Distribution and Risk of Mycolactone-Producing Mycobacteria Transmission within Buruli Ulcer Endemic Communities in Côte d’Ivoire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Questionnaire Administration
2.2. Study Communities
2.3. Administration of Questionnaire
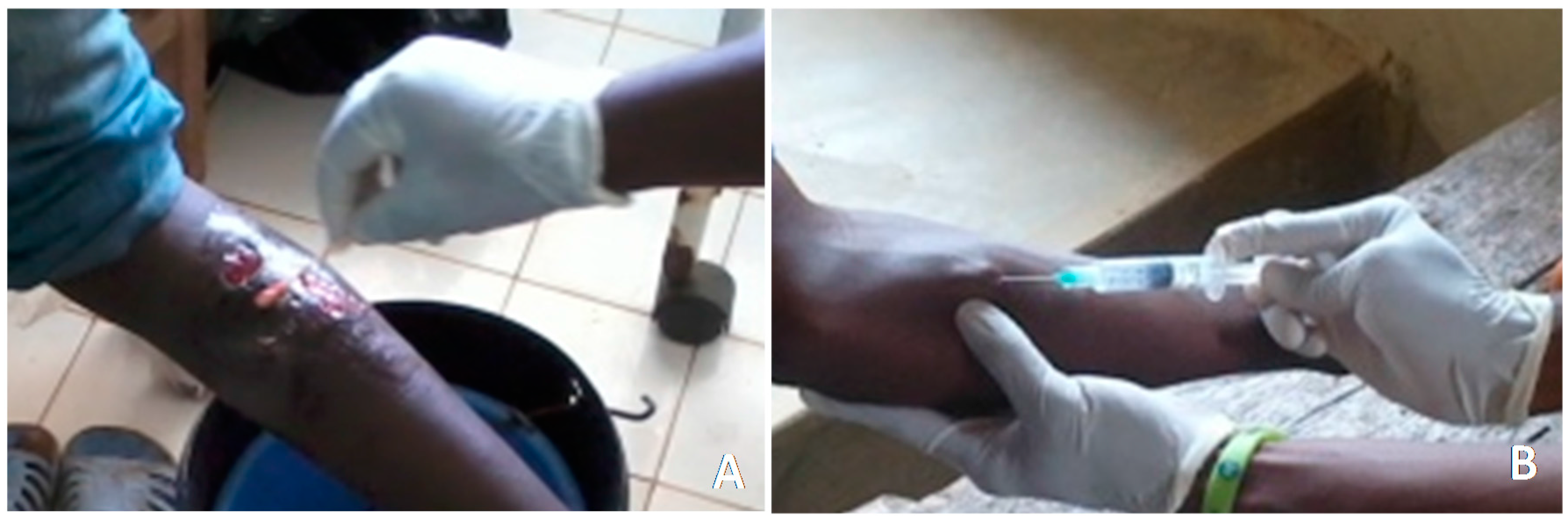
2.4. Collection of Human Samples
2.5. Environmental Sampling
2.6. DNA Extraction, Conventional PCR and Sequencing Analysis
2.7. Statistical and Phylogenetic Analysis
3. Results
3.1. Demographic Profile and Socio-Economic Activities Related to Water Bodies
3.2. Confirmation of Clinical Cases and Incidence of BU within Communities
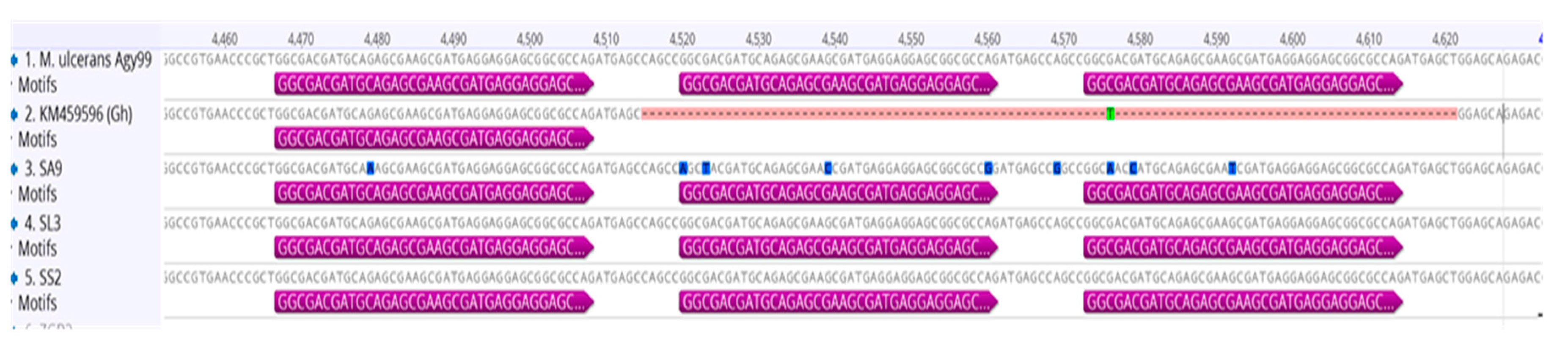
3.3. Genotyping and Discrimination of Human Strains
3.4. Distribution of MPMs in Environmental Samples from Water Bodies
3.5. Comparison of Human and Environmental MPM Strains
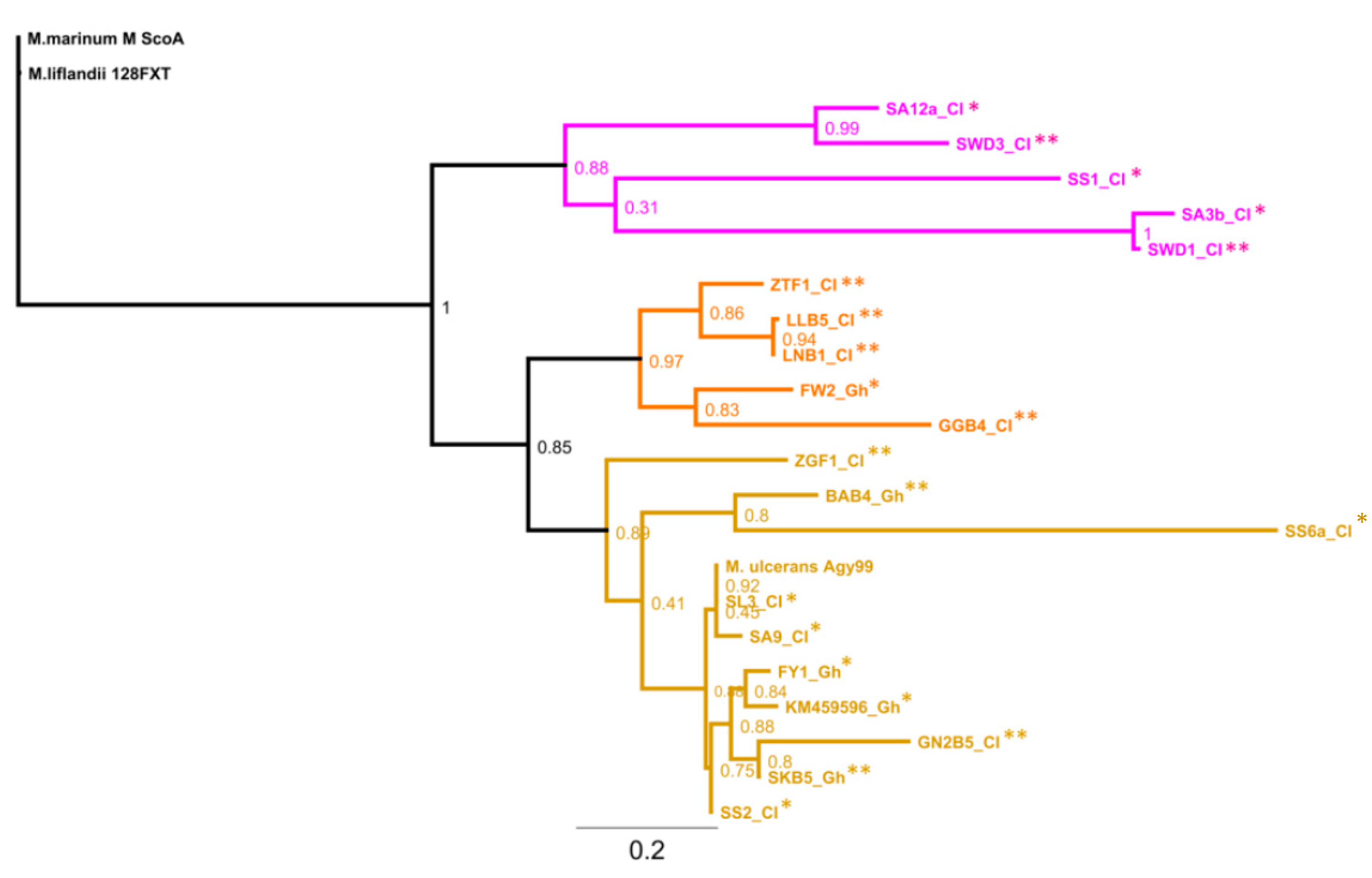
3.6. Genotypes from Côte d’Ivoire Share Homology with Ghanaian Genotypes, but Maintain Unique Sub-Clusters
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stienstra, Y.; van der Graaf, W.T.; Asamoa, K.; van der Werf, T.S. Beliefs and attitudes toward Buruli ulcer in Ghana. Am. J. Trop. Med. Hyg. 2002, 67, 207–213. [Google Scholar] [PubMed]
- Walsh, D.S.; Portaels, F.; Meyers, W.M. Recent advances in leprosy and Buruli ulcer (Mycobacterium ulcerans infection). Curr. Opin. Infect. Dis. 2010, 23, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, M.W.; Kator, H.; Kotob, S.; van Berkum, P.; Kaattari, I.; Vogelbein, W.; Floyd, M.M.; Butler, W.R.; Quinn, F.D.; Ottinger, C.; et al. A unique Mycobacterium species isolated from an epizootic of striped bass (Morone saxatilis). Emerg. Infect. Dis. 2001, 7, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Mve-Obiang, A.; Lee, R.E.; Umstot, E.S.; Trott, K.A.; Grammer, T.C.; Parker, J.M.; Ranger, B.S.; Grainger, R.; Mahrous, E.A.; Small, P.L. A newly discovered mycobacterial pathogen isolated from laboratory colonies of Xenopus species with lethal infections produces a novel form of mycolactone, the Mycobacterium ulcerans macrolide toxin. Infect. Immun. 