First Report of the L925I kdr Mutation Associated with Pyrethroid Resistance in Genetically Distinct Triatoma dimidiata, Vector of Chagas Disease in Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. DNA Extraction
2.3. Amplification and Sequencing of cyt b and ND4
2.4. Genetic Diversity and Population Differentiation
2.5. Haplotype Network Construction
2.6. Screening for kdr-Type Mutations in the VGSC (Para) Gene
3. Results
3.1. Genetic Diversity and Population Structure
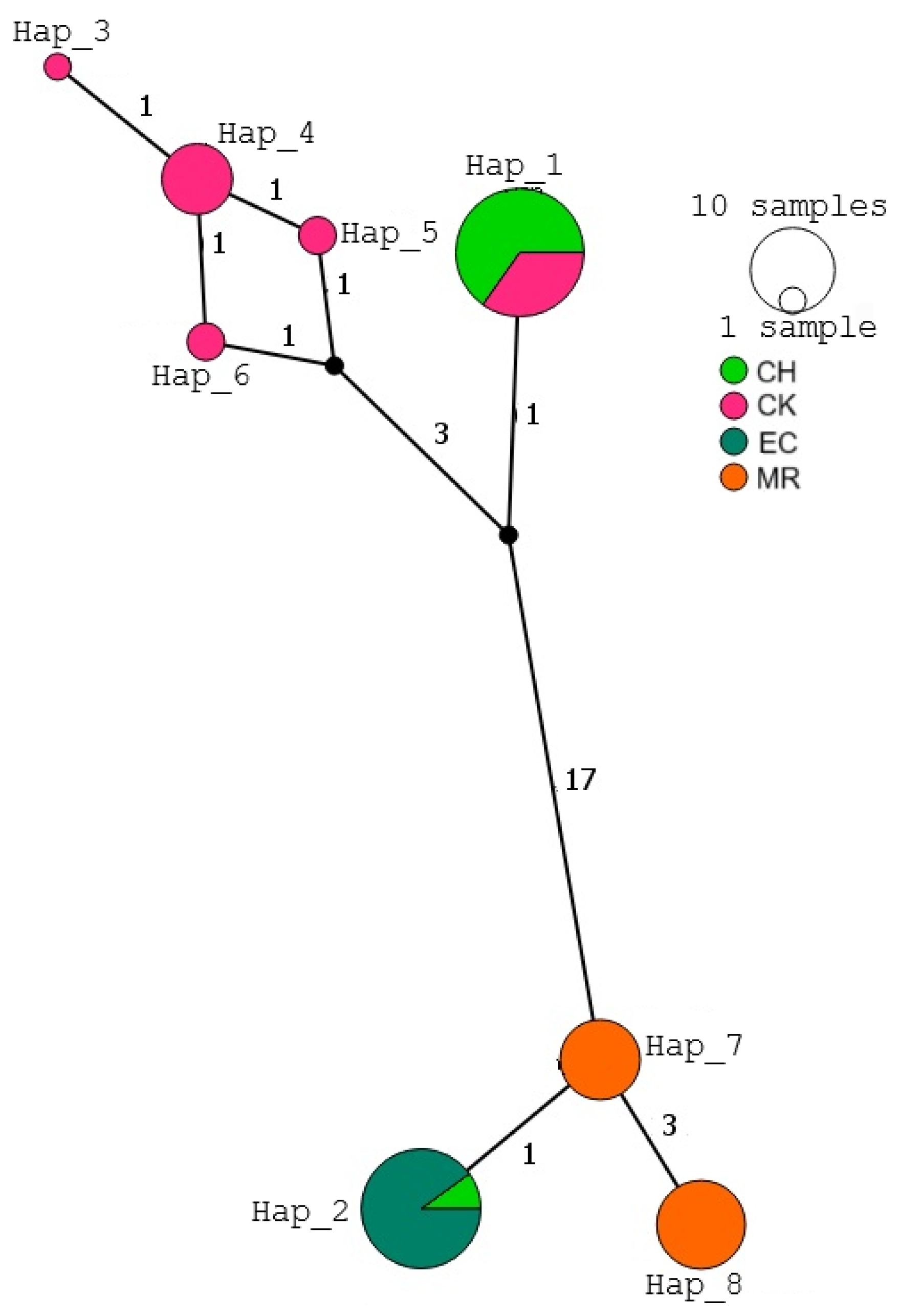
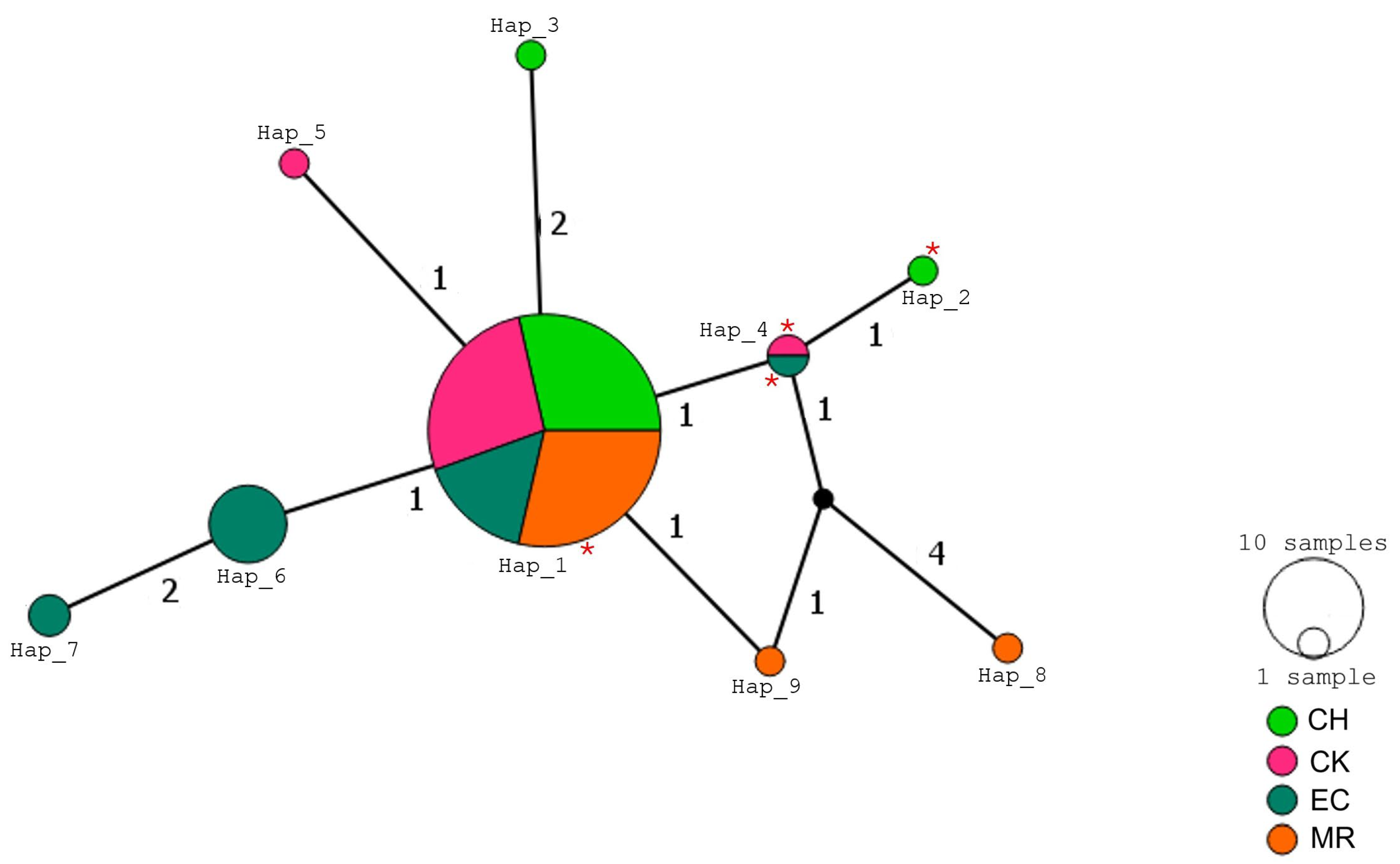
3.2. Mitochondrial Haplotype Networks
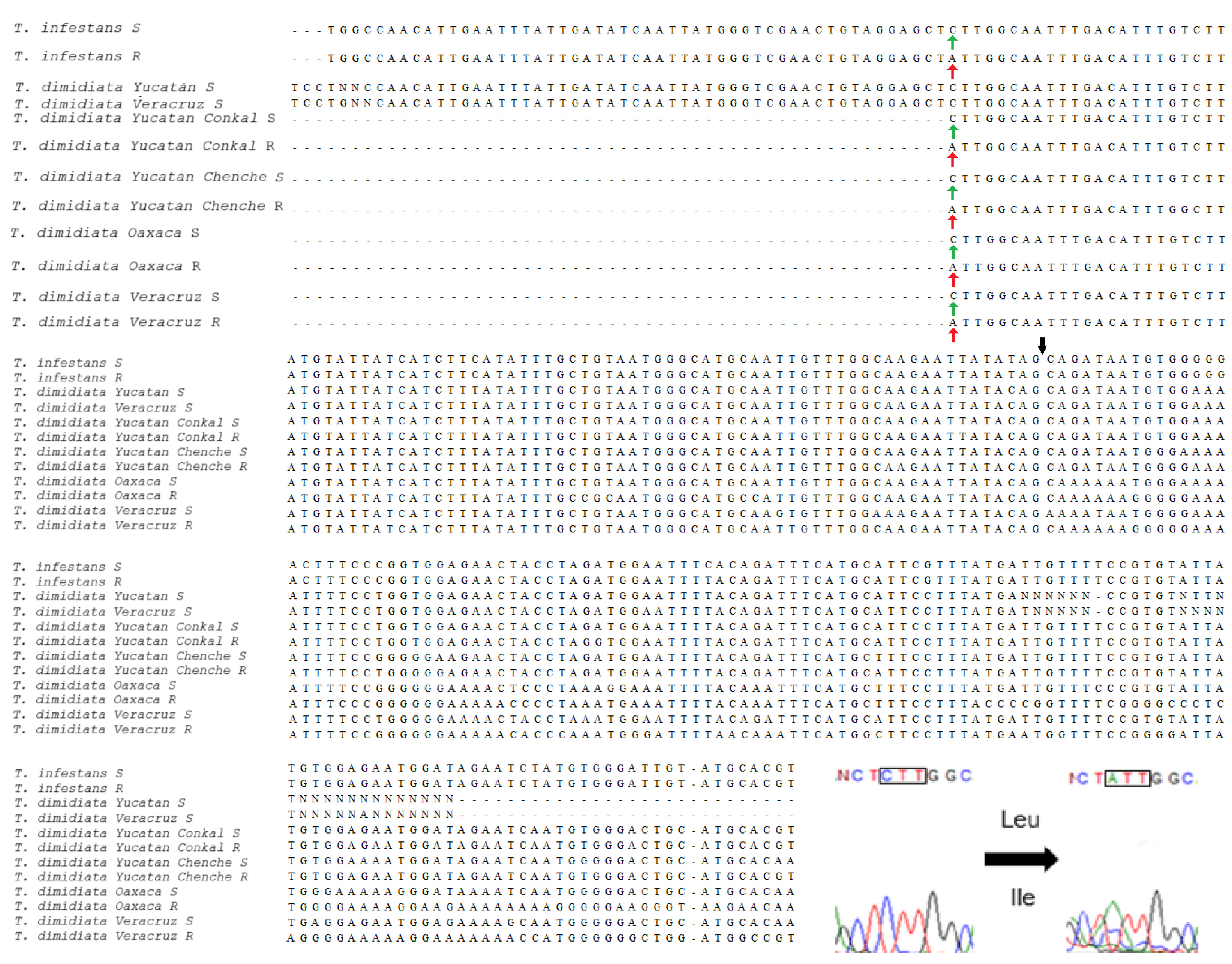
3.3. Analysis of VGSC Mutations
4. Discussion
4.1. Genetic Diversity and Population Structure
4.2. Haplotype Networks and Geographic Differentiation
4.3. Knockdown Resistance Mutations in the VGSC Para Gene
4.4. Implications for Vector Control
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Chagas Disease. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 10 April 2024).
- Coura, J.R. The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions-a comprehensive review. Mem. Inst. Oswaldo Cruz 2015, 110, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Cholewiński, M.; Derda, M.; Hadaś, E. Parasitic diseases in humans transmitted by vectors. Ann. Parasitol. 2015, 61, 137–157. [Google Scholar] [PubMed]
- Bravo-Ramírez, I.E.; Pech-May, A.; May-Concha, I.J.; Ramsey, J.M. Conocimientos actuales sobre Trypanosoma cruzi y la enfermedad de Chagas en México: Una revisión sistemática. Salud Publ. Mex. 2023, 65, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Cesaretto, N.R.; dos Reis, Y.V.; de Oliveira, J.; Galvão, C.; Alevi, K.C.C. Revisiting the genetic, taxonomic and evolutionary aspects of Chagas disease vectors of the Triatoma phyllosoma subcomplex (Hemiptera, Triatominae). Diversity 2022, 14, 978. [Google Scholar] [CrossRef]
