aedes spp. (esp. A. aegypti, A. albopictus) | Daytime | Chikungunya, dengue, Zika (also heartworm in animals) |
| Anopheles spp. | Nighttime | Malaria, filariasis |
| Culex quinquefasciatus | Nighttime | Filariasis |
| Country | Major Plasmodium Species | Major Anopheles Species | Reported Confirmed Cases/Deaths |
|---|---|---|---|
| Cambodia | P. falciparum (64%), P. vivax (36%) | An. dirus, An. minimus, An. macultus, An. sundaicus | 25,152/18 |
| China | P. falciparum (11%), P. vivax (88%) | An. sinensis, An. anthropophagus, An. dirus, An. minimus | 2921/24 |
| Lao People’s Democratic Republic | P. falciparum (62%), P. vivax (38%) | An. dirus, An. minimus, An. maculatus, An. jeyporiensis | 48,071/4 |
| Malaysia a | P. falciparum (7%), P. vivax (8%) | An. balabacensis, An. donaldi, An. maculatus, An. sundaicus, An. flavirostris | 3923/9 |
| Papua New Guinea | P. falciparum (56%), P. vivax (41%) | An. farauti, An. punctulatus, An. koliensis | 281,182/203 |
| Philippines | P. falciparum (81%), P. vivax (17%) | An. flavirostris, An. maculatus, An. balabacensis, An. litoralis | 4903/10 |
| Republic of Korea | P. vivax (100%) | An. sinensis | 638/0 |
| Solomon Islands | P. falciparum (54%), P. vivax (46%) | An. farauti, An. punctulatus, An. koliensis | 18,404/23 |
| Vanuatu | P. falciparum (12%), P. vivax (88%) | An. farauti | 982/0 |
| Viet Nam | P. falciparum (54%), P. vivax (46%) | An. minimus, An. dirus, An. sundaicus | 15,752/6 |
| Total | 401,928/297 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardy, M.C.; Barrington, D.J. A Transdisciplinary Approach to Managing Emerging and Resurging Mosquito-Borne Diseases in the Western Pacific Region. Trop. Med. Infect. Dis. 2017, 2, 1. https://doi.org/10.3390/tropicalmed2010001
Hardy MC, Barrington DJ. A Transdisciplinary Approach to Managing Emerging and Resurging Mosquito-Borne Diseases in the Western Pacific Region. Tropical Medicine and Infectious Disease. 2017; 2(1):1. https://doi.org/10.3390/tropicalmed2010001
Chicago/Turabian StyleHardy, Margaret C., and Dani J. Barrington. 2017. "A Transdisciplinary Approach to Managing Emerging and Resurging Mosquito-Borne Diseases in the Western Pacific Region" Tropical Medicine and Infectious Disease 2, no. 1: 1. https://doi.org/10.3390/tropicalmed2010001
APA StyleHardy, M. C., & Barrington, D. J. (2017). A Transdisciplinary Approach to Managing Emerging and Resurging Mosquito-Borne Diseases in the Western Pacific Region. Tropical Medicine and Infectious Disease, 2(1), 1. https://doi.org/10.3390/tropicalmed2010001