2005, 73, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.D.; Stinear, T.; Small, P.L.; Pluschke, G.; Merritt, R.W.; Portaels, F.; Huygen, K.; Hayman, J.A.; Asiedu, K. Buruli ulcer (M. ulcerans infection): New insights, new hope for disease control. PLoS Med. 2005, 2, e108. [Google Scholar] [CrossRef] [PubMed]
- Kanga, J.; Kacou, E.; Kouamé, K.; Kassi, K.; Kaloga, M.; Yao, J.M.; Dion-Lainé, M.; Avoaka, L.; Yoboué-Yao, P.; Sangaré, A.; et al. La lutte contre l’ulcère de Buruli. Expérience de la Côte d’Ivoire. Bull. Soc. Pathol. Exot. 2005, 99, 34–38. [Google Scholar] [CrossRef]
- Ahoua, L.; Aka, N.; Ekaza, E.; Bouzid, S.; N’Guessan, R.; Dosso, M. Risk factors for Buruli ulcer in Côte d’Ivoire: Results of a case-control study. Afr. J. Biotechnol. 2009, 8, 536–546. [Google Scholar]
- Marston, B.J.; Diallo, M.O.; Horsburgh, C.R., Jr.; Diomande, I.; Saki, M.Z.; Kanga, J.M.; Patrice, G.; Lipman, H.B.; Ostroff, S.M.; Good, R.C. Emergence of Buruli ulcer disease in the Daloa region of Côte d’Ivoire. Am. J. Trop. Med. Hyg. 1995, 52, 219–224. [Google Scholar] [PubMed]
- Asiedu, K.; Etuaful, S. Socioeconomic implications of Buruli ulcer in Ghana: A three-year review. Am. J. Trop. Med. Hyg. 1998, 59, 1015–1022. [Google Scholar] [PubMed]
- Debacker, M.; Portaels, F.; Aguiar, J.; Steunou, C.; Zinsou, C.; Meyers, W.; Dramaix, M. Risk factors for Buruli ulcer, Benin. Emerg. Infect. Dis. 2006, 12, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Brou, T.; Broutin, H.; Elguero, E.; Asse, H.; Guegan, J.F. Landscape diversity related to Buruli ulcer disease in Côte d’Ivoire. PLoS Negl. Trop. Dis. 2008, 2, e271. [Google Scholar] [CrossRef] [PubMed]
- Portaels, F.; Meyers, W.M.; Ablordey, A.; Castro, A.G.; Chemlal, K.; de Rijk, P.; Elsen, P.; Fissette, K.; Fraga, A.G.; Lee, R.; et al. First cultivation and characterization of Mycobacterium ulcerans from the environment. PLoS Negl. Trop. Dis. 2008, 2, e178. [Google Scholar] [CrossRef] [PubMed]