- Magallón-Gastelum, E.; Lozano, F.; Gutierréz, M.; Flores, A.; Sánchez, B.; Espinoza, B.; Brenière, S. Epidemiological risk for Trypanosoma cruzi transmission by species of Phyllosoma complex in the occidental part of Mexico. Acta Trop. 2006, 97, 331–338. [Google Scholar] [CrossRef]
- Bargues, M.D.; Klisiowicz, D.R.; Gonzalez-Candelas, F.; Ramsey, J.K.; Monroy, C.; Ponce, C.; Salazar-Schettino, P.M.; Panzera, F.; Abad-Franch, F.; Sousa, O.E.; et al. Phylogeography and genetic variation of Triatoma dimidiata, the main Chagas disease vector in Central America, and its position within the genus Triatoma. PLoS Negl. Trop. Dis. 2008, 2, e233. [Google Scholar] [CrossRef]
- Ramsey, J.M.; Peterson, A.T.; Carmona-Castro, O.; Moo-Llanes, D.A.; Nakazawa, Y.; Butrick, N.; Tun-Ku, E.; De la Cruz-Félix, K.; Ibarra-Cerdeña, C.N. Atlas of Mexican Triatominae (Reduviidae: Hemiptera) and vector transmission of Chagas disease. Mem. Inst. Oswaldo Cruz 2015, 110, 339–352. [Google Scholar] [CrossRef]
- Salazar-Schettino, P.; Bucio-Torres, M.; Cabrera-Bravo, M.; Alba-Alvarado, M.; Castillo-Saldaña, D.; Zenteno-Galindo, E.; Perera-Salazar, M.G. Enfermedad de Chagas en México. Rev. Fac. Med. 2016, 59, 6–16. [Google Scholar]
- Badel-Mogollón, J.; Rodríguez-Figueroa, L.; Parra-Henao, G. Análisis espacio temporal de las condiciones biofísicas y ecológicas de Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) en la región nororiental de los Andes de Colombia. Biomédica 2017, 37, 106–123. [Google Scholar] [CrossRef]