- Williamson, H.R.; Benbow, M.E.; Nguyen, K.D.; Beachboard, D.C.; Kimbirauskas, R.K.; McIntosh, M.D.; Quaye, C.; Ampadu, E.O.; Boakye, D.; Merritt, R.W.; et al. Distribution of Mycobacterium ulcerans in Buruli ulcer endemic and non-endemic aquatic sites in Ghana. PLoS Negl. Trop. Dis. 2008, 2, e205. [Google Scholar] [CrossRef] [PubMed]
- Doannio, J.; Konan, K.; Dosso, F.; Kone, A.; Konan, Y.; Sankare, Y.; Ekaza, E.; Coulibaly, N.; Odehouri, K.; Dosso, M.; et al. Micronecta sp (Corixidae) et Diplonychus sp (Belostomatidae), deux hémiptères aquatiques hôtes et/ou vecteurs potentiels de Mycobacterium ulcerans agent pathogène de l’ulcère de Buruli en Côte d’Ivoire. Med. Trop. 2011, 71, 53–57. [Google Scholar]
- Williamson, H.R.; Benbow, M.E.; Campbell, L.P.; Johnson, C.R.; Sopoh, G.; Barogui, Y.; Merritt, R.W.; Small, P.L.C. Detection of Mycobacterium ulcerans in the Environment Predicts Prevalence of Buruli Ulcer in Benin. PLoS Negl. Trop. Dis. 2012, 6, e1506. [Google Scholar] [CrossRef] [PubMed]
- Narh, C.A.; Mosi, L.; Quaye, C.; Dassi, C.; Konan, D.O.; Tay, S.C.K.; de Souza, D.K.; Boakye, D.A.; Bonfoh, B. Source Tracking Mycobacterium ulcerans Infections in the Ashanti Region, Ghana. PLoS Negl. Trop. Dis. 2015, 9, e0003437. [Google Scholar] [CrossRef] [PubMed]
- Yeboah-Manu, D.; Röltgen, K.; Opare, W.; Asan-Ampah, K.; Quenin-Fosu, K.; Asante-Poku, A.; Ampadu, E.; Fyfe, J.; Koram, K.; Ahorlu, C.; et al. Sero-Epidemiology as a Tool to Screen Populations for Exposure to Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2012, 6, e1460. [Google Scholar] [CrossRef] [PubMed]
- Ablordey, A.; Swings, J.; Hubans, C.; Chemlal, K.; Locht, C.; Portaels, F.; Supply, P. Multilocus variable-number tandem repeat typing of Mycobacterium ulcerans. J. Clin. Microbiol. 2005, 43, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.; Yeboah-Manu, D.; Boakye, D.; Mensah-Quainoo, E.; Rondini, S.; Schelling, E.; Ofori-Adjei, D.; Portaels, F.; Zinsstag, J.; Pluschke, G. Genetic diversity in Mycobacterium ulcerans isolates from Ghana revealed by a newly identified locus containing a variable number of tandem repeats. J. Bacteriol. 