- Killets, K.C.; Wormington, J.; Zecca, I.; Chaves, L.F.; Hamer, G.L.; Hamer, S.A. Comparative feeding and defecation behaviors of Trypanosoma cruzi-infected and uninfected triatomines (Hemiptera: Reduviidae) from the Americas. Insects 2025, 16, 188. [Google Scholar] [CrossRef]
- Gourbière, S.; Dorn, P.; Tripet, F.; Dumonteil, E. Genetics and evolution of triatomines: From phylogeny to vector control. Heredity 2012, 108, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ferrer, A.; Waleckx, E.; Rascalou, G.; Dumonteil, E.; Gourbière, S. Trypanosoma cruzi transmission dynamics in a synanthropic and domesticated host community. PLoS Negl. Trop. Dis. 2019, 13, e0007902. [Google Scholar] [CrossRef]
- Moo-Millan, J.I.; Arnal, A.; Pérez-Carrillo, S.; Hernandez-Andrade, A.; Ramirez-Sierra, M.J.; Rosado-Vallado, M.; Dumonteil, E.; Waleckx, E. Disentangling Trypanosoma cruzi transmission cycle dynamics through the identification of blood meal sources of natural populations of Triatoma dimidiata in Yucatán, Mexico. Parasites Vectors 2019, 12, 572. [Google Scholar] [CrossRef]
- Dumonteil, E.; Gourbière, S.; Barrera-Perez, M.; Rodriguez-Felix, E.; Ruiz-Piña, H.; Baños-Lopez, O.; Ramirez-Sierra, M.J.; Menu, F.; Rabinovich, J.E. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatan Peninsula of Mexico. Am. J. Trop. Med. Hyg. 2002, 67, 176–183. [Google Scholar] [CrossRef]
- Gamboa-León, R.; Ramirez-Gonzalez, C.; Pacheco-Tucuch, F.S.; O’Shea, M.; Rosecrans, K.; Pippitt, J.; Dumonteil, E.; Buekens, P. Seroprevalence of Trypanosoma cruzi among mothers and children in rural Mayan communities and associated reproductive outcomes. Am. J. Trop. Med. Hyg. 2014, 91, 348–353. [Google Scholar] [CrossRef]
- Pech-May, A.; Mazariegos-Hidalgo, C.; Izeta, A.; López, S.; Tun-Ku, E.; De la Cruz-Félix, K.; Ramsey, J. Genetic variation and phylogeography of the Triatoma dimidiata complex evidence a potential center of origin and recent divergence of haplogroups having differential Trypanosoma cruzi and DTU infections. PLoS Negl. Trop. Dis. 2019, 13, e0007044. [Google Scholar] [CrossRef]
- Monteiro, F.A.; Peretolchina, T.; Lazoski, C.; Harris, K.; Dotson, E.M.; Abad-Franch, F.; Tamayo, E.; Pennington, P.M.; Monroy, C.; Cordon-Rosales, C.; et al. Phylogeographic pattern and extensive mitochondrial DNA divergence disclose a species complex within the Chagas disease vector Triatoma dimidiata. PLoS ONE 2013, 8, e70974. [Google Scholar] [CrossRef]
- Dorn, P.L.; de la Rúa, N.M.; Axen, H.; Smith, N.; Richards, B.R.; Charabati, J.; Suarez, J.; Woods, A.; Pessoa, R.; Monroy, C.; et al. Hypothesis testing clarifies the systematics of the main Central American Chagas disease vector, Triatoma dimidiata (Latreille, 1811), across its geographic range. Infect. Genet. Evol. 2016, 44, 431–443. [Google Scholar] [CrossRef]
- Justi, S.A.; Galvão, C.; Schrago, C.G. Geological changes of the Americas and their influence on the diversification of the Neotropical kissing bugs (Hemiptera: Reduviidae: Triatominae). PLoS Negl. Trop. Dis. 2016, 10, e0004527. [Google Scholar] [CrossRef]
- DOF (Diario Oficial de la Federación). NOM-032-SSA2-2014 Para la Vigilancia Epidemiológica, Promoción, Prevención Y Control de las Enfermedades Transmitidas por Vectores; Diario Oficial de la Federación: Ciudad de México, México, 2015; pp. 1–43. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5389045&fecha=16/04/2015#gsc.tab=0 (accessed on 10 May 2025).