2006, 188, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Stragier, P.; Ablordey, A.; Bayonne, L.; Lugor, Y.; Sindani, I.; Suykerbuyk, P.; Wabinga, H.; Meyers, W.; Portaels, F. Heterogeneity among Mycobacterium ulcerans Isolates from Africa. Emerg. Infect. Dis. 2006, 12, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Ablordey, A.; Fonteyne, P.-A.; Stragier, P.; Vandamme, P.; Portaels, F. Identification of a new variable number tandem repeat locus in Mycobacterium ulcerans for potential strain discrimination among African isolates. Clin. Microbiol. Infect. 2007, 13, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Yip, M.J.; Porter, J.L.; Fyfe, J.A.M.; Lavender, C.J.; Portaels, F.; Rhodes, M.; Kator, H.; Colorni, A.; Jenkin, G.A.; Stinear, T. Evolution of Mycobacterium ulcerans and Other Mycolactone-Producing Mycobacteria from a Common Mycobacterium marinum Progenitor. J. Bacteriol. 2007, 189, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Kaser, M.; Gutmann, O.; Hauser, J.; Stinear, T.; Cole, S.; Yeboah-Manu, D.; Dernick, G.; Certa, U.; Pluschke, G. Lack of insertional-deletional polymorphism in a collection of Mycobacterium ulcerans isolates from Ghanaian Buruli ulcer patients. J. Clin. Microbiol. 2009, 47, 3640–3646. [Google Scholar] [CrossRef] [PubMed]
- Roltgen, K.; Qi, W.; Ruf, M.T.; Mensah-Quainoo, E.; Pidot, S.J.; Seemann, T.; Stinear, T.P.; Kaser, M.; Yeboah-Manu, D.; Pluschke, G. Single nucleotide polymorphism typing of Mycobacterium ulcerans reveals focal transmission of buruli ulcer in a highly endemic region of Ghana. PLoS Negl. Trop. Dis. 2010, 4, e751. [Google Scholar] [CrossRef] [PubMed]
- Dassi, C.; Mosi, L.; Akpatou, B.; Narh, A.C.; Quaye, C.; Konan, O.D.; Djaman, A.J.; Bonfoh, B. Detection of Mycobacterium ulcerans in Mastomys natalensis and Potential Transmission in Buruli ulcer Endemic Areas in Côte d’Ivoire. Mycobact. Dis. 2015, 5. [Google Scholar] [CrossRef]
- Portaels, F.; WHO. Laboratory diagnosis of Buruli ulcer: A manual for health care providers. Available online: http://www.who.int/iris/handle/10665/111738 (accessed on 29 December 2016).
- Ablordey, A.; Hilty, M.; Stragier, P.; Swings, J.; Portaels, F. Comparative nucleotide sequence analysis of polymorphic variable-number tandem-repeat loci in Mycobacterium ulcerans. J. Clin. Microbiol. 2005, 43, 5281–5284. [Google Scholar] [CrossRef] [PubMed]
- Stragier, P.; Ablordey, A.; Meyers, W.M.; Portaels, F. Genotyping Mycobacterium ulcerans and Mycobacterium marinum by using mycobacterial interspersed repetitive units. J. Bacteriol. 2005, 187, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Hilty, M.; Kaser, M.; Zinsstag, J.; Stinear, T.; Pluschke, G. Analysis of the Mycobacterium ulcerans genome sequence reveals new loci for variable number tandem repeats (VNTR) typing. Microbiology 2007, 153, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Lavender, C.J.; Stinear, T.P.; Johnson, P.D.; Azuolas, J.; Benbow, M.E.; Wallace, J.R.; Fyfe, J.A. Evaluation of VNTR typing for the identification of Mycobacterium ulcerans in environmental samples from Victoria, Australia. FEMS Microbiol. Lett. 2008, 287, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.-I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Ngazoa-Kakou, E.S.; Coulibaly-N’Golo, D.; Aka, N.; Vakou, S.; Aoussi, S.; Dosso, M. Clonality of Mycobacterium ulcerans by Using VNTR-MIRU Typing in Ivory Coast (Côte d’Ivoire), West Africa. Int. J. Trop. Dis. Health 2015, 7, 163–171. [Google Scholar]
- Williamson, H.; Phillips, R.; Sarfo, S.; Wansbrough-Jones, M.; Small, P. Genetic Diversity of PCR-Positive, Culture-Negative and Culture-Positive Mycobacterium ulcerans Isolated from Buruli Ulcer Patients in Ghana. PLoS ONE 2014, 9, e88007. [Google Scholar] [CrossRef] [PubMed]
- Narh, C.; Mosi, L.; Quaye, C.; Tay, S.; Bonfoh, B.; de Souza, D. Genotyping Tools for Mycobacterium ulcerans Drawbacks and Future Prospects. Mycobact. Dis. 2014, 4, 1000149. [Google Scholar] [CrossRef] [PubMed]
- WHO. Number of new cases of Buruli ulcer reported. Available online: http://apps.who.int (accessed on 11 March 2015).