- Reyes, M.; Angulo, V.; Sandoval, C. Toxic effect of b-cipermethrin, deltamethrin and fenitrothion in colonies of Triatoma dimidiata (Latreille, 1811) and Triatoma maculata (Erichson, 1848) (Hemiptera, Reduviidae). Biomédica 2007, 27, 75–82. [Google Scholar] [CrossRef][Green Version]
- Santo-Orihuela, P.L.; Carvajal, G.; Picollo, M.I.; Vassena, C.V. Toxicological and biochemical analysis of the susceptibility of sylvatic Triatoma infestans from the Andean Valley of Bolivia to organophosphate insecticide. Mem. Inst. Oswaldo Cruz 2013, 108, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Fabro, J.; Sterkel, M.; Capriotti, N.; Mougabure-Cueto, G.; Germano, M.; Rivera-Pomar, R.; Ons, S. Identification of a point mutation associated with pyrethroid resistance in the para-type sodium channel of Triatoma infestans, a vector of Chagas’ disease. Infect. Genet. Evol. 2012, 12, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, N.; Mougabure-Cueto, G.; Rivera-Pomar, R.; Ons, S. L925I mutation in the para-type sodium channel is associated with pyrethroid resistance in Triatoma infestans from the Gran Chaco region. PLoS Negl. Trop. Dis. 2014, 8, e2659. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, F.D.; Du, Y.; Dong, K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013, 106, 93–100. [Google Scholar] [CrossRef]
- Corbel, V.; N’Guessan, R. Distribution, Mechanisms, Impact and Management of Insecticide Resistance in Malaria Vectors: A Pragmatic Review. In Anopheles Mosquitoes—New Insights into Malaria Vectors; Manguin, S., Ed.; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Dong, K.; Du, Y.; Rinkevich, F.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef]
- Davila-Barboza, J.; Villanueva-Segura, O.K.; Lopez-Monroy, B.; Ponce-Garcia, G.; Bobadilla-Utrera, C.; Montes-Rincon, M.; Molina-Garza, Z.J.; Arredondo-Jimenez, J.I.; Rodriguez-Sanchez, I.P.; Manrique-Saide, P.C.; et al. Novel Kdr mutations (K964R and A943V) in pyrethroid-resistant populations of Triatoma mazzottii and Triatoma longipennis from Mexico and detoxifying enzymes. Insect Sci. 2019, 26, 809–820. [Google Scholar] [CrossRef]
- Davila-Barboza, J.; Villanueva-Segura, O.K.; Ponce-Garcia, G.; Lopez-Monroy, B.; Rodiguez-Sanchez, P.; Flores, A.E. First report of two kdr mutations L1014F/S in natural populations of Triatoma pallidipennis Stal and Triatoma picturata Usinger vectors of Chagas disease in Mexico. J. Vector Ecol. 2019, 44, 285–289. [Google Scholar] [CrossRef]
- Lent, H.; Wygodzinsky, P.W. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas disease. Bull. Am. Mus. Nat. Hist. 1979, 163, 123–520. [Google Scholar]
- Coen, E.; Strachan, T.; Dover, G. Dynamics of concerted evolution of ribosomal DNA and histone gene families in the melanogaster species subgroup of Drosophila. J. Mol. Biol. 1982, 158, 17–35. [Google Scholar] [CrossRef]
- Martínez, F.; Villalobos, G.; Cevallos, A.; De la Torre, P.; Laclette, J.; Alejandre, R.; Espinoza, B. Taxonomic study of the Phyllosoma complex and other triatomine (Insecta: Hemiptera: Reduviidae) species of epidemiological importance in the transmission of Chagas disease: Using ITS-2 and mtCytB sequences. Mol. Phylogenet. Evol. 2006, 41, 279–287. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Davila-Barboza, J. Mutaciones “kdr” y Enzimas Asociadas a la Resistencia a Piretroides en Triatominos (Hemimptera: Reduvidae) Vectores de la Enfermedad de Chagas en México. Ph.D. Thesis, Universidad Autónoma de Nuevo León, Nuevo León, México, 2018. [Google Scholar]