- Vandelannoote, K.; Durnez, L.; Amissah, D.; Gryseels, S.; Dodoo, A.; Yeboah, S.; Addo, P.; Eddyani, M.; Leirs, H.; Ablordey, A.; et al. Application of real-time PCR in Ghana, a Buruli ulcer-endemic country, confirms the presence of Mycobacterium ulcerans in the environment. FEMS Microbiol. Lett. 2010, 304, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.; Williamson, H.; Silverman, J.; Small, P.L. Newly identified Mycobacterium species in a Xenopus laevis colony. Comp. Med. 2007, 57, 97–104. [Google Scholar] [PubMed]
- Tobias, N.J.; Doig, K.D.; Medema, M.H.; Chen, H.; Haring, V.; Moore, R.; Seemann, T.; Stinear, T.P. Complete genome sequence of the frog pathogen Mycobacterium ulcerans ecovar Liflandii. J. Bacteriol. 2013, 195, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Primm, T.P.; Lucero, C.A.; Falkinham, J.O. Health impacts of environmental mycobacteria. Clin. Microbiol. Rev. 2004, 17, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.; Benbow, M.E.; Brenden, T.O.; Qi, J.; Johnson, R.C. Buruli ulcer disease prevalence in Benin, West Africa: Associations with land use/cover and the identification of disease clusters. Int. J. Health Geogr. 2008, 7, 25. [Google Scholar] [CrossRef] [PubMed]


| Clinical History | PCR Test | |||||||
|---|---|---|---|---|---|---|---|---|
| District | Community | Sample Code | Sex | Age (years) | Lesion | Specimen | IS2404 | ER |
| Daloa | Zaïbo | SZ1 | M | 27 | ulcer | swab | Pos | Neg |
| SZ2 | F | 65 | ulcer | swab | Neg | Neg | ||
| SZ3 | F | 27 | ulcer | swab | Neg | Neg | ||
| Gorodi | FG1 | M | 21 | nodule | FNA | Pos | Neg | |
| SG2 | F | 29 | ulcer | swab | Pos | Neg | ||
| SG3 | F | 70 | ulcer | swab | Pos | Neg | ||
| SG4 | F | 48 | ulcer | swab | Pos | Neg | ||
| Tiassalé (Taabo) | Léléblé | SL1 | M | 7 | ulcer | swab | Pos | Neg |
| SL2 | M | 7 | ulcer | swab | Pos | Neg | ||
| SL3 | F | 43 | ulcer | swab | Pos | Pos | ||
| SL4a | M | 10 | ulcer | swab | Pos | Neg | ||
| SL4b | M | 10 | ulcer | swab | Pos | Neg | ||
| Sokrogbo | SS1 | M | 60 | ulcer | swab | Pos | Neg | |
| SS2 | F | 10 | ulcer | swab | Pos | Pos | ||
| SS3 | F | 56 | ulcer | swab | Pos | Neg | ||
| SS4 | F | 19 | ulcer | swab | Pos | Neg | ||
| SS5 | F | 30 | ulcer | swab | Pos | Neg | ||
| SS6a | M | 17 | ulcer | swab | Pos | Neg | ||
| SS6b | M | 17 | ulcer | swab | Pos | Neg | ||
| SS7 | F | 20 | ulcer | swab | Pos | Neg | ||
| SS8 | F | 30 | ulcer | swab | Pos | Neg | ||
| SS9 | M | 36 | ulcer | swab | Pos | Neg | ||
| Ahondo | SA1 | M | 12 | ulcer | swab | Pos | Pos | |
| SA2 | M | 50 | ulcer | swab | Pos | Neg | ||
| SA3a | M | 25 | ulcer | swab | Pos | Neg | ||