- Melgar, S.; Castellanos, S.; Stevens, L.; Monroy, M.C.; Dorn, P.L. Genetic diversity of the Chagas vector Triatoma dimidiata s.l. (Hemiptera: Reduviidae) across geographic scales in a top-priority area for control. J. Med. Entomol. 2024, 61, 1309–1321. [Google Scholar] [CrossRef]
- Gómez-Palacio, A.; Triana, O. Molecular Evidence of demographic expansion of the chagas disease vector Triatoma dimidiata (Hemiptera, Reduviidae, Triatominae) in Colombia. PLoS Negl. Trop. Dis. 2014, 8, e2734. [Google Scholar] [CrossRef]
- Velásquez-Ortiz, N.; Hernández, C.; Cantillo-Barraza, O.; Medina, M.; Medina-Alfonso, M.; Suescún-Carrero, S.; Muñoz, M.; Vega, L.; Castañeda, S.; Cruz-Saavedra, L.; et al. Estimating the genetic structure of Triatoma dimidiata (Hemiptera: Reduviidae) and the transmission dynamics of Trypanosoma cruzi in Boyacá, eastern Colombia. PLoS Negl. Trop. Dis. 2022, 16, e0010534. [Google Scholar] [CrossRef]
- Pech-May, A.; Ramsey, J.M.; González, R.E.; Giuliani, M.; Berrozpe, P.; Quintana, M.G.; Salomon, O.D. Genetic diversity, phylogeography and molecular clock of the Lutzomyia longipalpis complex (Diptera: Psychodidae). PLoS Negl. Trop. Dis. 2018, 12, e0006614. [Google Scholar] [CrossRef]
- Sierra, I.; Capriotti, N.; Fronza, G.; Mougabure-Cueto, G.; Ons, S. Kdr mutations in Triatoma infestans from the Gran Chaco are distributed in two differentiated foci: Implications for pyrethroid resistance management. Acta Trop. 2016, 158, 208–213. [Google Scholar] [CrossRef]
- Fronza, G.; Roca-Acevedo, G.; Mougabure-Cueto, G.A.; Sierra, I.; Capriotti, N.; Toloza, A.C. Insecticide resistance mechanisms in Triatoma infestans (Reduviidae: Triatominae): The putative role of enhanced detoxification and knockdown resistance (kdr) allele in a resistant hotspot from the Argentine Chaco. J. Med. Entomol. 2020, 57, 837–844. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, S.; Zhou, J.; Du, Y.; Zhang, Y.; Wang, J. Status of insecticide resistance and associated mutations in Q-biotype of whitefly, Bemisia tabaci, from eastern China. Crop Prot. 2012, 31, 67–71. [Google Scholar] [CrossRef]
- Palenchar, D.; Gellatly, K.; Yoon, K.; Mumcuoglu, K.; Shalom, U.; Clark, J. Quantitative sequencing for the determination of kdr-type resistance allele (V419L, L925I, I936F) frequencies in common bed bug (Hemiptera: Cimicidae) populations collected from Israel. J. Med. Entomol. 2015, 52, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Ilias, A.; Tsagkarakou, A. Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J. Apic. Res. 2017, 56, 625–630. [Google Scholar] [CrossRef]
- Usherwood, P.N.; Davies, T.G.; Mellor, I.R.; O’Reilly, A.O.; Peng, F.; Vais, H.; Khambay, B.P.; Field, L.M.; Williamson, M.S. Mutations in DIIS5 and the DIIS4–S5 linker of Drosophila melanogaster sodium channel define binding domains for pyrethroids and DDT. FEBS Lett. 2007, 581, 5485–5492. [Google Scholar] [CrossRef]
- Chanon, K.E.; Mendez-Galvan, J.F.; Galindo-Jaramillo, J.M.; Olguin-Bernal, H.; Borja-Aburto, V.H. Cooperative actions to achieve malaria control without the use of DDT. Int. J. Hyg. Environ. Health 2003, 206, 387–394. [Google Scholar] [CrossRef]
- Flores, A.E.; Ponce, G.; Silva, B.G.; Gutierrez, S.M.; Bobadilla, C.; Lopez, B.; Mercado, R.; Black, W.C., IV. Widespread cross-resistance to pyrethroids in Aedes aegypti (L.) from Veracruz State, Mexico. J. Econ. Entomol. 2013, 106, 959–969. [Google Scholar] [CrossRef]
- Samake, J.N.; Yared, S.; Getachew, D.; Mumba, P.; Dengela, D.; Yohannes, G.; Chibsa, S.; Hee, C.S.; Spear, J.; Irish, S.R.; et al. Detection and population genetic analysis of kdr L1014F variant in eastern Ethiopian Anopheles stephensi. Infect. Genet. Evol. 2022, 99, 105235. [Google Scholar] [CrossRef]
- Remón, C.; Fronza, G.; Maza, Y.; Sartor, P.; Weinberg, D.; Mougabure-Cueto, G. Resistance to deltamethrin in Triatoma infestans: Microgeographical distribution, validation of a rapid detection bioassay and evaluation of a fumigant canister as control alternative strategy. Bull. Entomol. Res. 2020, 110, 645–653. [Google Scholar] [CrossRef]
- Zuluaga, S.; Fernandez, G.J.; Mejía-Jaramillo, A.M.; Lowenberger, C.; Triana-Chavez, O. Exploring novel pyrethroid resistance mechanisms through RNA-seq in Triatoma dimidiata from Colombia. Curr. Res. Insect Sci. 2024, 7, 100103. [Google Scholar] [CrossRef]
- Lobbia, P.A.; Rodríguez, C.; Mougabure-Cueto, G. Can infection with Trypanosoma cruzi modify the toxicological response of Triatoma infestans susceptible and resistant to deltamethrin? Acta Trop. 2023, 245, 106969. [Google Scholar] [CrossRef]
- Cuncunubá, Z.M.; Gutiérrez-Romero, S.A.; Ramirez, J.D.; Velásquez-Ortiz, M.M.; Ceccarelli, S.; Parra-Henao, G.; Henao-Martínez, A.F.; Rabinovich, J.; Basáñez, M.G.; Nouvellet, P.; et al. The epidemiology of Chagas disease in the Americas. Lancet Reg. Health 2024, 17, 100881. [Google Scholar] [CrossRef]
- Hernández, M.L.; Dujardin, J.P.; Villacís, A.G.; Yumiseva, C.A.; Remón, C.; Mougabure-Cueto, G. Resistance to deltamethrin in Triatoma infestans (Hemiptera: Reduviidae): Does it influence the phenotype of antennae, wings, and heads? Acta Trop. 2023, 245, 106976. [Google Scholar] [CrossRef]
- Mougabure-Cueto, G.; Picollo, M.I. Insecticide Resistance in Triatomines. In Triatominae—The Biology of Chagas Disease Vectors; Guarneri, A.A., Lorenzo, M.G., Eds.; Entomology in Focus; Springer: Cham, Switzerland, 2021; Volume 5, pp. 537–555. [Google Scholar] [CrossRef]
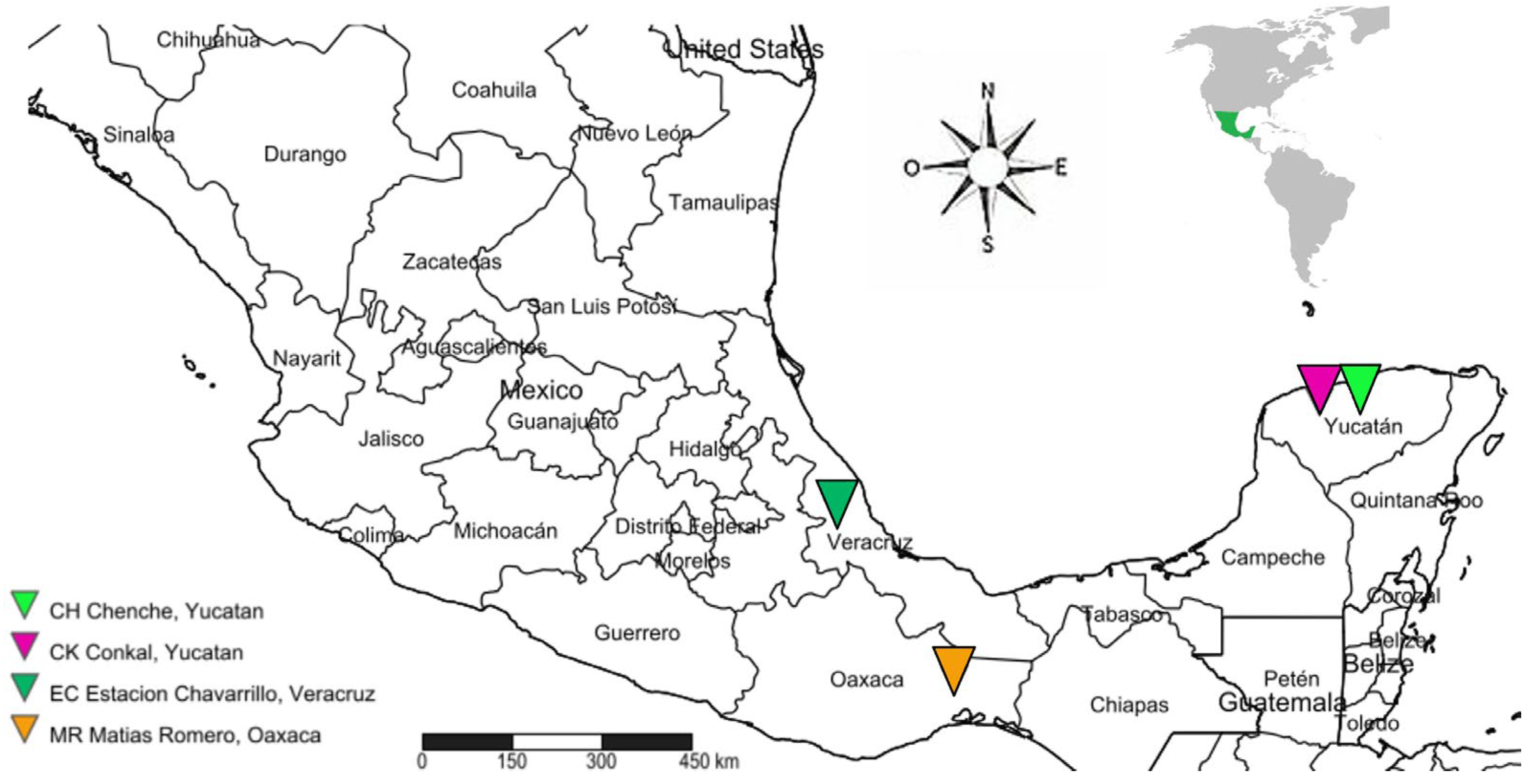

| Molecular Marker | Indices | Chenche | Conkal | Estacion Chavarrillo | Matias Romero | Overall |
|---|---|---|---|---|---|---|
| cyt b | N | 17 | 20 | 18 | 20 | 75 |
| η | 19 | 7 | 0 | 3 | 27 | |
| S | 19 | 7 | 0 | 3 | 27 | |
| Su | 0 | 1 | 0 | 0 | 1 | |
| K | 4.191 | 3.174 | 0 | 1.563 | 11.189 | |
| Nh | 2 | 5 | 1 | 2 | 8 | |
| Hd ± SD | 0.221 ± 0.121 | 0.732 ± 0.064 | 0 | 0.521 ± 0.042 | 0.799 ± 0.023 | |
| π | 0.016 | 0.013 | 0 | 0.006 | 0.044 | |
| θ | 0.022 | 0.008 | - | 0.003 | 0.022 | |
| Fu’s Fs test | 8.772 ** | 2.116 | - | 4.362 | 14.407 **** | |