| SA3b | M | 25 | ulcer | swab | Pos | Neg | ||
| FA4 | M | 49 | nodule | FNA | Pos | Neg | ||
| SA6 | F | 13 | ulcer | swab | Pos | Pos | ||
| SA7 | F | 18 | ulcer | swab | Pos | Neg | ||
| SA8 | M | 44 | ulcer | swab | Pos | Neg | ||
| SA9 | F | 6 | ulcer | swab | Pos | Pos | ||
| SA10 | M | 12 | ulcer | swab | Pos | Pos | ||
| SA11 | M | 10 | ulcer | swab | Pos | Pos | ||
| SA12a | M | 44 | ulcer | swab | Pos | Neg | ||
| SA12b | M | 44 | ulcer | swab | Pos | Neg | ||
| BU Confirmed Clinical Cases | |||||||
| Community | Sample Code | Allelic Profiles | Genotype | Reference | |||
| MIRU1 | Locus 6 | ST1 | Locus 19 | ||||
| Léléblé | SL2 | 1 | 1 | N/D | N/D | undetermined | current study |
| SL3 | 3 | N/D | 2 | 2 | C- | [13,34] | |
| Sokrogbo | SS1 | 9 | 1 | N/D | N/D | undetermined | current study |
| SS2 | 3 | N/D | 2 | 2 | C- | [13,34] | |
| Ahondo | SA2 | N/D | 1 | N/D | 4 | undetermined | current study |
| SA9 | 3 | 1 | N/D | N/D | C- | [13,34] | |
| SA10 | 3 | 1 | 2 | 2 | C | [13,34] | |
| Published African MPM Genotypes | |||||||
| Allelic Profiles | Genotype | Reference | |||||
| MPM Strain | MIRU1 | Locus 6 | ST1 | Locus 19 | |||
| M. ulcerans | 1 | 1 | 1 | 2 | A | [35] | |
| 3 | 1 | 1 | 2 | B | |||
| 3 | 1 | 2 | 2 | C | |||
| 1 | 1 | 2 | 2 | D | |||
| Mycolactone-producing M. marinum and M. pseudoshottsii | 1 | 4 | 2 | 2 | MPM | ||
| Mycolactone-producing M. liflandii | 1 | 2 | 2 | 1 | MPML | ||
| Communities | Water Bodies | 16S rRNA N° (%) | IS2404 N° (%) | |
|---|---|---|---|---|
| Daloa | Zaïbo | Gbouwa pond | 9/13 (69.23%) | 4/9 (44.44%) |
| Tourou pond | 13/13 (100%) | 1/13 (7.69%) | ||
| La Lobo River | 11/13 (84.62%) | 2/11 (18.18%) | ||
| Gorodi | Godo River | 7/13 (53.85%) | 1/7 (14.26%) | |
| Nidrou 1 pond | 5/13 (38.46%) | 0/5 (0%) | ||
| Nidrou 2 pond | 10/13 (76.92%) | 2/10 (20%) | ||
| Sub-total | 55/78 (71.0%) | 10/55 (18.2%) | ||
| Tiassalé | Léléblé | N’ziba pond | 11/13 (84.62%) | 1/11 (9.09%) |
| Do pond | 7/13 (53.85%) | 0/7 (0%) | ||
| Barrage pond | 5/13 (38.46%) | 1/5 (20%) | ||
| Lahôbloua pond | 10/13 (76.92%) | 1/10 (10%) | ||
| Sokrogbo | Woudigné pond | 4/13 (30.77%) | 2/4 (50%) | |
| Barrage 1 pond | 4/13 (30.77%) | 0/4 (0%) | ||
| Barrage 2 pond | 2/13 (15.38%) | 0/2 (0%) | ||
| Ahondo | Djapipo Barrage pond | 0/13 (0%) | 0/0 (0%) | |
| Bandama River | 5/13 (38.46%) | 2/5 (40%) | ||
| sub-total | 48/117 (41%) | 7/48 (14.5%) | ||
| Overall Prevalence | 103/195 (52.82%) | 17/103 (16.50%) | ||
| Sample Matrices | 16S rRNA N° (%) | IS2404 N° (%) | |
|---|---|---|---|
| Sample Type | Plant biofilm | 38/75 (50.67 %) | 7/38 (18.42%) |
| Water filtrand | 20/30 (66.67 %) | 5/20 (25%) | |