| Tajima’s Test D | −1.002 | 2.000 | - | 2.266 * | 3.196 ** | |
| ND4 | N | 19 | 20 | 18 | 20 | 77 |
| η | 7 | 2 | 2 | 5 | 13 | |
| S | 7 | 2 | 2 | 5 | 13 | |
| Su | 0 | 0 | 0 | 0 | 0 | |
| K | 1.719 | 0.674 | 1.046 | 2.626 | 4.779 | |
| Nh | 3 | 4 | 2 | 4 | 10 | |
| Hd ± SD | 0.608 ± 0.070 | 0.505 ± 0.125 | 0.523 ± 0.047 | 0.784 ± 0.035 | 0.889 ± 0.012 | |
| π | 0.009 | 0.004 | 0.005 | 0.0137 | 0.025 | |
| θ | 0.010 | 0.003 | 0.003 | 0.007 | 0.014 | |
| Fu’s Fs test | 2.627 | −0.899 | 2.953 | 2.686 | 2.782 | |
| Tajima’s Test D | −0.473 | 0.457 | 1.949 | 2.642 ** | 2.258 * |
| CytB/ND4 | Chenche | Conkal | Estacion Chavarrillo | Matias Romero |
|---|---|---|---|---|
| Chenche | 0.322 ** | 0.846 ** | 0.878 ** | |
| Conkal | 0.326 ** | 0.894 ** | 0.922 ** | |
| Estacion Chavarrillo | 0.753 ** | 0.797 ** | 0.694 ** | |
| Matias Romero | 0.746 ** | 0.939 ** | 0.674 ** |
| cyt b | |||||
|---|---|---|---|---|---|
| Source | df | Sum of Squares | Variance Components | Variation (%) | p Value |
| Among populations | 31 | 6879.388 | 91.249 | 88.458 | 0.001 |
| Within populations | 71 | 845.359 | 11.906 | 11.542 | |
| TOTAL | 74 | 7724.747 | 103.155 | ||
| ND4 | |||||
| Among populations | 3 | 1061.155 | 13.667 | 82.331 | 0.001 |
| Within populations | 73 | 214.117 | 2.933 | 17.670 | |
| TOTAL | 76 | 1275.273 | 16.600 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saucedo-Montalvo, M.C.; Davila-Barboza, J.A.; Gutierrez-Rodriguez, S.M.; Lopez-Monroy, B.; Favela-Lara, S.; Rodriguez-Sanchez, I.P.; Reyes-Solis, G.d.C.; Bobadilla-Utrera, C.; Flores, A.E. First Report of the L925I kdr Mutation Associated with Pyrethroid Resistance in Genetically Distinct Triatoma dimidiata, Vector of Chagas Disease in Mexico. Trop. Med. Infect. Dis. 2025, 10, 182. https://doi.org/10.3390/tropicalmed10070182
Saucedo-Montalvo MC, Davila-Barboza JA, Gutierrez-Rodriguez SM, Lopez-Monroy B, Favela-Lara S, Rodriguez-Sanchez IP, Reyes-Solis GdC, Bobadilla-Utrera C, Flores AE. First Report of the L925I kdr Mutation Associated with Pyrethroid Resistance in Genetically Distinct Triatoma dimidiata, Vector of Chagas Disease in Mexico. Tropical Medicine and Infectious Disease. 2025; 10(7):182. https://doi.org/10.3390/tropicalmed10070182
Chicago/Turabian StyleSaucedo-Montalvo, Mario C., Jesus A. Davila-Barboza, Selene M. Gutierrez-Rodriguez, Beatriz Lopez-Monroy, Susana Favela-Lara, Iram P. Rodriguez-Sanchez, Guadalupe del C. Reyes-Solis, Cristina Bobadilla-Utrera, and Adriana E. Flores. 2025. "First Report of the L925I kdr Mutation Associated with Pyrethroid Resistance in Genetically Distinct Triatoma dimidiata, Vector of Chagas Disease in Mexico" Tropical Medicine and Infectious Disease 10, no. 7: 182. https://doi.org/10.3390/tropicalmed10070182
APA StyleSaucedo-Montalvo, M. C., Davila-Barboza, J. A., Gutierrez-Rodriguez, S. M., Lopez-Monroy, B., Favela-Lara, S., Rodriguez-Sanchez, I. P., Reyes-Solis, G. d. C., Bobadilla-Utrera, C., & Flores, A. E. (2025). First Report of the L925I kdr Mutation Associated with Pyrethroid Resistance in Genetically Distinct Triatoma dimidiata, Vector of Chagas Disease in Mexico. Tropical Medicine and Infectious Disease, 10(7), 182. https://doi.org/10.3390/tropicalmed10070182