| Plant detritus | 25/45 (55.56 %) | 4/25 (16%) | |
| Soil | 20/45 (44.44 %) | 1/20 (5%) | |
| Total | 103/195 (52.82 %) | 17/103 (16.50%) |
| Clinical Isolates | ||||
| District | Community | Type of Sample (ID) | IS2404 Identity | |
| Tiassalé (Taabo) | Léléblé | Lesion swab (SL3) | 98% for M. liflandii 128 FXT | |
| Sokrogbo | Lesion swab (SS1) | 97% for M. pseudoshottsii L15 | ||
| Lesion swab (SS2) | 97% for M. liflandii 128 FXT | |||
| Lesion swab (SS6a) | N/D | |||
| Ahondo | Lesion swab (SA2) | 96% for M. ulcerans Agy99 | ||
| Lesion swab (SA3b) | N/D | |||
| Lesion swab (SA9) | N/D | |||
| Lesion swab (SA10) | 95% for M. pseudoshottsii L15 | |||
| Lesion swab (SA12a) | N/D | |||
| Environmental Isolates | ||||
| Daloa | Zaïbo | Gbouwa pond/water filtrand (ZGF1) | N/D | |
| Tourou pond/water filtrand (ZTF1) | 89% for M. liflandii 128 FXT | |||
| Gorodi | Godo River/plant biofilm (GGB4) | 96% for Mycobacterium sp. YM-1 | ||
| Nidrou2 pond/plant biofilm (GN2B5) | 97% for M. liflandii 128 FXT | |||
| Tiassalé (Taabo) | Léléblé | N’Ziba pond/plant biofilm (LNB1) | 99% for M. liflandii 128 FXT | |
| Lahôbloua pond/plant biofilm (LLB5) | N/D | |||
| Sokrogbo | Woudigné pond/plant detritus (SWD1) | N/D | ||
| Woudigné pond/plant detritus (SWD3) | 99% for M. liflandii 128 FXT | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dassi, C.; Mosi, L.; Narh, C.A.; Quaye, C.; Konan, D.O.; Djaman, J.A.; Bonfoh, B. Distribution and Risk of Mycolactone-Producing Mycobacteria Transmission within Buruli Ulcer Endemic Communities in Côte d’Ivoire. Trop. Med. Infect. Dis. 2017, 2, 3. https://doi.org/10.3390/tropicalmed2010003
Dassi C, Mosi L, Narh CA, Quaye C, Konan DO, Djaman JA, Bonfoh B. Distribution and Risk of Mycolactone-Producing Mycobacteria Transmission within Buruli Ulcer Endemic Communities in Côte d’Ivoire. Tropical Medicine and Infectious Disease. 2017; 2(1):3. https://doi.org/10.3390/tropicalmed2010003
Chicago/Turabian StyleDassi, Christelle, Lydia Mosi, Charles A. Narh, Charles Quaye, Danièle O. Konan, Joseph A. Djaman, and Bassirou Bonfoh. 2017. "Distribution and Risk of Mycolactone-Producing Mycobacteria Transmission within Buruli Ulcer Endemic Communities in Côte d’Ivoire" Tropical Medicine and Infectious Disease 2, no. 1: 3. https://doi.org/10.3390/tropicalmed2010003
APA StyleDassi, C., Mosi, L., Narh, C. A., Quaye, C., Konan, D. O., Djaman, J. A., & Bonfoh, B. (2017). Distribution and Risk of Mycolactone-Producing Mycobacteria Transmission within Buruli Ulcer Endemic Communities in Côte d’Ivoire. Tropical Medicine and Infectious Disease, 2(1), 3. https://doi.org/10.3390/tropicalmed2010003






